Abstract
The aims of this study were to evaluate the activity of Lactococcus garvieae of dairy origin against pathogenic bacteria during cheese manufacture and its suitability and safety as a probiotic on Nile tilapia (Oreochromis niloticus). For these purposes, Lactococcus garvieae isolated from raw cow milk was tested to control the growth of Staphylococcus aureus in artificially contaminated cheese during storage under refrigeration. Also a feeding experiment was conducted on 120 Oreochromis niloticus using a diet containing Lactococcus garvieae as a probiotic bacteria against pathogenic S. aureus. The findings of this study showed that Lactococcus garvieae of dairy origin produced inhibitory substances against pathogenic microorganisms. The selected strain had a good inhibitory activity against Staphylococcus aureus in artificially contaminated cheese during refrigerated storage. Concerning fish experiment, it showed no evidence of disease in fish that were fed a diet containing Lactococcus garvieae, and showed a higher survival rate than others. Further investigations for purification of the produced inhibitory substance and confirming that is a bacteriocin-like substance are needed. Nonetheless, it is the first report of using L. garvieae of dairy origin as a probiotic for controlling the pathogenic Staphylococcus aureus in Oreochromis niloticus.
Introduction
Lactococcus garvieae (L. garvieae) is one of the genus Lactococcus species [Citation1]. In the past, the species of this genus were known as the lactic acid producing members of streptococci. They are not pathogenic for human or even animals [Citation2], except L. garvieae, which is considered the only pathogenic Lactococcus species. It causes a septicemic process called lactococcosis, that was defined in rainbow trout in Japan for the first time [Citation3]. Since then, L. garvieae has been identified as the main cause for many outbreaks in other fish species in several countries [Citation4]. Currently, it was discovered that L. garvieae is not limited to aquatic species; as it has been caused mastitis in cows and has been found in some dairy products such as goat cheese and raw cow milk [Citation5]. Additionally, L. garvieae is considered also an emerging zoonotic pathogen [Citation6]. Thus, the importance of L. garvieae is increasing in all fields of life either in human or in animals, but the available data for this new pathogen in foods other than fish products are still very scarce [Citation5].
In contrast, the bacteriocins produced by lactococci have been studied extensively; these substances are antimicrobial peptides synthesized by the bacterial ribosome that act mainly against closely related species [Citation7]. Nisin is possibly the most important known bacteriocin. It is produced by Lactococcus lactis strains and used as a food preservative [Citation8]. During a survey of Lactic Acid Bacteria (LAB) for other bacteriocins, antimicrobial substances produced by L. garvieae strain were identified and termed garviecin L1-5 [Citation9]. Then in the last ten years, several new bacteriocins from L. garvieae have been reported including, garvicin ML [Citation10], garvieacin Q [Citation11], garvicin A [Citation12], and garvicin KS [Citation13]. Likewise, there are other non-purified bacteriocins produced by L. garvieae detected by Suneel and Kaliwal [Citation14].
L. garvieae isolated from raw milk and dairy products have been reported to inhibit indicator strains due to the production of bacteriocin [Citation15], while Alomar [Citation16] suggested that hydrogen peroxide may play a role in the inhibition of Staphylococcus aureus (S. aureus) by L. garvieae. The efficiency of L. garvieae for inhibition of S. aureus may also depend on the interactions of both these organisms with the raw milk microflora [Citation17].
Staphylococcus aureus is one of the most prevalent enterotoxin producing microbes, and it is considered the main cause for staphylococcal food poisoning and gastroenteritis worldwide. Enterotoxin production by this strain at levels hazardous to public health has been reported in different cheese varieties [Citation18]. Cheese manufacturing from raw milk can lead to staphylococcal outbreaks, especially when the curd is insufficiently acidified, or when the cheese manufacture occurred under poor hygienic conditions [Citation18]. New biopreservation strategies based on using the inhibitory effect of some bacterial strains, including some strains of microbial communities of raw milk such as LAB, could help in the control of pathogenic S. aureus strain in cheese by several ways including, bacteriocin production, lower pH [Citation19], and H2O2 [Citation20]. On the other hand, Staphylococci are not part of the normal fish microflora and its presence on fish is an indicator for a disease [Citation21,Citation22]. Currently, S. aureus has been recorded recently in Oreochromis niloticus (O. niloticus) causing high mortalities with different histopathological changes [Citation23]. Also, it causes a health hazard for fish handlers and consumers [Citation24]. For these reasons, we choose S. aureus as an indicator to test the effects of the L. garvieae bacteriocin. It is worth mentioning that the administration of probiotics during tilapia fish farming through feed can improve their feed conversion ratio (FCR) and can reduce mortality among the fish by 20% [Citation25]. Furthermore, in finfish, immune responses can be increased using many probiotics through the stimulation of innate and cellular immunity [Citation26].
Therefore, the aims of the present study were evaluation of the ability of L. garvieae strain to control the pathogenic effects of S. aureus during cheese manufacture, and assessment its activity against pathogenic S. aureus in O. niloticus. Also this study aimed to assess its safety and its potential use as a probiotic in dairy products and fish. Finally, we evaluated the ability of this strain to produce inhibitory substances to explain the possible cause of the inhibitory effect on pathogenic bacteria.
Materials and methods
.1 Bacterial strains and culture conditions
A lyophilized stock culture of pathogenic S. aureus strain (ATCC 6538) was obtained as a reference pathogenic strain from the Microbiology Department in the Faculty of Veterinary Medicine at Zagazig University, Egypt. The culture was grown in broth (brain heart infusion; Oxoid) at 37 °C for 18–24 h. L. garvieae strain was obtained from our previous work (we chose the strain that carry only Fbp gene) [Citation27] in which it was isolated, identified, and stored frozen at −80 °C in broth culture contained 15% (w/v) glycerol. Then it was cultured on M17 medium anaerobically at 37 °C for 48 h.
.2 Assessment of the antimicrobial activity of L. garvieae during cheese manufacturing
.2.1 Preparation of cultures [Citation28]
The used bacterial strains were preserved at −80 °C in frozen broth with 15% glycerol. S. aureus and L. garvieae strains were subcultured for two times and then exposed to decimal serial dilutions, after which they were plated on specific agar media to determine the viable cell numbers, on Baird Parker agar at 37 °C for 24–48 h and M17 agar anaerobically at 30 °C for 24 h, respectively.
.2.2 Preparation of cheeses containing L. garvieae
For cheese preparation, we must use only milk free from the used cultures thus, pasteurized milk was first bacteriologically tested for the presence of S. aureus and L. garvieae using Baird Parker agar at 37 °C for 24 h, and M17agar at 30 °C for 24 h, respectively. The cheese was then manufactured according to the method previously described by Abou-Donia [Citation29], with adding 108 colony forming units (CFU)/mL of the L. garvieae strain. To contaminate cheese with S. aureus culture, 103 CFU/g of it was added during the agitation step to the salted curd. Overall, we prepared three different batches of fresh cheese:
| A) | Cheese made from Pasteurized milk that was experimentally contaminated with S. aureus only. | ||||
| B) | Cheese made from Pasteurized milk that was experimentally contaminated with S. aureus and containing L. garvieae. | ||||
| C) | Control cheese that was prepared with no added cultures. | ||||
.2.3 Microbial examination of the prepared cheeses
The experimentally contaminated cheeses, and non-contaminated control cheese were subjected to counts of S. aureus and L. garvieae, at day zero and then every two days, till the end of the experiment duration at day ten. For this purpose, 25 g of each cheese batch were resuspended with 225 mL of peptone water at concentration 0.1% and were then subjected to several serial dilutions in the same broth, followed by spread plating (0.1 mL) on Baird Parker agar at 37 °C for 48 ± 2 h and on M17 agar at 30 °C for 24 h to enumerate S. aureus and L. garvieae, respectively. Growing colonies were enumerated, and the results were expressed as CFU/g. The experiments were repeated three times in separated occasions.
.3 Assessment of pathogenicity of the examined S. aureus in fish
.3.1 Assessment of pathogenicity of the examined S. aureus in fish
A total number of 60 O. niloticus that seemed healthy with an average body weight of 50 ± 5 g were randomly selected and divided in six glass fish tanks (80 × 60 × 30 cm) containing 80 L of water, resulting in a stocking rate of ten fish per tank. The fish tanks were provided with everyday refreshed dechlorinated tap water with the temperature maintained at 22 ± 2 °C during the experimental period, and an air pump for continuous aeration. The fish were adapted in this environment for 14 days and were provided with a basic food two times a day. A bacterial suspension was prepared by culturing the bacterium for 24 h in tryptic soy broth (TSB). One mL of inoculums contained approximately 1010 CFU/mL was diluted in 1 L distilled water to get a final adjustment to the bacterial culture to 107 CFU /mL [Citation23]. We classified the fish into 2 groups (each with three replicates “N = 10”). The first group of fish was inoculated intraperitoneally (I.P) with 0.5 mL of the prepared bacterial suspension, the inoculation dose was selected according to a biological test (data not shown). The second group of fish was served as a negative control by inoculating fish I.P with 0.5 mL of sterile saline solution. The fish groups were checked regularly, and mortalities were recorded for 14 days (). Dead fish were examined bacteriologically for bacterial re-isolation.
able 2 Mortality rates record in pathogenicity experiment with S. aureus and in feeding experiment using L. garvieae.
.3.2 Re-isolation of S. aureus from morbid fish
We examined the newly dead or moribund fish. Bacteria were isolated under aseptic conditions from some internal organs. Samples were inoculated on Tryptone Soya Broth, then incubated at 37 °C for 24 h. Loopfuls were taken from the broth media and streaked on the surface of Baird parker agar plates, then incubated at 37 °C for 24 h. The grown colonies on the plates were identified using biochemical tests [Citation30]. Gram staining, Oxidase and Catalase tests were performed to confirm the cause of morbidity or mortality [Citation31].
.4 Assessment of the effect of L. garvieae on fish
.4.1 Assessment of the effect of L. garvieae on fish in vivo to ensure its safety
A total number of 60 O. niloticus that seemed healthy with an average body weight of 50 ± 5 g were randomly selected and divided them in six glass fish tanks (80 × 60 × 30 cm) containing 80 L of water, resulting in a stocking rate of ten fish per tank. The fish were adapted for 14 days, and then we put the fish into 2 groups (each with three replicates). The first group was inoculated I.P with 0.5 mL of the suspension containning 107 L. garvieae [Citation32], and the second group was served as a negative control by inoculating it I.P with 0.5 mL of sterile saline solution. The fish groups were checked regularly, and the living and dead fish numbers were recorded for 14 days. Dead fish were examined bacteriologically for bacterial re-isolation.
.4.2 Assessment of the probiotic activity of L. garvieae in fish in vivo [Citation33]
.4.2.1 Preparation of feed with probiotic
L. garvieae cells were prepared by inoculating the bacterium in TSB and incubating for 48 h at 30 °C. The culture was centrifuged at 3000 rpm for 30 min, then the bacteria were cleaned with sterile saline solution for two times. The final bacterial concentration in this saline suspension was adjusted to 107 cells/mL as mentioned before [Citation32]. For the feeding experiment, the bacterial suspension containing L. garvieae isolate was added to the marketed food using an automatic mixer to give 1 × 107 bacterial cells/g. The pellets were subjected to air for 24 h at room temperature to dry and then refrigerated till using (4 °C). Then the viability of L. garvieae in the stored feed was evaluated twice during the period of the experiment (once every week) according to Irianto and Austin [Citation34].
.4.2.2 Feeding experiment
A total of 120 O. niloticus that seemed healthy with an average body weight of 50 ± 5 g were distributed in four equal groups, each with three replicates (10 fish per replicate), which were divided in three glass fish tanks (80 × 60 × 30 cm), resulting in stocking rate 10 fish per tank. The fish were adapted for 2 weeks during which they were fed an artificial diet, and supplied with continuously aerated dechlorinated water with the temperature kept up at 20 ± 2 °C. Fish in the first and third groups were fed on a diet without bacterial supplementation during the feeding experiment. Fish in the second and fourth groups were received a diet containing 107 L. garvieae bacterial cells/g at 5% biomass/day two times a day. After 14 days of the feeding experiment, the fish in the first and second groups were served as controls, so they were injected I.P with 0.5 mL of sterile saline solution. We made I.P injection for the third and fourth groups with 0.5 mL of the S. aureus suspension contained 107 bacterial cells/mL which was prepared according to Gaafar et al. [Citation23]. The injected fish were observed regularly during the period of the experiment, and the mortality rate was recorded; dead fish were examined bacteriologically for bacterial re-isolation. At the end of the experimental period, blood samples were collected from the caudal blood vessel of each fish in each group [Citation35]. The blood samples were centrifuged at 3000 g for 15 min and the supernatant serum was collected and stored at −20 °C until used for biochemical factors include Lysozyme, IgM and immunoglobulin.
.4.2.3 Humoral immunological studies
Serum Lysozyme activity was measured using a modified turbidimetry method described by Ellis [Citation36]. Nitric Oxide Assay: The nitric oxide (NO) level in each tested serum sample was measured using the method described by Ragaraman et al. [Citation37]. Immunoglobulin M (IgM) was determined by nephelometry method (MININEPH TM Human Kit, the binding Site Ltd, Birmingham, UK).
.5 Preparation of L. garvieae culture supernatant
Isolated L. garvieae bacterial strain was inoculated into 100 mL of M17 broth and incubated at 37 °C for 24 h. Then, centrifugation of the broth was applied at 12,000 rpm for 20 min, where the cell residues were discarded, giving rise to a clear supernatant free from cells. Adjustment of the supernatant pH to 5.0 was made with 1 N NaOH, and it was then evaporated using rotary flash evaporation; sterilized with 0.22-μm filter paper (Millipore, India). This solution was used to assess the antimicrobial activity of L. garvieae.
.6 Assessment of the activity of the inhibitory substances produced by L. garvieae in vitro
The activity of the supernatant was evaluated via the agar well diffusion method [Citation38]. Lyophilized stock cultures of S. aureus were used as an indicator strain. Briefly, molten M17agar media (45 °C) was first injected (1% v/v) with a standardized suspension of S. aureus, then this medium was rapidly distributed into sterile Petri dishes. After solidification of the medium, 3 wells of 6 mm diameter for each were made into the agar. These wells were filled with different amount of previously produced BLS as, 15 μL, 30 μL, and 45 μL, where the effects of organic acids as antimicrobial were excluded by adding 1 N NaOH to make an adjustment to pH until reach 6.5 [Citation39]. The plates were allowed to diffuse for 2 h at 4 °C, then they were incubated at 37 °C as it was the best condition for the indicator strain growth and examined after 24 h [Citation40]. The zones of inhibition were detected in millimeters by the scale used for zone interpretation (HiMedia, Mumbai).
.7 Statistical analysis
All data were analyzed using SPSS software (v. 16). Analysis of data was performed using one-way analysis of variance (ANOVA). Tukey’s honest significant difference (HSD) multiple comparison test was used to check the significance of differences between the mean values. The alpha level for the determination of significance was set to .05. Means in the same column followed by different letters are significantly different, and the highest value is represented by the letter (a).
Results
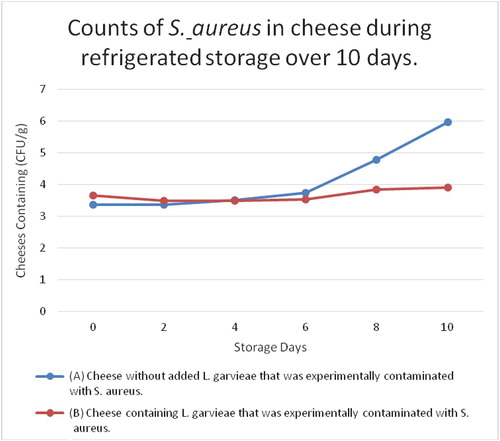
Microbiological examination of the Pasteurized milk used in cheese manufacture showed undetectable levels of S. aureus and L. garvieae. As shown in , S. aureus could be grown in cheeses injected with 103 CFU/g of the strain during refrigerated storage as the log count of S. aureus was 5.97 ± 0.06 CFU/g at the end of the experiment refrigeration time. When the cheeses were prepared with both, the L. garvieae and S. aureus strains, inhibition of S. aureus growth was detected, and the average counts of this pathogen at the end of the experiment refrigeration time were lower than that of the other category of cheese where the L. garvieae strain was absent (3.90 ± 0.02 CFU/g, ). These results showed that the inhibition of S. aureus in cheese might have occurred due to the production of inhibitory substances.
able 1 Counts of S. aureus in cheese during refrigerated storage over 10 days.
On the other hand, as it was shown in (), the used S. aureus strain gave a mortality rate in the second day post-inoculation. Then increased gradually to reach 100% at the end of the experiment in the group of fish which were injected I.P with this bacterium. While no mortality occurred in the control group. These results indicated that this strain was extremely pathogenic to O. niloticus. Postmortem lesions were enlargement and congestion of kidneys, spleen, and liver. After re-isolation of bacteria from internal organs (kidneys, liver, and spleen) of fish, S. aureus was isolated and identified as yellow halo colony surrounding the yellow zone on mannitol salt agar media. The gram staining of these colonies revealed typical gram positive cocci in grape like clusters, Oxidase negative and catalase positive.
Additionally, the examined L. garvieae strain after I.P injection gave no signs of disease or caused mortality, thus we can evaluated L. garvieae as harmless to O. niloticus, and it was therefore considered safe for use in these fish.
Our results also showed no evidence of mortality or signs of disease in the second group of fish that received a diet containing L. garvieae bacteria during the period of the feeding experiment, and the survival rate of this group was 100%, unlike the control group. While the survival rate was 10% in the third group which fed on an ordinary diet and injected with S. aureus and was 50% in the fourth group which fed on a diet containing L. garvieae and injected with S. aureus. Re-isolation of S. aureus was done from internal organs of morbid fish in the third and fourth groups as mentioned before. 3 showed that, group 2, which received a diet containing L. garvieae, showed a highly significant increase in IgM levels, lysozyme activity, and nitric oxide levels, however, these levels were highly significantly decreased in group 3, which was infected with S. aureus. Group 4, which was infected with S. aureus and fed a diet containing the probiotic bacterium was higher in Lysozyme activity, IgM and nitric oxide compared to the control group which fed an ordinary diet. So, these results confirmed the role of L. garvieae in improving the fish defense mechanism by elevating the immunological parameters and show the bad effect of S. aureus through decreasing the immunological parameters of O. niloticus.
able 3 Immunological parameters following the feeding experiment.
To know the possible cause of the inhibitory activity of L. garvieae against a pathogenic S. aureus strain in vitro, we prepared the culture supernatant, then assessed the antimicrobial activity using the agar well diffusion method. The L. garvieae strain was found to have antimicrobial activity. As the supernatant derived from it showed moderate zones of inhibition with different amounts; for example, with 15 μL, 30 μL and 45 μL of supernatant, zones of inhibition were 18 mm, 20 mm and 23 mm, respectively, formed against S. aureus. This inhibition may be occurred due to proteinous substances produced mainly by LAB. Inhibition due to other causes including, hydrogen peroxide or organic acids was prevented through culturing the producer strain under anaerobic condition, and neutralizing the culture supernatant before applying the antimicrobial activity.
Discussion
Over the last few years, members of the genus lactococcus such as Lactococcus lactis and other strains producing antimicrobial substances have been isolated from various products, including meat, meat products, vegetable products, dairy products, and raw milk [Citation41,Citation42]. They are considered as a technological control method for controlling pathogenic bacteria like S. aureus inspite of the traditional chemical one. Also, many authors have been reported L. garvieae as a component of the natural microbiota of different dairy products manufactured from raw milk [Citation43–Citation45].
Regarding the first part of our work in cheese, the results cleared that the average counts of S. aureus in cheese contained both S. aureus and L. garvieae strains, at the end of the experimental refrigeration time, were lower than that of the cheese in which the L. garvieae strain was absent. Other studies applied on the bacteriocins producing L. garvieae suggest that their effects on S. aureus in cheese can vary in accordance with the type of cheese and the bacteriocin-producing strain. S. aureus increased by 1.8 log CFU/g in Manchego cheese when it was made from milk without bacteriocin-producing bacteria [Citation46]; however, this increase was lower than the 2 log CFU/g, and 3 log CFU/g in case of Feta cheese, and camembert-type goat cheese [Citation18,Citation47] respectively. Conversely, other authors found that L. garvieae had a bacteriostatic effect on S. aureus in both shaken and static buffered BHI cultures [Citation17]. Others found that the addition of bacteriocinogenic strains of Lactic Acid Bacteria to milk during cheese manufacture ended with only slight inhibition of S. aureus [Citation19].
Effects of other bacteriocins as nisin on S. aureus in cheese have been previously reported in cheese manufacturing [Citation48–Citation50].
Several previous works have been applied on L. garvieae, where bacteriocins have been detected and purified. The bacteriocin, termed garvicin KS (GarKS), is produced by L. garvieae strains isolated from raw milk and it has a wide inhibitory spectrum against important pathogens belonging to the genera Staphylococcus, Bacillus, Listeria, and Enterococcus [Citation9]. Also, Garviecin L1-5 is a small bacteriocin, with a molecular mass of about 2.5 kDa, produced by L. garvieae L1-5 isolated from a raw cow’s milk sample. It inhibits bacteria from the Lactococcus, Listeria, Enterococcus, and Clostridium genera [Citation13]. L. garvieae IPLA 31405, isolated from among the normal microbiota of a raw-milk cheese [Citation51]. It lacks hemolysin and gelatinase activities, and produces a bacteriocin active against food-borne pathogens [Citation5]. Bacteriocins can be produced also from L. garvieae of nondairy origin and they have different antimicrobial activities against bacteria [Citation10–Citation12].
However, a slight genetic relation between dairy isolates and fish isolates of L. garvieae was detected [Citation52]. The strains of dairy origin were evaluated to have a weak lactose acidifying capacity and a low incidence of known virulence factors. While those of fish origin have not any acidifying properties and have many virulence factors [Citation53]. Although, L. garvieae might contribute to improve physical properties of dairy products, and no reports have been recorded about the association between consumption of raw-milk cheese and L. garvieae infections in human [Citation5,Citation52,Citation53]. The safety of L. garvieae of dairy origin should be detected before using it as a biopreservative in food.
On the other hand, the experimental challenge with S. aureus showed its higher pathogenicity to O. niloticus because of the high mortality rates recorded (). These results are coordinated with Gaafar et al. [Citation23] who recorded remarkable mortalities in O. niloticus after applying pathogenicity test with S. aureus.
Regarding the fact that the diseases have been spread in the aquatic environments every year, it clears that it is preferable to protect fish against the infection with pathogenic bacteria to prevent losses of fish. This can be achieved by adding a probiotic bacteria to fish diet to increase the resistance against the disease and to minimize damages or losses. In our work, we used L. garvieae of dairy origin to evaluate its effect on S. aureus. The results showed that L. garvieae had an inhibitory effect on S. aureus in vivo with no disease signs or mortality after I.P injection into O. niloticus. These results are similar to those detected by some authors [Citation54–Citation56] as they recorded a new L. garvieae subspecies strain has a probiotic activity. Also, they mentioned that L. garvieae is able to produce a novel bioactive peptide and a volatile phenol compound that can be used as food additives to improve food safety due to their antifungal and antioxidant properties.
In our work, there were no signs of disease on fish fed a diet supplemented with L. garvieae after being challenged with S. aureus and we also observed a higher survival rate in these fish. Similar findings were reported by Robertson et al. [Citation57], who observed a higher survival rate in the fish group fed probiotics for a period of time in spite of the presence of pathogenic bacteria. Furthermore, other studies reported the same effect of feed supplemented with probiotics in fish challenged with other microbes such as Aeromonas hydrophila [Citation33]. On the other hand, dietary supplementation with L. garvieae appeared to elevate the serum lysozyme activity, immunoglobulins, and nitric oxide, thus it improved the O. niloticus immune status. Also, fish group infected with S. aureus and fed a diet containing the L. garvieae bacterium had a higher Lysozyme activity, IgM and nitric oxide compared to the control group, so L. garvieae has an immunological role in reducing the infectivity with S. aureus. Similar results were recorded by Nikoskelainen et al. [Citation58], who reported an improvement in the immunity of rainbow trout through using probiotics by stimulating phagocyte activity, production of immunoglobulin, and complement-mediated bacterial killing. Also, it was found that the serum lysozyme activity in fish was elevated after adding Bacillus subtilis and Lactobacillus acidophilus to their diet [Citation59]. Moreover, Lactococcus lactis was used as a probiotic treatment against A. hydrophila in tilapia fish and resulted in elevations of respiratory burst activity, lysozyme activity and superoxide dismutase [Citation60]. However, the fish group which infected only with S. aureus strain, showed the lowest levels of the measured immunological parameters. This can be explained by the presence of extraordinary numbers of virulence factors that allow S. aureus to resist extreme conditions, and affect host cell [Citation61].
As known before L. garvieae had an inhibition effect on some types of bacteria especially, coagulase-positive bacteria due to nutritional competition or H2O2 production. Hydrogen peroxide has a destructive effect on the microbial populations as it may cause a rapid bacteriostatic or even bactericidal effect especially gram-negative bacteria [Citation62], but in our work we excluded this effect as discussed before. So, we tried to detect and obtain the inhibitory substances produced by L. garvieae strain isolated from raw dairy products. For this purpose, we prepared the culture supernatant. This solution was used to assess the antimicrobial activity of L. garvieae, using the agar well diffusion method.
Our results revealed the production of moderate zones of inhibition with different amounts of supernatant containing the inhibitory substance produced by L. garvieae. These results are in line with the fact shows that bacteriocins are expressed at low levels, due to the interactions between bacteriocins and milk components, and the availability of nutrients necessary for bacterial growth and the manufacture of these materials [Citation63]. This moderate inhibitory effect can be influenced by different factors exist in food, inactivation by food pH or enzymes, poor solubility, unequal distribution in the food matrix. Additionally, low stability during food shelf life, the diversity, and sensitivity to the microbial load of food also play an important role [Citation64]. Our results are nearly similar to that detected by Suneel and Kaliwal [Citation14]. Another author found that, the antimicrobial substance produced by L. garvieae strain was active against closely related species, tested Gram-positive bacteria, and Gram-negative strains [Citation15]. Finally, we can detect that our work can be considered the first one that demonstrates the great potential of using L. garvieae of dairy origin in the treatment of diseases in fish caused by pathogenic bacteria like S. aureus.
Conclusions
L. garvieae of dairy origin can produce inhibitory substance which can control pathogenic S. aureus during cheese manufacture. Further studies on purification of this substance and applying tests to confirm it as a bacteriocin are needed. Also, more studies to investigate the activity of pure one on pathogenic microorganisms are recommended to open new possibilities for its application on the improvement of dairy product industry. This study is the first report of using L. garvieae of dairy origin as a probiotic for controlling the pathogenic S. aureus in O. niloticus, as the results demonstrate the great potential of using this L. garvieae strain for the treatment of diseased fish. Other works in the future for applying it as an alternative to the existing antibiotics used in treatment of fish diseases are also recommended.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
None declared.
Ethical approval
This study was approved by the Committee of Animal Welfare and Research Ethics, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Acknowledgements
The authors thank the participants in this work. This work was performed using the facilities of the Faculty of Veterinary Medicine, Zagazig University.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- Y.CaiJ.YangH.PangM.KitaharaLactococcus fujiensis sp. Nov, a lactic acid bacterium isolated from vegetable matterInt J Syst Evol Microbiol61201115901594 10.1099/ijs.0.025130-0
- K.L.RuoffLeuconostoc, Pediococcus, Stomatococcus, and miscellaneous gram-positive cocci that grow aerobicallyP.R.MurrayE.J.BaronM.A.PfallerF.C.TenoverR.H.YolkenManual of clinical microbiology6th ed.1995American Society for MicrobiologyWashington, D.C.315323 ISBN: 9781555810863
- D.VendrellJ.L.BalcázarI.Ruiz-ZarzuelaI.de BlasO.GironésJ.L.MúzquizLactococcus garvieae in fish: a reviewComp Immunol Microbiol Infect Dis292006177198 10.1016/j.cimid.2006.06.003
- J.J.EvansP.H.KlesiusC.A.ShoemakerFirst isolation and characterization of Lactococcus garvieae from Brazilian Nile tilapia, Oreochromis niloticus (L.), and pintado, Pseudoplathystoma corruscans (Spix & Agassiz)J Fish Dis322009943951 10.1111/j.1365-2761.2009.01075.x
- E.FernándezA.AlegríaS.DelgadoB.MayoPhenotypic, genetic and technological characterization of Lactococcus garvieae strains isolated from a raw milk cheeseInt Dairy J202010142148 10.1016/j.idairyj.2009.11.004
- G.López-CamposM.Aguado-UrdaM.Mar BlancoA.GibelloM.T.CutuliV.López-AlosoLactococcus garvieae: a small bacteria and a big dataHealth Inform Sci Syst3201519
- P.D.CotterC.HillR.P.RossBacteriocins: developing innate immunity for foodNat Rev Microbiol32005777788 10.1038/nrmicro1273
- J.Delves-BroughtonNisin and its uses as a food preservativeFood Technol J1001990106112 10.1111/j.1471-0307.1990.tb02449.x
- f.VillaniM.AponteG.BlaiottaG.MaurielloO.PepeG.MoschettiDetection and characterization of a bacteriocin, garviecin L1–5, produced by Lactococcus garvieae isolated from raw cow's milkJ Appl Microbiol902001430439 10.1046/j.1365-2672.2001.01261.x
- J.BorreroD.A.BredeM.SkaugenD.B.DiepC.HerranzI.F.NesCharacterization of garvicin ML, a novel circular bacteriocin produced by Lactococcus garvieae DCC43, isolated from mallard ducks (Anas platyrhynchos)Appl Environ Microbiol772011369373 10.1128/AEM.01173-10 Epub 2010 Nov 5
- A.TosukhowongT.ZendoW.VisessanguanS.RoytrakulL.PumpuangJ.JaresitthikunchaiGarvieacin Q a novel class II bacteriocin from Lactococcus garvieae BCC 43578Appl Environ Microbiol78201216191623 10.1128/AEM.06891-11
- B.A.MaldonadoN.CárdenasB.MartínezJ.L.Ruiz-BarbaJ.F.Fernández-GarayzábalJ.M.RodríguezNovel class IId bacteriocin from Lactococcus garvieae that inhibits septum formation in L. garvieae strainsAppl Environ Microbiol79201343364346 10.1128/AEM.00830-13
- K.V.OvchinnikovH.ChiI.MehmetiH.HoloI.NesD.B.DiepNovel group of leaderless multipeptide bacteriocins from gram-positive bacteriaAppl Environ Microbiol82201652165224 10.1128/AEM.01094-16
- D.SuneelB.KaliwalIdentification and characterization of Lactococcus garvieae and antimicrobial activity of its bacteriocin isolated from cow’s milkAsian J Pharm Clin Res62013104108 ISSN – 0974-2441
- F.VillaniM.AponteG.BlaiottaG.MaurielloO.PepeG.MoschettiDetection and characterization of a bacteriocin, garviecin L1–5, produced by Lactococcus garvieae isolated from raw cow’s milkJ Appl Microbiol902001430439
- J.AlomarStudy of physiological properties of Lactococcus lactis and Lactococcus garvieae for the control of Staphylococcus aureus in technology Cheese [Ph.D. dissertation]2007National Polytechnic Institute of LorraineNancy
- C.Delbes-PausG.DorchiesZ.ChaabnaC.CallonM.C.MontelContribution of hydrogen peroxide to the inhibition of S. aureus by Lactococcus garvieae in interaction with raw milk microbial communityFood Microbiol272010924932 10.1016/j.fm.2010.05.031
- A.MeyrandS.Boutrand-LoeiS.Ray-GueniotC.MazuyC.E.GaspardG.JaubertGrowth and enterotoxin production of Staphylococcus aureus during the manufacture and ripening of Camembert-type cheeses from raw goats’ milkJ Appl Microbiol851998537544 10.1046/j.1365-2672.1998.853531.x
- J.L.ArquésE.RodriguezP.GayaM.MedinaB.GuamisM.NuñezInactivation of S. aureus in raw milk cheese by combinations of high pressure treatments and bacteriocin-producing lactic acid bacteriaJ Appl Microbiol982005254260 10.1111/j.1365-2672.2004.02507.x
- M.OteroM.E.Nader-MaciasInhibition of S. aureus by H2O2 – producing Lactobacillus gasseri isolated from the vaginal tract of cattleAnim Reprod Sci9620063546 10.1016/j.anireprosci.2005.11.004
- M.A.AtyahM.Zamri-SaadA.Siti-ZahrahFirst report of methicillin-resistant Staphylococcus aureus from cage-cultured tilapia (Oreochromis niloticus)Vet Microbiol1442010502504 10.1016/j.vetmic.2010.02.004
- A.M.HammadW.WatanabeT.FujiiT.ShimamotoOccurrence and characteristics of methicillin-resistant and -susceptible Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci from Japanese retail ready-to-eat raw fishInt J Food Microbiol1562012286289
- A.Y.GaafarM.K.SolimanH.F.EllakanyN.A.AffrA.K.ElbialyS.Z.MonaComparative pathogenecity of methicillin-resistant Staphylococcus aureus (MRSA) in Nile tilapia (Oreochromis niloticus) and Tilapia zilliLife Sci J122015186194
- A.G.HafsatA.G.YaqubB.G.GaladimaA.A.JamesS.AbubakarMethicillin resistant S. aureus (MRSA) and methicillin resistant coagulase negative Staphylococci (MRCoNS) isolated from fish and fish handlers in Maiduguri, NigeriaAdv J Food Sci Technol92015494502 10.19026/ajfst.9.1954
- S.M.AlyM.F.MohamedG.JohnEffect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus)Aquacult Res392008647656 10.1111/j.1365-2109.2008.01932.x
- W.S.HeoY.R.KimE.Y.KimS.C.BaiI.KongEffect of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder (Paralichthys olivaceus)Aquaculture37620132024 10.1016/j.aquaculture.2012.11.009
- E.N.AbdelfatahA.B.TahounIdentification of lactic acid bacteria in raw milk and kariesh cheese with special reference to Lactococcus garvieaeJ Food Nutr Sci32015203208 10.11648/j.jfns.20150306.11
- J.L.ArquésE.RodriguezP.GayaM.MedinaB.GuamisM.NuñezInactivation of S. aureus in raw milk cheese by combinations of highpressure treatments and bacteriocin-producing lactic acid bacteriaJ Appl Microbiol982005254260 10.1111/j.1365-2672.2004.02507.x
- S.A.Abou-DoniaEgyptian Domiati soft white pickled cheese. Review. NewZealandJ Dairy Sci Technol211986167190
- E.J.NogaFish disease: diagnosis and treatment2010John Wiley & Sons2062 In our work, fish fed a diet supplemented with L.garvieae showed no evidence of disease
- N.B.BullerBacteria from fish and other aquatic animals: a practical identification manualCABI2004114160
- P.H.ChangJ.A.PlumbHistopathology of experimental Streptococcus sp. infection in tilapia, Oreochromis niloticus(L.), and channel catfish, Ictalurus punctatus (Rafinesque)J Fish Dis191996235241 10.1111/j.1365-2761.1996.tb00130.x
- S.M.AlyA.M.Abd-El-RahmanG.JohnM.F.MohamedCharacterization of some bacteria isolated from Oreochromis niloticus and their potential use as probioticsAquaculture277200816 10.5897/AJB11.1871
- A.IriantoB.AustinProbiotics in aquacultureJ Fish Dis252002633642 10.1046/j.1365-2761.2002.00422.x
- G.K.OstranderThe laboratory fish. Book1st ed.2000Academic Press/Elsevier516518 ISBN: 0125296509
- Ellis AE. Lysozyme assays: lysozyme assay. In Stolen JS, Fletcher EC, Aderson DP, Robetrson DS, van Muiswinkel WB, editors. Techniques in fish immunology. USA: SOS publications. p. 101–103.
- V.RajaramanB.J.NonneckeS.T.FranklinD.C.HammellR.L.HorstEffect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacerJ Dairy Sci81199832783285 10.3168/jds.S0022-0302(98)75892-8
- VijVijaiPalM.JamunaK.JeevarathnamIsolation and characterization of bacteriocin producing lactic acid bacteria from a south indian special dosa (Appam) batterJ Cult Collect420055360 ISSN: 1310-8360
- A.SavadogoC.A.T.OuattaraI.H.N.BassoleAntimicrobial activity of lactic acid bacteria strains isolated from Burkina Faso fermented milkPakistan J Nutr32004174179 10.3923/pin.2004.174.179
- U.SchillingerF.K.LuckeIdentification of lactobacilli from meat and meat productsFood Microb41987199208 10.1016/0740-0020(87)90002-5
- D.J.C.Vanden-BergA.SmitsB.PotA.M.LedeboerK.KerstersJ.M.A.VerbakelIsolation, screening and identification of lactic-acid bacteria from traditional food fermentation processes and cultureFood Biotechnol71993189205 10.1080/08905439309549857
- Z.YildirimM.G.JohnsonDetection and characterization of a bacteriocin produced by Lactococcus lactis subsp. cremoris R isolated from radishLett Appl Microbiol.261998297304 10.1046/j.1472-765X.1998.00335.x
- A.AlegríaP.Alvarez-MartínN.SacristánE.FernándezS.DelgadoB.MayoDiversity and evolution of microbial populations during manufacture and ripening of Casín, a traditional Spanish, starterfree cheese made from cow’s milkInt J Food Microbiol13620094451 10.1016/j.ijfoodmicro.2009.09.023
- G.El-BaradeiA.Delacroix-BuchetJ.-C.OgierBacterial diversity of traditional Zabady fermented milkInt J Food Microbiol1212008295301 10.1016/j.ijfoodmicro.2007.11.014
- R.FoschinoC.PicozziM.BorghiM.C.CerlianiE.CresciInvestigation on the microflora of Caprino Lombardo cheese from raw goat milkItal J Food Sci1820063349
- M.NuñezL.BautistaM.MedinaP.GayaStaphylococcus aureus, thermostable nuclease and staphylococcal enterotoxins in raw ewes milk Manchego cheeseJ Appl Bacteriol6519882934 10.1111/j.1365-2672.1988.tb04313.x
- O.ErkmenBehavior of S. aureus in Turkish Feta cheese during manufacture and ripeningJ Food Prot58199512011205 10.4315/0362-028X-58.11.1201
- O.M.AbdallaP.M.DavidsonG.L.ChristenSurvival of selected pathogenic bacteria in white pickled cheese made with lactic acid bacteria or antimicrobialsJ Food Prot561993972976 10.4315/0362-028X-56.11.972
- E.A.ZottolaT.L.YezziD.B.AjaoR.F.RobertsUtilization of cheddar cheese containing nisin as an antimicrobial agent in other foodsInt J Food Microbiol241994227238 10.1016/0168-1605(94)90121-X
- E.RodrĺguezJ.L.ArquésP.GayaJ.TomilloM.NuñezM.MedinaBehaviour of S. aureus in semi-hard cheese made from raw milk with nisin-producing starter culturesMilchwissenschaft552000633635
- A.B.FlórezP.ReimundoS.DelgadoE.FernándezÁ.AlegríaJ.A.GuijarroGenome sequence of Lactococcus garvieae IPLA 31405, a bacteriocin producing, tetracycline-resistant strain isolated from a raw-milk cheeseJ Bacteriol194201251185119
- R.FoschinoD.NuceraG.VolponiC.PicozziM.OrtoffiM.T.BotteroComparison of Lactococcus garvieae strains isolated in northern Italy from dairy products and fishes through molecular typingJ Appl Microbiol1052008652662 10.1111/j.1365-2672.2008.03780.x
- M.G.FortinaG.RicciR.FoschinoC.PicozziP.DolciG.ZeppaPhenotypic typing, technological properties and safety aspects of Lactococcus garvieae strains from dairy environmentsJ Appl Microbiol1032007445453 10.1111/j.1365-2672.2006.03265.x
- K.VarshaS.PriyaL.DevendraK.NampoothiriControl of spoilage fungi by protective lactic acid bacteria displaying probiotic propertiesAppl Biochem Biotechnol1722014112 10.1007/s12010-014-0779-4
- K.K.VarshaL.DevendraG.ShilpaS.PriyaA.PandeyK.M.Nampoothiri2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus spInt J Food Microbiol21120154450 10.1016/j.ijfoodmicro.2015.06.025 Epub 2015 Jul 2
- K.K.VarshaK.M.NampoothiriLactococcus garvieae subsp. bovis subsp. nov., lactic acid bacteria isolated from wild gaur (Bos gaurus) dung, and description of Lactococcus garvieae subsp. garvieae subsp. novInt J Syst Evol Microbiol66201638053809 10.1099/ijsem.0.001268 Epub 2016 Aug 4
- P.A.W.RobertsonC.O'DowdC.BurrellsP.WilliamsB.AustinUse Carnobacterium sp. as probiotic for Atlantic salmon (Salmo salar L) and rainbow trout (Oncorhynchus mykiss, Walbaum)Aquaculture1852000235243 10.1016/S0044-8486(99)00349-X
- S.NikoskelainenA.C.OuwehandG.BylundS.SalminenE.M.LiliusImmune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus)Fish Shellfish Immunol152003443452 10.1016/S1050-4648(03)00023-8
- S.M.AlyY.A.G.AhmedA.A.A.GhareebM.F.MohamedStudies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infectionsFish Shellfish Immunol252008128136 10.1016/j.fsi.2008.03.013
- X.ZhouZ.TianY.WangW.LiEffect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune responseFish Physiol Biochem362010501509
- G.Y.LiuMolecular pathogenesis of Staphylococcus aureus infectionPediatric Res65201071R77R 10.1203/PDR.0b013e31819dc44d
- L.BjorckC.G.RosenV.MarshallB.ReiterAntibacterial activity of the lactoperoxidase system in milk against pseudomonads and other gram-negative bacteriaAppl Environ Microbiol301975199204
- D.N.FurtadoS.D.TodorovM.LandgrafM.T.DestroB.D.FrancoBacteriocinogenic Lactococcus lactis subsp. lactis DF04Mi isolated from goat milk: application in the control of Listeria monocytogenes in fresh Minas-type goat cheese. BrazJ Microbiol462015201206 10.1590/S1517-838246120130761
- A.GálvezR.L.LópezH.AbriouelE.ValdiviaN.B.OmarApplication of bacteriocins in the control of foodborne pathogenic and spoilage bacteriaCrit Rev Biotechnol282008125152 10.1080/07388550802107202