?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In our previous research, the immobilization of formaldehyde dehydrogenase (FDH) onto mesoporous silica (MPS: pore size = 12.3 nm) was investigated. However, this method could not obtain the thermal stability of the enzyme. To solve this problem, FDH was immobilized on mesoporous zirconia material (MPZ) with a lower thermal conductivity. MPZ was synthesized using [poly(ethylene glycol)–poly(propylene glycol)–poly(ethylene glycol) (Pluronic P123, EO20PO70EO20)], zirconium(IV) n-propoxide (ca. 75% in 1-propanol), acetylacetone, 1, 3, 5-trimethylbenzene, and ethanol. The material retained high surface area (113.6 m2/g) and pore volume (0.27 cm3/g). It was characterized by Brunauer–Emmett–Teller surface area, Barrett–Joyner–Halenda pore size distribution, X-ray diffraction, field-emission scanning electron microscopy, transmission electron microscopy, and energy-dispersive X-ray spectroscopy.
1 Introduction
Among these pollutants, volatile organic compounds (VOCs) have drawn significant interest since they have been reported to induce sick house syndrome and to have chronic and acute health effects [Citation1,Citation2]. In particular, formaldehyde, one of the harmful VOCs, is of great significance. Even low concentration of formaldehyde, such as 10 μM, adversely affects the human body, for example, anger and cancer [Citation2–Citation5]. Therefore, development of a sensor is highly required for measuring VOCs, such as formaldehyde, with high sensitivity and selectivity from the environmental and medical viewpoints.
Currently, standard methods for the detection of formaldehyde include visible absorption, HPLC, gas chromatography, and fluorometry [Citation6–Citation8]. Even though these formaldehyde sensors can be measured at a very low concentration of <0.1 nM, the size of equipments is very large, and it takes a long time for measurements. Smaller size of sensors unfortunately results in lower sensitivity.
From the above reasons, we pay attention to high sensitivity and selectivity of enzymes. Campanella et al. [Citation9,Citation10] have developed an enzyme electrode for phenol determination which is capable of being used also in non-aqueous solvents and a recently published paper investigated the sensitivity of biosensors to phenol or p-cresol, working in n-hexane, chloroform or mixtures thereof. Formaldehyde dehydrogenase (FDH) is known to react to formaldehyde with high selectively and sensitivity. FDH derived from Pseudomonas putida (molecular size: 8.6 nm × 8.6 nm × 19.0 nm) is a dimeric molecule with a molecular weight of 85,757.2 Da, which has a Zn[II] ion within its active site. Therefore, we are working on the development of formaldehyde biosensor using FDH.
While enzymes can be used as biosensors, enzymes are generally known to be unstable under high temperature and to organic solvents besides having a higher cost, thus limiting their practical application. In order to find a solution to stability issue, we have worked on the enzyme immobilization onto mesoporous silica (MPS), with the aim of enhancing the enzyme activity and stability under a wide range of practical conditions [Citation11]. In recent years, MPS was verified to be one of the most effective solid supports to immobilize a wide variety of enzymes owing to its large specific surface area and uniform pore size [Citation12–Citation18]. In a previous research, we immobilized an enzyme in MPS to be used in various environments [Citation11]. However, thermal stability of the enzyme immobilized on MPS did not improve. It was considered that the thermal energy was transmitted to the enzyme by thermal conductance of silica [Citation11]. From the above results, we turned our focus on the zirconia with a lower thermal conductivity as an enzyme immobilization support.
Zirconia has high boiling point, strength, toughness, hardness, wear resistance, and good frictional resistance, thus making it an excellent refractory material. It acts as a non-magnetic electrical insulator with low thermal conductivity, and has good corrosion resistance in acidic and alkaline environments [Citation19]. Due to its high oxygen ion conductivity it is also used in structural ceramics, soled catalysts, oxygen sensors, electric materials for high temperature fuel cells [Citation19–Citation28]. The most important application of the zirconia is that it can be used as a catalyst. It has a high melting point and high thermal stability under reducing or oxidizing environments, marking it an excellent catalyst support even under harsh conditions [Citation29]. It is chemically more stable than the traditional support materials such as γ-alumina, zeolite, and MPS [Citation30]. Additionally, it also possesses unique bifunctional characteristics of weak basic and acidic properties, and can function as a catalyst support or catalyst by itself [Citation19,Citation31,Citation32].
The effective use of zirconia as a catalyst and catalyst support requires a high specific surface area with suitable pore structure. Thus, synthesis of mesoporous zirconia (MPZ) by using liquid crystal template technique was demonstrated by Sudhakar Reddy and Sayari [Citation33] in 1996 for the first time. Most of the synthesized pure MPZ can be pore size controlled only a small size. However, Rezaei et al. [Citation34] succeeded in the synthesis of MPZ with large pore size by using ethylene diamine along with P123 block copolymer as a template. For the materials calcined at 600 °C, the surface area, pore volume, and pore size ranged from 128.5 to 138.2 m2/g, 0.1 to 0.4 cm3/g, 4.00 to 11.3 nm, respectively. In recent years, those studies are progressing for a wide range of applications such as thin film of MPZ on a substrate.
Next, we presented the improved activity and cost performance of enzyme immobilized on MPS with an interparticle mesoporous structure (IPMPS), in order to solve the problem of high cost [Citation35]. IPMPS materials have a uniform pore size with very low volume compared to conventional MPS. Using such a material, the aggregation of the enzymes immobilized on this support is spatially suppressed. Simultaneously, the substrate affinity is greater in the case of enzymes immobilized on conventional supports. Thus, the enzymes immobilized on IPMPS exhibited a high relative activity, despite the small amount of adsorbed enzyme. We achieved improvement of enzyme activity and cost reduction.
To the best of our knowledge, there was no report on improvement in the thermal stability and structural change of enzyme in the immobilized FDH on zirconia. We synthesized MPZ with interparticle pore structure using Pluronic P123, zirconium(IV) n-propoxide (ca. 75% in 1-propanol), acetylacetone, trimethylbenzene (TMB), and ethanol. In this study, we present that FDH raised substrate affinity and thermal stability though immobilization on the MPZ with interparticle pore structure.
2 Experimental procedure
2.1 Materials
FDH from P. putida (molecular size: 8.6 nm × 8.6 nm × 19.0 nm) obtained from Toyobo. Co., Japan. Triblock co-polymer of poly(ethylene glycol)–poly(propylene glycol)–poly(ethylene glycol) (Pluronic P123, EO20PO70EO20, Mn ca. 5800 g/mol) was purchased from Sigma–Aldrich Co., St. Louis, MO. Phenyltriethoxysilane was purchased from Shin-Etsu Chemical Co., Tokyo, Japan. Zirconium(IV) n-propoxide (ca. 75% in 1-propanol), acetylacetone, 25% ammonia solution, and 99.5% ethanol were purchased from Wako Co., Tokyo, Japan. All the reagents are special-grade chemicals.
2.2 Synthesis of MPZ
The following cocktails C-1 were employed: C-1: 0.03 M of zirconium(IV) n-propoxide, 0.017 M of acetylacetone, and 0.17 M of ethanol were mixed.
MPZ was prepared as follows: 1 g of triblock copolymer (Pluronic P123) was dissolved in 26 mL of deionized water and added to 0.75 g of 28% aqueous ammonia solution. The solution was stirred for 2 h at room temperature, and then C-1 was added to the mixture. The suspension was stirred for 3 h at RT and for another 48 h at 90 °C in temperature-controlled bath. After cooling, solid material was collected by the centrifugation, and washed with de-ionized water and acetone. The final product was dried at 100 °C for 24 h and calcined at 500 °C for 4 h.
2.3 Synthesis of MPS
The MPS materials were prepared by using the triblock copolymer Pluronic P123, TEOS, and n-decane as reagents for controlling the morphology. In a typical procedure, TEOS (2.13 g) was added drop wise to a solution of P123 (1 g) in 10 M HCl (3.7 mL) and H2O (31.3 mL). This mixture was stirred at 40 °C for 20 h and then transferred to an autoclave for further reaction at 100 °C for 24 h. The product was filtered, dried at 80 °C for 10 h, and then calcined at 550 °C for 4 h in air (heating rate of 1 °C/min). In some cases, n-decane (7.5 g) was added to the HCl solution containing P123 before addition of the TEOS.
2.4 Synthesis of MPS with IPMPS
The IPMPS materials were prepared as follows: a 28% ammonium solution (400 μL) was added to deionized water (2.6 mL). After gently stirring this solution, TEOS (3 mL) was added to the mixed solution. The resulting mixture was stirred for 1 day at room temperature and then frozen at −30 °C for one day. The frozen sample was then freeze-dried to obtain the IPMPS.
2.5 Characterization of porous materials
The particle morphology of the MPS materials was observed via field-emission scanning electron microscopy (FE-SEM: S-4300, Shimadzu Co., Kyoto, Japan) with an accelerating voltage of 10.0 kV. Transmission electron microscopy (TEM) and scanning TEM (STEM) images were taken using a JEOL JEM 2010 (JOEL Ltd., Tokyo, Japan) operated at 200 kV. The surface area, pore diameter, and pore volume were determined from nitrogen adsorption/desorption measurements using a Shimadzu TriStar 3000 system. Pore diameter distributions were calculated from the desorption branches using the Barrett–Joyner–Halenda (BJH) method. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method based on the desorption isotherms. Small-angle X-ray diffraction (SAXRD) spectra were recorded on an X-ray diffractometer (RINT-2550VB3L, Rigaku Co., Tokyo, Japan) with CuKα, radiation at 40 kV, and 200 mA. The particle diameter and surface potential in the buffer solution were measured using dynamic light scattering (DLS, Otsuka Electronics Co., Tokyo, Japan) and zeta-potential (ζ-potential, Otsuka Electronics Co., Tokyo, Japan) analyses, respectively.
2.6 Immobilization of FDH onto porous materials
Facile preparation of immobilized FDH was performed as follows: 100 μL of FDH/50 mM–pH 7.0–phosphate buffer solution (0.5 mg/100 μL) was added to 700 μL of the same buffer. Three milligrams of porous materials were added in FDH solution and the mixture was stirred at 4 °C for overnight. The precipitate was separated by centrifuge at 4 °C with 12,000 rpm for 10 min. Then, FDH immobilized on MPZ was washed with cold deionized water, and stored at 4 °C. FDH immobilized MPZ are named, for example, MPZ–FDH.
2.7 Assay of FDH activities
Three milligrams of immobilized FDH were added to a mixture containing 100 μL of 7.68 mM formaldehyde solution as a substrate, 100 μL of nicotinamide adenine dinucleotide (NAD) solution (4 mg/mL), and 800 μL of 50 mM–pH 7.0–phosphate buffer solution. The mixture was stirred at room temperature for 8 min. After the enzyme reaction, immobilized enzyme was separated using centrifuge at 4 °C with 12,000 rpm for 2 min. Amount of NADH was determined with UV–vis spectrophotometer at fixed wavelength of 340 nm (BioSpec-1600, Shimadzu, Kyoto, Japan), and the enzyme reaction is as under Eq. Equation(I)(I)
(I) :
(I)
(I)
The activity of non-immobilized FDH (the native) was measured as a positive control. The enzyme activity (Unit) of FDH was determined by using Eq. Equation(II)(II)
(II) :
(II)
(II)
2.8 Stability of FDH under various conditions
Thermal stability of immobilized FDH was investigated. Three hundred micrograms of the native or 3.0 mg of immobilized FDH was added to 800 μL of 50 mM–pH 7.0–phosphate buffer solution. The suspension was incubated at 40 °C for at a constant interval (0, 10, 20, 30, 40, and 50 min), and then cooled to room temperature. After the treatment, immobilized FDH was washed using cool, deionized water, and then remaining activity of enzyme was measured. The thermal-treated suspension was added to the mixture of 100 μL of NAD solution (4 mg/mL) and 100 μL of 7.68 mM HCHO solution, and then remaining activity of FDH was measured. The activity of native FDH was measured as a positive control. Denaturation temperature of the native, immobilized FDH on MPZ, and MPS were measured by DSC (DSC-120U, Seiko Instrument Inc., Tokyo, Japan).
2.9 Analyses on structural orderliness of immobilized FDHs
The secondary structures of the native and immobilized FDH were analyzed using FT-IR. The FT-IR spectra of native FDH and immobilized FDH were recorded at room temperature on a Perkin-Elmer Spectrum GX FT-IR Spectrometer System using a mercury–cadmium–tellurium detector (MA, resolution, 4 cm−1; number of scans, 64).
The secondary structure of native and immobilized FDH was determined by circular dichroism (CD) spectropolarimeter (J-820K, JASCO Co., Tokyo, Japan) in the wavelength range 190–260 nm using a quartz cell (optical path length 0.1 cm) and an integration number of 16. CD spectra were measured when concentrations of the native, FDH immobilized on MPZ, MPS, and IPMPS were prepared at 6.0 × 10−7, 4.1 × 10−6, 4.1 × 10−6, and 4.1 × 10−6 M, respectively.
3 Results and discussion
3.1 Properties of mesoporous materials
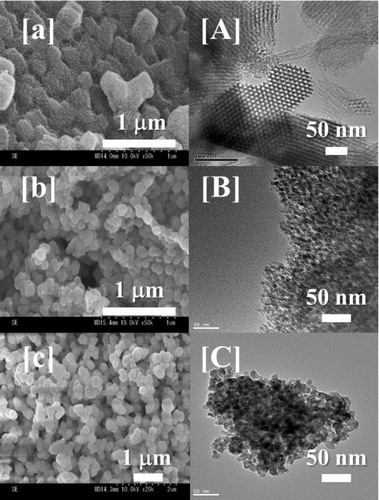
The structures of the MPZ, MPS, and IPMPS samples were characterized based on their BET surface areas, BJH pore-size distributions, TEM, FE-SEM images, and ζ-potentials. The structural properties of the MPZ, MPS, and IPMPS materials as determined based on the BET, SAXRD, ζ-potential measurements, and average particle diameter analyses are summarized in .
Table 1 Structural properties of mesoporous materials.
In , the pore-size distribution curves and the nitrogen adsorption–desorption isotherms obtained using the BJH and the BET methods, respectively, are plotted for the MPZ [circular], MPS [square], and IPMPS [triangle] samples. The MPS material exhibited type IV [H1] curves, indicating the presence of a cylindrical mesoporous structure on its surface [Citation35]. On the other hand, MPZ and IPMPS did not have a type IV [H1] structure. The surface areas of synthesized MPZ, MPS, and IPMPS materials exhibited 70.55, 723.7, and 291.8 m2/g and pore volumes of 0.2, 1.8, and 0.9 cm3/g, respectively. The average pore diameters of the MPZ, MPS, and IPMPS samples were controlled at approximately 13 nm, respectively. The suitability of this MPS pore size for the molecular size of FDH has been demonstrated previously [Citation11], and thus all samples had an optimum pore size for FDH immobilization. In , it can be seen that the MPZ and IPMPS materials had a significantly reduced pore volume because of its noncylindrical pore structure. FE-SEM images of the MPZ, MPS, and IPMPS materials are shown in (a)–(c). The MPS sample was composed of large particles (∼500 nm), while the MPZ and IPMPS materials were composed of aggregates of differently sized particles (100–400 nm). This result indicates that a heterotypic sol–gel reaction occurred because of the presence of an excessive amount of ammonia in the case of the MPZ and IPMPS materials. The TEM images of the MPS and IPMPS materials are presented in (A)–(C), respectively. As can be seen in these images, the MPS material was formed as uniform and small domains of cylindrical small pores (13.2 nm), while the mesopores of MPZ and IPMPS were not regularly arranged. Thus, the porous structure of the MPZ and IPMPS sample was determined from the TEM images. Next, the pore structures were investigated using SAXRD analysis, and the results are shown in Fig. S1. The MPS material exhibited diffraction peaks (1 0 0, 1 1 0, and 2 0 0) corresponding to an ordered two-dimensional hexagonal mesostructure (p6mm) [Citation36]. In contrast, the respective mesoporous structure of the MPZ and IPMPS materials was not well-ordered (Fig. S1). It was thus concluded that the shape of the pore structure of the MPZ and IPMPS materials is regular, but with varying pore spaces. Therefore, the supports have interparticle pore structure appears to have a “disordered pore structure” based on the low-angle X-ray diffraction analysis.
Fig. 1 Nitrogen adsorption–desorption isotherms and BJH analysis [inset] for MPZ [circular], MPS [square] and IPMPS [triangle].
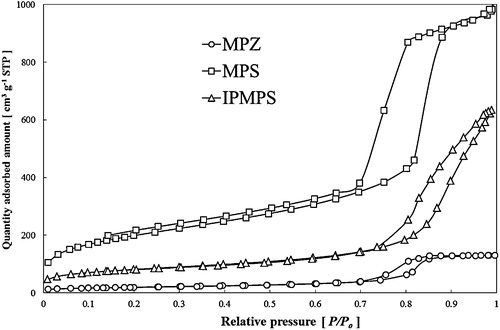
Fig. 2 FE-SEM (a–c) and TEM (A–C) images of the synthesized mesoporous materials. (A, a): MPS, (B, b): IPMPS, (C, c): MPZ.
We investigated the surface composition and elemental composite by wide-angle XRD and EDX, respectively. The XRD patterns of MPZ material are shown in Fig. S2. The sample exhibited the diffraction peak (1 0 1, 1 1 0, 1 1 2, and 2 1 1) corresponding to crystal structure of tetragonal phase with high thermal, mechanical, and structural stability [Citation37]. shows an EDX, scanning TEM (STEM) image, and several elemental mapping images. Not only the zirconium mapping images but also the oxygen ones conform to the STEM image.
3.2 Activity evaluation of FDH immobilized on porous materials
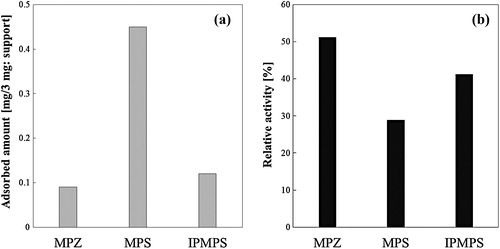
We compared the activity of the FDH immobilized on those supports. FDH was immobilized on MPZ, IPMPS, and MPS with the highest activity in previous study [Citation11]. shows the relative activity and adsorbed amount of FDH immobilized on MPZ, MPS, and IPMPS. Adsorbed amount of enzyme on MPZ and IPMPS was less than that on MPS, because MPZ (surface area: 70.55 m2/g, pore volume: 0.20 cm3/g) and IPMPS (surface area: 291.8 m2/g, pore volume: 0.90 cm3/g) have lower surface area and pore volume than MPS (surface area: 723.7 m2/g, pore volume: 1.8 cm3/g). Activity of native FDH was measured as a positive control. Relative activity to the native was lower than 100%, but activity had a maximum when FDH was immobilized on MPZ and IPMPS. In the previous study, we showed that substrate affinity of FDH is improved by immobilizing on a support having an interparticle pore structure [Citation34]. Therefore, we performed a kinetic analysis of FDH, which was immobilized on the MPZ.
Fig. 4 (a) FDH adsorption on different supports. Immobilized method was shown in Section 2.6. (b) Relative activities of FDH immobilized on each mesoporous materials.
3.3 Kinetic studies of native FDH and immobilized FDH
The kinetic properties of FDH immobilized on MPZ and each MPSs, including the Michealis constant, Km; the maximum rate, Vmax; and the turn over number, Kcat; were determined for the enzyme reaction (). The Kcat/Km value of FDH immobilized on MPZ was comparable to that of the native. These results show that FDH immobilized on MPZ has better effect on enzyme activity than that on MPS. Particularly, Km value of MPZ–FDH was conspicuously higher than that of others. This is consistent with our hypothesis that interparticle voids can facilitate substrate diffusion and, therefore, enhance the apparent enzymatic activity. However, the Kcat value of MPZ was lower than that of IPMPS. From these results, we suggested that FDH immobilized on MPZ led to denaturation.
Table 2 Apparent Km, Kcat, and Kcat/Km values at three mesoporous materials, calculated using the Michaelis–Menten equation.
3.4 Structural changes in the immobilized FDH
Next, we analyzed the structure of FDH in order to study the influence of the electrostatic interactions between the enzyme and the silica materials. Changes in the secondary structure of FDH immobilized onto the three porous materials were analyzed using FT-IR and CD spectra.
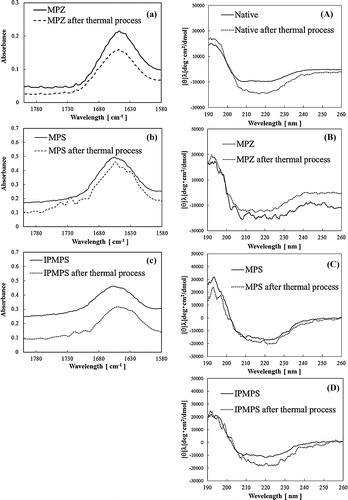
According to the literature [Citation38–Citation43], the contribution of the β-sheet, unordered, and α-helix content can be identified based on the amide I peaks at approximately 1670, 1660, and 1650–1640 cm−1, respectively, while peaks in the 1680–1690 cm−1 region reflect the contribution of the antiparallel β-sheet. (a) shows the amide I region of FT-IR spectra measured over the wavelengths range 1700–1580 cm−1 for native and immobilized FDH samples. The α-helix and β-sheet conformation of FDH are presented in Scheme S1. The bands at 1650, 1690, and 1630 cm−1 suggest the presence of the α-helix, antiparallel β-sheet, and β-sheet, respectively [Citation43,Citation44]. The band indicating the presence of a random coil arises near 1540 cm−1. The maximum peak for the native FDH [solid], FDH immobilized on MPZ [dashed line], MPS [dotted line], and IPMPS [dot–dot dashed line] occur at 1655, 1648, 1655, and 1655 cm−1. This result suggested that FDH immobilized on MPZ increased content of the β-sheet. These results indicate that the enzyme did not alter any structural changes when immobilized on the two pore structures, but enzyme led to structural changes by immobilization on zirconia.
Fig. 5 (a) FT-IR spectra of the native and immobilized FDHs on various MPS materials. The native FDH is denoted by the solid line. The FDHs on MPZ, MPS and IPMPS are denoted by the dashed, dotted and dot–dot dashed lines, respectively. (b) Circular dichroism spectra of the native and immobilized FDHs on various absorbents. Native FDH is denoted by solid line. FDHs on MPZ, MPS and IPMPS are denoted by dashed, dotted and dot–dot dashed lines, respectively.
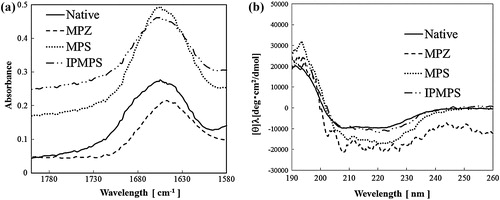
CD spectra measurements were carried out to analyze the changes in secondary structure of the enzyme. (b) shows the CD spectra measured at wavelengths of 190–260 nm for native and immobilized FDH suspended in 50 mM–pH 7.0–phosphate buffer solution. The spectrum of the native [solid line] shows broad negative peaks from 208 to 222 nm, suggesting a combination of two different structures: α-helix with the inverted peaks 222 and 208 nm, and β-sheets with the inverted peak at 217 nm. Similar to the results of the FT-IR analysis, FDH immobilized on MPZ supports undergo a structural change.
Enzyme immobilization by physical adsorption is mainly affected by the electrostatic interaction [Citation44]. Table S1 shows the ζ-potential of FDH, MPZ, MPS, and IPMPS. The value is −17.4, 19.5, −13.8, and −30.9, respectively. As a result, the ζ-potential of only MPZ will have a positive charge. The interaction between the FDH and MPZ is very strong, because surface potential of enzyme has a negative charge. Therefore, FDH immobilized on MPZ caused the structural change.
3.5 Assays of FDH stability after thermal treatment
Numerous papers have been published to point out that the stability of enzymes can be enhanced by immobilizing onto inorganic materials [Citation45]. Therefore, we also evaluated the stability of immobilized FDH after thermal treatment.
(a) illustrates the residual activities of native and immobilized FDH after thermal treatments at 40 °C for different time periods. FDH immobilized on MPZ showed improved thermal stability, but with FDH immobilized on MPS and IPMPS thermal stability was not improved. FDH immobilized on the MPS had a denaturalization owing to thermal energy transmitted from silica wall (thermal conductivity of silica and zirconia: 8 and 3 W/m K), respectively.
Fig. 6 (a) Thermal stability as a function of time for the native [circle] and immobilized FDHs on MPZ [white circle], MPS [triangle] and IPMPS [square] at 40 °C in 50 mM–pH 7.0–phosphate buffer. Initial activities are considered as 100%. (b) Differential scanning calorimetry curves for the Native [solid line] and immobilized, FDH on MPZ [dashed line], MPS [dotted line] and MPZ [dot–dot dashed line].
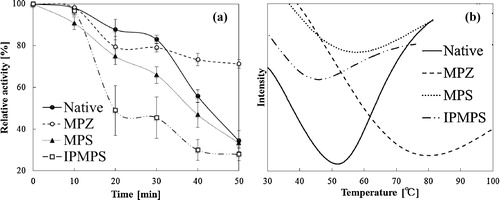
The starting temperature of denaturation of the native and FDH immobilized on MPZ, MPS, and IPMPS was measured using DSC ((b)). The denaturation temperature of the native was 51.8 °C. In contrast, those temperatures of FDH immobilized on MPZ, MPS, and IPMPS were 78.9, 57.7, and 46.8 °C, respectively. This also indicated that thermal stability of FDH was improved by encapsulated on MPZ. We believe that structural change of the enzyme was less likely to occur by the strong interaction with the zirconia and the zirconia prevented the heat transfer from external to immobilized FDH.
3.6 Structural changes in the immobilized FDH after thermal process
In the next step, we incubated the native and immobilized FDH at 40 °C for 50 min, and the thermal treated enzymes were carried out to analyze the changes in secondary structure of the enzyme. (a)–(c) demonstrated IR spectrum of immobilized FDH before and after thermal treatment. The maximum peak for FDH immobilized on MPZ, MPS, and IPMPS occur at 1649, 1648, and 1646 cm−1. By comparing the data obtained before and after thermal treatment, it can be observed that FDH immobilized on MPSs induced an increase in the β-sheet content. On the other hand, FDH immobilized on MPZ did not alter any structural changes after thermal treatment. (A)–(C) shows the CD spectra measured of native FDH and immobilized FDH before and after thermal treatment. From this result, native FDH increase the β-sheet at the same time occur the thermal denaturation. FDH immobilized on MPSs was also increased β-sheet by a thermal treatment. Therefore, we suggested that enzyme activity decreased by enzyme structural changes due to thermal treatment.
3.7 Cycle test
The cycle performance of MPZ, MPS, and IPMPS was compared. Fig. S3 illustrates the cycle tests of FDH immobilized on MPZ, MPS, and IPMPS. After each cycle, the remaining activity was recorded. FDH immobilized on MPZ was only retained ∼80% of its initial activity even after seven repeated reactions. These results indicate that FDH immobilized on MPZ had higher cycle performance than that on MPSs. FDH immobilized on MPZ decreased enzyme release because of the strong electrostatic interaction with the zirconia.
4 Conclusions
FDH was immobilized through physical adsorption into the carefully controlled, size-optimized pores of three absorbents, and the performance of these catalysts was evaluated based on their enzyme activity, cycling characteristics, kinetic behavior, and enzyme structure analyses in order to determine the optimal structure as the carrier.
FDH immobilized on the MPZ support exhibited a higher activity than that on the MPS material because of an increase in the substrate affinity resulting from the interparticle pore space. However, FDH immobilized on MPZ was occurred a structural change by a strong interaction of the zirconia has positive charge.
In addition, we also evaluated the stability of immobilized FDH after thermal treatment. As a result, we suggested that structural change of the enzyme was less likely to occur by the strong interaction with the zirconia and the zirconia prevented the heat transfer from external to FDH.
Finally, we examined structural changes in the immobilized FDH after thermal process. It was found that the enzyme immobilized on MPZ is not affected even thermal treatment by the above experiments. It should also be noted that the potential of new MPZ immobilization carriers was also demonstrated.
Appendix A Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:10.1016/j.jascer.2013.12.003.
Supplementary data
Download MS Word (37.3 KB)Acknowledgment
This study is supported by A-STEP (Adaptable & Seamless Technology Transfer Program through Target-driven R&D) Project No. AS242Z00150M, Japan Science and technology Agency (JST).
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- National Air Quality and Emission Trend Report: Air Toxics1996US Environment Protection Agency (Chapter 5)
- World Health Organization, Environmental Health Criteria 89, Formaldehyde1989WHOGeneva
- M.A.FlyvholmP.AndersenAm. J. Ind. Med.241993533552
- I.J.ChasnoffJ.W.EllisZ.S.FainmanFamily Health and Medical Guide1989Publications InternationalLincoln-Wood, IL
- H.KimY.D.KimS.H.ChoArch. Environ. Health5421999115118
- G.R.MohlmannAppl. Spectrosc.39198598101
- T.DumasJ. Chromatogr.2471982289295
- B.MannJ.GrajeskiJ. Chromatogr.3861987149158
- L.CampanellaM.P.SammartinoM.TomassettiSens. Actuators B71992383388
- L.CampanellaG.FaveroM.P.SammartinoM.TomassettiTalanta6199410151023
- Y.MasudaS.KugimiyaK.MuraiA.HayashiK.KatoColloids Surf. B10120132633
- R.M.BlancoP.TerrerosM.Fernández-PérezC.OteroG.Díaz-GonzálezJ. Mol. Catal. B: Enzym.3020048393
- S.ShenP.S.ChowS.KimK.ZhuR.B.H.TanJ. Colloid Interface Sci.3212008365372
- K.KatoR.IrimescuT.SaitoY.YokogawaH.TakahashiBiosci. Biotechnol. Biochem.672003203206
- K.KatoS.SeelanT.SaitoJ. Biosci. Bioeng.1082009310313
- R.AtluriY.SakamotoA.E.Garcia-BennettLangmuir25200931893191
- J.Y.LeeJ.Y.LimH.K.LimAbdom. Imaging302005744747
- J.HuangY.LiuX.WangJ. Mol. Catal. B: Enzym.5720091015
- S.JaenickeG.K.ChuahV.RajuY.T.NieCatal. Surv. Asia122008153169
- M.J.MayoJ.R.SeidenstickerD.C.HagueA.H.CarimNanoStruct. Mater.111999271282
- X.YangR.E.JentoftF.C.JentoftCatal. Lett.1062006196202
- Y.SunS.WalspurgerB.LouisJ.SommerAppl. Catal. A2922005200207
- M.FaticantiN.CioffiS.De RossiN.DitarantoP.PortaL.SabbatiniT.Bleve-ZacheoAppl. Catal. B6020057382
- O.K.TanaW.CaobY.HuaW.ZhuSolid State Ionics1722004309316
- G.LiuY.LinAnal. Chem.77200558945901
- Q.ZhuB.FanSolid State Ionics1762005889894
- S.T.ArunaK.S.RajamScr. Mater.482003507512
- V.G.DeshmaneY.G.AdewuyiMicroporous Mesoporous Mater.148201288100
- R.GopalanC.H.ChangY.S.LinJ. Mater. Sci.30199530753081
- B.Q.XuT.YamaguchiK.TanabeChem. Lett.17198816631666
- K.TanabeT.YamaguchiCatal. Today201994185197
- J.Sudhakar ReddyA.SayariCatal. Lett.381996219223
- M.RezaeiS.M.AlaviS.SahebdelfarL.XinmeiZ.F.YanJ. Mater. Sci.42200770867092
- Y.MasudaS.KugimiyaY.KawachiK.KatoChem. Lett.42201312521254
- R.YadavK.A.SinghA.SakthivelChem. Lett.42201311601162
- M.RezaeiS.M.AlaviS.SahebdelfarZ.-F.YanJ. Porous Mater.152008171179
- S.F.ParkerProteins and Polypeptides1971Plenum PressNew York (Chapter 10)
- T.A.TuRaman Spectroscopy in Biology: Principles and Applications1982John Wiley and Sons, Ltd.Chichester
- M.D.BylerBiopolymers251986469487
- H.ToriiM.TasumiJ. Chem. Phys.96199233793387
- S.SpassovM.BeekesD.NaumannBiochim. Biophys. Acta1760200611381149
- T.MiyazawaR.E.BloutJ. Am. Chem. Soc.831961712719
- M.J.WinninghamD.Y.SogahMacromolecules301997862876
- Y.Chang-ChengA.S.SaritD.MrinmoyK.J.MichaelR.M.VincentJ. Am. Chem. Soc.12820061461214618
- T.OritaK.KatoM.TomitaJ. Ceram. Soc. Jpn.1192011238245