Abstract
Freeze casting and cryogenic treatment both low temperature process have been employed to fabricate nanobiocomposite hydroxyapatite (HA)–gelatin–polyvinyl alcohol (PVA) macroporous scaffolds from synthesized three different spherical, rod and fibrous HA nanoparticles and composition optimized vis-á-vis porosity architecture, content and compressive strength. A critical HA morphology, solid loading and liquid nitrogen interaction time have a significant effect to enhance the mechanical response of developed scaffolds. Cryo-treated 40 wt.% nanorod HA–gelatin–PVA scaffold posses interconnected pore structure with 80 vol.% porosity, average pore diameter 50–200 μm and highest 5.8 MPa compressive strength. Different degree of the apatite deposition phenomenon in simulated body fluid solution at 37 °C and pH ∼ 7.4 varies with respect to time. In vitro cytotoxicity and L929 mouse fibroblast cell culture in the presence of Dulbecco's Modified Eagle Medium and 10% Fetal Bovine Serum at 37 °C and 5% CO2 atmosphere exhibit excellent cytocompatibility and cell viability at low extract concentration up to 25%.
1 Introduction
Auto graft limitation and probability of allograft induced disease transmission in recipient influence the artificial biomaterial demand for tissue engineering [Citation1]. As a consequence, the interest has been attracted toward the use of synthetic implantable biomaterial that reproduces the bond and morphology of natural bone. Matured bone contains 65% hydroxyapatite (HA) mineral, 25–30% collagen and rest being the matrix. Cancellous bone has high 50–80 vol.% porosity and average pore size ∼125 μm, in which HA nanocrystals provide rigidity through insertion within collagen matrix, whereas high ordered fibrous collagen supports the tensile strength and flexibility of bone [Citation2]. Synthetic HA has excellent protein adsorption capability and biocompatibility consist of similar mineral constituent of bone and teeth [Citation3,Citation4]. In another end, gelatin is a cytocompatible and biodegradable material which is nothing but an irreversibly hydrolyzed form of collagen and has similar chemical composition, easy availability and low cost. In addition, gelatin has several clinical utility factors such as temporary defect filler and wood dressing [Citation5]. Polymeric binder polyvinyl alcohol (PVA) is a biocompatible, biodegradable, highly water soluble and chemical resistance material most widely employed for biomedical application [Citation6]. Thus, HA–gelatin–PVA nanobiocomposite has great potential to consider for the selection of suitable artificial biomaterial to mimic the nature cancellous bone composition. This class of macroporous HA scaffold has extensive use to repair and in the reconstruction of the musculoskeletal system because of their excellent bioactivity and biocompatibility with natural bone. High surface area, suitable pore size and interconnectivity simulate the human bone structure for the migration and cell proliferation, vascularization and as a support material for growth of new bone [Citation7,Citation8]. In order to produce highly porous and mechanically robust bioceramics, different approaches are considered such as gel casting, freeze casting, foaming, incorporation of pore formers, dual phase mixing and salt leaching [Citation9–Citation12]. Freeze casting has attracted much more attention among other methods since solvent water itself acts as porogen to avoid the use of other organic pore formers. Low temperature comprises fabrication of identical bone as nanobiocomposite matrix made of HA and collagen, which can minimize the several fabrication steps compared to other processes including high temperature assisted porogen elimination. The detailed process and advantages over formation of porous architecture are found elsewhere [Citation13]. Heinemann et al. prepared HA-collagen nanobiocomposite scaffold through freeze drying technique to mimic the cancellous bone structure, which exhibits 85 vol.% porosity, pore size in the range of 100–200 μm and ∼0.085 MPa compressive strength [Citation14]. In another study, Kane and Roeder [Citation15] have also employed a similar technique to fabricate HA-collagen scaffold with 30 wt.% HA solid loading and achieved 0.02 MPa compressive strength when scaffold has 90 vol.% porosity and 50 μm elongated pore. However, the morphological effect of different HA nanoparticles on the mechanical response, porosity and bioactivity of freeze casted scaffold are not well documented to justify the efficacy of HA nanoparticles for the fabrication of scaffolds. In a recent attempt, an optimized freeze casted scaffold has been further cryo-treated to enhance the mechanical properties and considered for biological assessment [Citation13]. Hulbert et al. [Citation16] have demonstrated the influence of pore size on bone regeneration, where pore size less than 10 μm inhibits cellular in-growth, while pore between 15 and 50 μm helps fibro-vascular colonization, pores between 50 and 150 μm determines osteoid growth and pores higher than 150 μm facilitates internal regeneration of mineralized bone. In vitro bioactivity has been assessed through the spherical apatite nucleation on the surface of macroporous HA scaffold in simulated body fluid (SBF) solution [Citation9]. Kim et al. [Citation17] investigated the in vitro cytocompatibility through osteoblastic cells (MG63), under dynamic cell culture conditions. The differentiation and proliferation of the bone cells are measured to be a higher degree on the gelatin–HA nanocomposite scaffold compared to the pure HA scaffold. In another study Li et al. [Citation18] also evaluated the cytotoxicity and cell viability on freeze casted nanoHA–chitosan composite scaffold through L929 mouse fibroblast cell line. The developed composite scaffold shows non-toxicity behavior to the L929 cells after 24 h incubation [Citation18]. In this perspective, the efficiency of optimized cryo-treated nanorod HA–gelatin–PVA macroporous scaffold has been evaluated through bioactivity in SBF solution, in vitro cytotoxicity and cell viability for the future scope of clinical application.
2 Experimental
2.1 Synthesis and characterization of HA nanoparticles
Three different HA nanoparticles prepared from common precursors (CH3COO)2Ca and KH2PO4. Both the solutions were prepared in deionized (DI) water and slowly added into 1 L DI water with adjustment of solution pH and temperature. NH4OH and tris-buffer (tri-methylhydroxy aminomethane) solutions were used to maintain the solution pH for spherical and rod morphology, respectively. Spherical HA nanoparticles (NHS), rod HA nanoparticles (NHR) and fibroid HA nanoparticles (NHF) were prepared at pH 12.25 and temperature = 298 K, pH 9.5 and temperature = 303 K, and pH 5.25 and temperature = 353 K, respectively. Detailed powder preparation procedure could be found elsewhere [Citation19]. Phase evaluation of HA nanoparticles was studied through room temperature powder X-ray diffraction, XRD (Philips PAN Analytical, Netherlands) with filtered 1.540 Å Cu K-α radiation. Samples were scanned in a continuous mode with a scanning rate of 0.02°/s. HA peaks were recognized by referring JCPDS file number 74-0565. Morphologies of synthesized HA nanoparticles were studied through transmission electron microscope (JEOL JEM-2100, TEM). The TEM samples were prepared by dispersing a small amount of powder in acetone using 20 kHz and 500 W ultrasonic energy for 30 min. The dispersed suspension was dropped on a carbon coated copper grid and dried to evaporate the solvent and images were taken in bright field mode. The surface area of nanoparticles was measured through BET surface area (Quanta chrome, Autosorb-I).
2.2 Fabrication and characterization of porous scaffold
Freeze casting technique was employed to fabricate porous HA scaffolds from three HA different morphologies such as NHS, NHR and NHF. PVA solution was prepared in DI water (10 wt.% PVA) at 80 °C by constant stirring. After the formation of a clear PVA solution, the solution was cooled down to 30 °C. Gelatin (15 wt.%) was mixed with PVA solution and stirred for 2 h. HA nanoparticles were slowly added into PVA–gelatin solution, and further continuously stirred for homogenous mixing. The resulting HA slurry was casted into a glass mold and pre-freezed for 12 h at −5 °C inside a refrigerator, followed by freeze drying for 24 h at −53 °C and 77 torr. Freeze casted HA–gelatin–PVA scaffolds were designed as HGPS, HGPR and HGPF for spherical, rod and fibroid morphologies, respectively. Different grade of scaffolds was prepared with solid loading variation of 30, 40 and 50 wt.%. Nanorod HA and their 40 wt.% solid loading scaffold exhibited highest compressive strength with 70 vol.% porosity and hence considered as optimized composition to enhance the mechanical strength through cryo-treatment at different time schedules. Optimized cryo-treatment time was considered for 5 h [Citation13]. The cryo-treated macroporous HA–gelatin–PVA scaffolds were designed as HGPS05, HGPR05 and HGPF05 for HGPS, HGPR and HGPF scaffolds, respectively. Both as freeze casted and cryo-treated HA–gelatin–PVA scaffold (ø-14 mm, h-16 mm) samples were uniaxially compressed by universal testing machine (H10 KS Tinius Olsen). Elastic modulus was calculated from the slope of the stress–strain curve. HA solid content was optimized from mechanical response and pore size phenomena were evaluated for the same. Surface morphology, microstructure and pore shape of cryo-treated HA–gelatin–PVA scaffold samples were studied through scanning electron microscope (SEM) (JEOL JSM 6480LV). The SEM images of platinum coated samples were observed in secondary electron mode at 20 kV. Porosity of scaffolds were measured by applying Archimedes’ principle using ethanol as solvent, as well as cross checked through mercury intrusion porosimetry (Quantachrome, Pore master-33). Mercury intrusion porosimetry was also employed to measure pore size and distribution of all scaffolds. The HA scaffold samples were placed in a penetrometer and infused with mercury under increasing pressure (1.0–33,000 psi) with Hg contact angle 140°.
2.3 In vitro bioactivity of the scaffold
A similar human blood plasma ion concentration was prepared at 7.4 solution pH and 37 °C temperature from different chemicals and designated as SBF solution [Citation20]. The HGPR05 scaffolds (0.5 g, 3 × 6 × 10 mm3) were soaked in 20 mL of SBF solution inside a closed polystyrene (Tarson) petridis and kept in an incubator at temperature 37 °C and solution pH ∼ 7.4 for the time interval of 1, 3 and 7 days to assess in vitro bioactivity. The SBF soaked samples were repeatedly cleaned with DI water and dried at 40 °C for 12 h prior to understanding the bioactivity. Feasibility of the apatite nucleation and deposition was studied through SEM attached with EDX.
2.4 Cytotoxicity assessment of scaffold
In vitro cytotoxicity test of steam sterilized HGPR05 scaffold was performed using mammalian mouse fibroblast cell line, L929 by direct contact method as per ISO-10993-5 guideline [Citation21]. L929 cells were used in the present study, because it can be easily cultured in a reproducible manner, and also this cell line is widely used for preliminary cytotoxicity evaluation for a wide range of biomaterials because of easy proliferation and adherence on most of the biomaterial surface. In the beginning, guide line L929 cells were subcultured, trypsinized and seeded on to multiwall tissue culture plates. The L929 fibroblast cells were cultured with Dulbecco's Modified Eagle Medium, 10% Fetal Bovine Serum and incubated at 37 °C in 5% CO2 atmosphere till formation of the cell monolayer. The test specimen (HGPR05) was incubated with monolayer cells at 37 °C for 24–26 h. The HGPR05 surface was examined using phase contrast microscope for cellular response after the requisite incubation. In vitro cytotoxicity of the test specimen was compared with the negative control (high density polyethylene), a nontoxic material and positive control (stabilized PVA disk), a toxic material. Cell monolayer was examined microscopically for the response around the test specimens.
2.5 Cell viability study on scaffolds
The MTT assay was performed to measure the metabolic activity of cells and estimated through ‘color-change’ phenomenon from yellow colored tetrazolium salt, MTT {3-(4,5-diamethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide} to purple colored formazan. Fresh test specimens (HGPR05) were sterilized by steam sterilization at 121 °C for 20 min and extract was prepared after 24–26 h incubation at 37 ± 1 °C in 1 mL culture medium with containing serum protein. The extract solution was further diluted to 50%, 25% and 12.5% in same culture medium. Equal volume (100 μL) of extract, as obtained from HGPR05, negative control (high density polyethylene), positive control (dilute phenol) and cell were placed on the subconfluent monolayer of L929 cells and incubated for 24 ± 2 h at 37 ± 1 °C. The cultured cells were treated with 50 μL of MTT and further incubated at 37 ± 1 °C for 4 h in humidified and 5% CO2 atmosphere. The excess amount of MTT was removed by aspiration and 100 μL of isopropanol was added in order to dissolve the formazan crystals. Cytotoxicity tests were performed in triplicate. The color exchange was quantified by measuring absorbance at 570 nm using a spectrophotometer.
3 Results and discussion
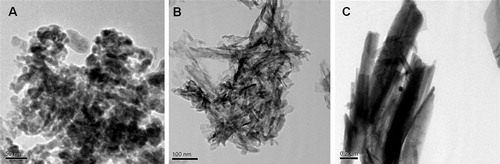
Phase content, purity and crystallinity of the synthesized HA nanoparticles are evaluated through XRD patterns and represented in . XRD pattern of NHS and NHR reveals the formation of HA pure phase with semicrystalline behavior at 25 °C, whereas NHF shows high crystallinity after synthesis at relatively high 80 °C temperature. The diffraction peaks for all HA nanoparticles confirm the hexagonal structure of HA. Low temperature synthesized HA nanoparticle appears without any secondary phase as tricalcium phosphates. The results reveal that the crystallinity of HA phase increases along with an increase in aspect ratio and decrease with the solution pH. Low solution pH favors anisotropic growth of calcium deficient fibrous apatite particles, whereas high solution pH facilitates isotropic growth of the apatite nuclei which result in semicrystalline behavior at low temperature (25 °C). The calculated crystallite size for NHS, NHR and NHF is recorded as 9 nm, 12 nm and 60 nm, respectively. shows a bright field HRTEM micrographs of the synthesized HA nanoparticles. NHS shows spherical morphologies with ∼20 nm diameter, rod morphologies show ∼15 nm diameters with aspect ratio ∼5, whereas fibroid morphologies of HA exhibits micron size length with 30–40 nm diameters. The measured BET specific surface area of the synthesized HA nanoparticles are 256 m2/g, 217 m2/g and 47 m2/g for NHS, NHR and NHF and their calculated particle size are 7, 9 and 70 nm, respectively.
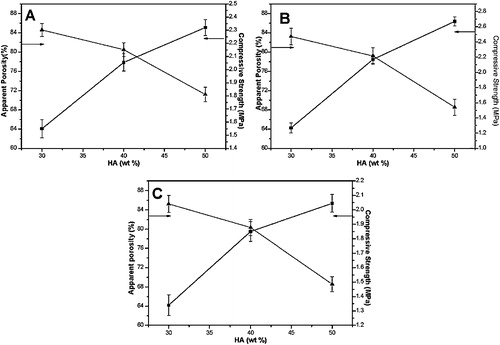
Solid loading effect of different HA nanoparticles on porosity and compressive strength are studied and represented in . It is observed that apparent porosity decreases with increase solid loading from 30 to 50 wt.%, but compressive strength enhances for the same scaffolds. However, both the properties follow non-linear behavior as also demonstrated by Fritsch et al. [Citation22]. Reasonable compressive strength and appropriate porosity of the scaffold is the result for 40 wt.% HA solid loading. The freeze casted HGPR scaffold reveals an average compressive strength of ∼2 MPa with the elastic modulus 70 MPa. Higher compressive strength for HGPR scaffold achieved because of moderate aspect ratio and semi-crystalline behavior, which favors high anchoring effect throughout the gelatin matrix. This optimized scaffold has been considered to enhance mechanical strength through cryo-treatment.
Fig. 3 Variation of porosity and compressive strength for (A) HGPS, (B) HGPR and (C) HGPF with respect to HA content.
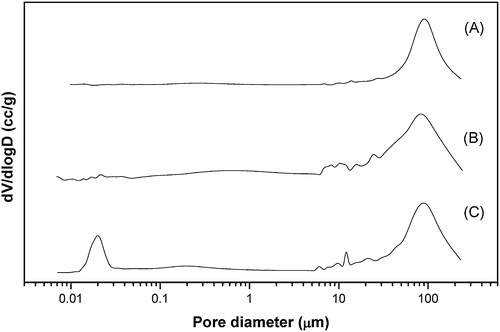
SEM microstructure of 5 h cryo-treated HA–gelatin scaffolds for optimum 40 wt.% solid loading is represented in . Adequate porosity, uniform pore size and reasonable strength are the key features for the selection of such a scaffold. HGPS05 scaffold results in irregular pore morphologies with partly connected open macroporous structure and average pore diameter in the range ∼170 μm as illustrated in A. Open macroporous architecture with average pore diameter in the range ∼160 μm is observed for the HGPR05 scaffold (B). C represents the SEM microstructure of HGPF05 scaffold. HGPF05 scaffold has relatively larger pore diameter than the counterpart HGPR05 and HGPS05 scaffolds. Elliptical open macropores with average pore diameter observe in the range ∼180 μm. HGPR05 scaffold demonstrates well pore size distribution with the reticulated open porous structure as compared to HGPS05 and HGPF05, which is further confirmed by mercury porosimeter analysis. Most of the pores are open macroporous and preferably have irregular elliptical shape. Different pore size, content and morphologies are influenced by the HA surface area, solid loading and PVA molecular interaction phenomena during freeze casting process as well as cryo-treatment. Pore size distribution after 5 h cryo-treated scaffold is analyzed through mercury intrusion porosimetry, as demonstrated in . Mono-mode pore size distribution is observed for HGPS05 with average pore diameter in the range ∼90 μm and some micro pores in the range ∼1–10 μm (A). Porosity of HGPS05 scaffold is measured 80 vol.% through mercury intrusion porosimeter. B exhibits multimodal pore size distribution for HGPR05 scaffold with average pore diameter in the range ∼85 μm, 78 vol.% porosity and micron size pores in the range ∼0.1–1 μm. The existence of micron size pore distribution is attributed to the growth of tiny ice crystals while freezing process [Citation23]. C illustrates the similar pore size distribution pattern for HGPF05 scaffold. The average pore diameter is observed in the range of ∼100 μm along with porosity of around 85 vol.%. Different gradation of micro pore is observed in the range of ∼0.1–10 μm. Strong anchoring of HA particles in the gelatin matrix develops ice crystals during freeze casting followed by cryo-treatment that facilitates diverse pore size distribution as revealed by porosimetry and SEM microstructure. Tiny closed pores are difficult to encounter through SEM microstructure, but mercury porosimeter evaluates all range of open and closed pores diameter (0.1–200 μm) and cumulative pore size shifted to the lower region. Multimodal pore size along with variation in pore diameter may suitable for the proliferation of osteoblast and mesenchymal stem cell as well as the easy passage of nutrients through the pores.
Fig. 4 SEM microstructure of scaffold (A) HGPS05, (B) HGPR05 and (C) HGPF05 for 40 wt.% solid loading.
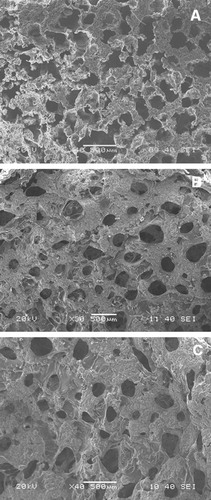
Fig. 5 Mercury intrusion pore size distribution of HA–gelatin–PVA scaffolds (A) HGPS05, (B) HGPR05 and (C) HGPF05 for 40 wt.% solid loading.
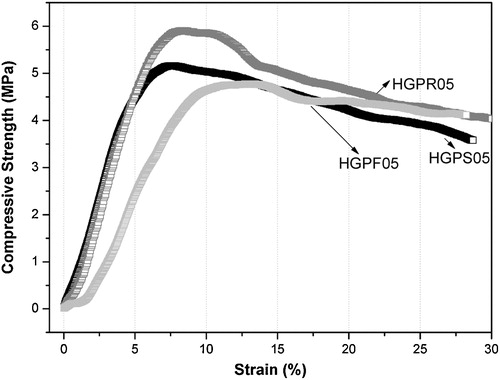
The optimum composition of HA–gelatin–PVA scaffold at liquid nitrogen environment shows the higher mechanical properties compared with the untreated HA scaffold. Stress–strain behavior of 40 wt.% solid content after 5 h cryo-treated HA–gelatin–PVA scaffolds under compression are represented in . The property of the cryo-treated HA–gelatin–PVA porous scaffolds and their detail mechanical behavior is shown in . Under identical loading condition, the cryo-treated HA–gelatin–PVA scaffold exhibits 5.2, 5.8 and 4.7 MPa stress and corresponding modulus are 155, 202 and 167 MPa for HGPS05, HGPR05 and HGPF05 scaffold, respectively. The rate of stain 5% is observed in HGPS05 and 8% in HGPR05, whereas 12% strain is calculated in HGPF05 scaffold. Asefnejad et al. [Citation24] demonstrated freeze casted nanoHA–polyurethane composite has 50–200 μm pore size, 80 vol.% porosity and 0.6 MPa compressive strength [Citation24]. In another study, Isikli et al. [Citation25] reported porous chitosan–gelatin–HA scaffold have moderate compressive strength ∼ 3 MPa and such scaffold also support attachment and proliferation of bone cells. Low strain rate and high compressive strength reveal that scaffold develops relatively ductile to brittle transition. Organic polymer matrix of the scaffold under goes ductile behavior to the brittle nature through the development of internal stress which increases the compressive strength at the cryogenic temperature (77 K).
Table 1 Physical properties of cryo-treated HA–gelatin–PVA scaffold.
In vitro bioactivity of the HGPR05 scaffolds in SBF solution is evaluated and represented in . A illustrates the nucleated spherical apatite layer on the HGPR05 scaffold surface at 37 °C and pH ∼ 7.4 after one day incubation. B represents the SEM microstructure after 3 days incubation of HGPR05 scaffold in SBF solution. However, minimum seven days are required for the complete deposition of near spherical apatite particles without existence of any bare scaffold surface as demonstrated in C. The mechanism of the apatite layer formation on the HA scaffold surface influence by the surface charge of the HA scaffold that absorbs Ca2+ and PO43− from the metastable SBF solution and forms amorphous apatite layer [Citation26]. In addition, the nucleated spherical shaped apatite layer on the surface of the HA scaffold in SBF is also confirmed through EDAX. EDAX images show the presence of prime elements such as Ca, P, O and C along with some trace elements of Mg and Na from SBF solution. Combined SEM and EDAX supports the bioaccessibility of such macroporous HA–gelatin–PVA scaffolds. The deposition of apatite layer begins on the porous surface as compared to the dense surface because of their high surface area. Aforementioned data illustrate that the apatite preferentially deposits along the macroporous architecture of the scaffold.
Fig. 7 Apatite nucleation on the HGPR05 scaffold in SBF solution associated with EDX (A) 1 day, (B) 3 days and (C) 7 days.

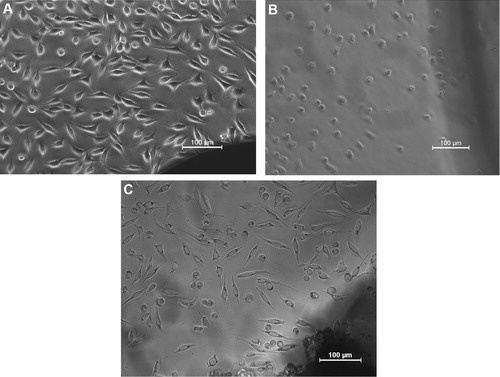
The cell adhesion/proliferation, L929 mouse fibroblast cells viability on HGPR05 scaffolds is investigated using phase contrast microscopy and MTT analysis, respectively. The results are also compared with positive control and negative control. Some representative microscopic images of cultured L929 cells are shown in . The cell density on the negative control (high density polyethylene) is very similar to the fibroblast-like morphology with cell-to-cell contacts and filopodia extension. In contrast, the L929 cells are almost dead on positive control (stabilized PVC disk) revealing severs toxicity. Similar to the negative control, the spindle shaped L929 cells are found to adhere and expand on HGPR05 (C). The fraction of globular shaped is similar on both positive control and HGPR05 scaffold. The observation of the globular shaped L929 cells can be the result of the initial degradation rate of the HGPR05 and the release of the polymeric small chains. represents MTT assay results on the cell viability and proliferation of L929 cells on the test material, HGPR05 at different concentrations. The metabolic activity of HGPR05 extracts in contact with L929 cell is near to 40% as the developed formazan is directly proportional to the number of mitochondrially active cells [Citation27]. Clearly, the MTT assay results reveal that the metabolic activity of L929 cells decreased with the increase in the extract concentration of HGPR05 in vitro, as shown in . As far as the quantification of cell viability concern, 90% of the cells are viable on the negative control and <10% cells on positive control. It is important to mention that, at the HGPR05 extract concentration at 12.5% and 25%, more than the 70% of the cells is mitochondrially viable. Although a significant decrease in cell viability at 100% extract is recorded, and near to four times on larger cells population are mitochondrially active than compared to the positive control. The slow degradation from the HGPR05 scaffold appears to cause toxicity to L929 cells at higher extract concentration up to 50% or more. The aforementioned study and relevant data confirms the cytocompatibility of HGPR05 with L929 fibroblast cell. The cytotoxicity testing using L929 mouse fibroblast cells over 24 h reveals the attachment and proliferation of connective tissue cells on the HGPR05 scaffold in a manner that is comparable to that on the negative control.
4 Conclusions
For the first time we developed such a highly porous with moderate compressive strength nanobiocomposite scaffolds. Three different morphologies have significant influence on the compressive strength; pore size and pore volume of cryogenically treated freeze casted HA–gelatin–PVA macroporous scaffolds. The combination of the freeze casting and cryogenic treatment HA–gelatin–PVA macroporous scaffolds develops anisotropic pore morphology. The macroporous scaffold exhibits 80 vol.% porosity with average pore diameter in the range ∼50–200 μm. Stress–strain behavior reveals the ductile to quasi-plastic nature at strain of 8%. Multimodal pore size distribution with optimum strength ∼5.8 MPa and compression modulus ∼200 MPa obtained at 40 wt.% of HA solid loading. In vitro apatite preferentially deposits around the porous architecture compared to dense matrix, however, optimum seven days incubation requires for the complete coverage on macroporous scaffolds. In vitro cytotoxicity and cell viability reveals excellent cytocompatibility and cell viability to L929 cells at low extract concentration up to 25%, whereas higher extract concentration induces slight toxic effect to L929 cells compared to positive and negative control. The anisotropic pore morphology, cytocompatibility and cell viability of the scaffold suggest the probable use for the healing of cancellous bone and delivery of bone growth factors.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- R.M.DeijkersR.M.BloemP.C.PetitR.BrandS.W.VehmeyerM.R.VeenJ. Bone Joint Surg.791997161166
- R.Z.WangF.Z.CuiH.B.LuH.B.WenC.L.MaH.D.LiJ. Mater. Sci. Lett.141995490492
- S.V.DorozhkinM.EppleAngew. Chem. Int. Ed.41200231303146
- S.K.SwainD.SarkarAppl. Surf. Sci.286201399103
- H.W.KangY.TabataY.IkadaBiomaterials20199913391344
- M.I.BakerS.P.WalshZ.SchwartzB.D.BoyanJ. Biomed. Mater. Res. B100201214511457
- L.CerroniR.FilocamoM.FabbriC.PiconiS.CaropresoS.G.CondoBiomol. Eng.192002119124
- B.BasuS.K.SwainD.SarkarRSC Adv.320131462214633
- S.K.SwainS.BhattachryyaD.SarkarMater. Sci. Eng. C31201112401244
- P.SepulvedaJ.G.P.BinnerS.O.RogeroJ. Biomed. Mater. Res.5020002734
- S.H.LiJ.R.de WijnP.LayrolleK.de GrootJ. Am. Ceram. Soc.8620036572
- G.TripathiB.BasuCeram. Int.382012341349
- S.K.SwainD.SarkarMater. Lett.922013252254
- S.HeinemannC.HeinemannM.JagerJ.NeunzehnH.P.WiesmannT.HankeACS Appl. Mater. Interfaces3201143234331
- R.J.KaneR.K.RoederJ. Mech. Behav. Biomed. Mater.720124149
- S.F.HulbertF.A.YoungR.S.MathewsJ.J.KlawitterC.D.TalbertF.H.StellingJ. Biomed. Mater. Res.41970433456
- H.KimH.E.KimV.SalihBiomaterials26200552215230
- X.Y.LiK.H.NanS.ShiH.ChenInt. J. Biol. Macromol.5020124349
- S.K.SwainS.V.DorozhkinD.SarkarMater. Sci. Eng. C32201212371240
- A.C.TasBiomaterials21200014291438
- I.S.O., 10993-5, Part 5 (1999).
- A.FritschL.DormieuxC.HellmichJ.SanahujaJ. Biomed. Mater. Res. A882009149161
- T.MoritzH.J.RichterJ. Eur. Ceram. Soc.27200745954601
- A.AsefnejadA.BehnamghaderM.T.KhorasaniB.FarsadzadehInt. J. Nanomed.6201193100
- C.IsikliV.HasirciN.HasirciJ. Tissue Eng. Regen. Med.62012135143
- H.M.KimT.HimenoT.KokuboT.NakamuraBiomaterials26200543664373
- A.CosijnsC.VervaetJ.LuytenS.MullensF.SiepmannL.V.HoorebekeB.MasschaeleV.CnuddeJ.P.RemonEur. J. Pharm. Biophar.672007498506