Abstract
Hydrothermal synthesis of Fe3O4 (magnetite) particles was carried out using organic compounds as morphology control agents to obtain magnetite crystals with uncommon facets. It was established that the morphology of Fe3O4 crystals obtained by hydrothermal treatment of an aqueous solution containing Fe2+ and organic compounds depended on the organic compound used. The shape of the Fe3O4 particles obtained when no additives were used was quasi-octahedral. In contrast, the addition of picolinic acid, citric acid or pyridine resulted in the formation of polyhedral crystals, indicating the presence of not only {1 1 1}, {1 0 0} and {1 1 0} facets but also high-index facets including at least {3 1 1} and {3 3 1}. When citric acid was used as an additive, octahedral crystals with {1 1 1} facets also appeared, and their size decreased as the amount of citric acid was increased. Thus, control of Fe3O4 particle morphology was achieved by a simple hydrothermal treatment using additives.
1 Introduction
Fe3O4 (magnetite) is an extremely important compound that is employed in a variety of applications such as MRI contrast agents [Citation1], high-density magnetic energy storage [Citation2], catalysts [Citation3], and pigments [Citation4]. It is well known that different crystal facets of Fe3O4 exhibit different functionalities. Therefore, control of which crystal facets are present is crucial for further improvement of Fe3O4 properties. Typically, {1 1 1} facetted octahedral Fe3O4 particles with sizes of 5–170 nm are obtained by wet chemical processes in the laboratory [Citation5]. The Fe3O4 particles generally assume an octahedral shape, since the compound has a face-centered cubic (fcc) structure and the order of stability of the facets is {1 1 1} > {1 0 0} > {1 1 0} ≫ other facets [Citation6]. However, recent reports have demonstrated the synthesis of Fe3O4 with facets other than the most stable {1 1 1} facets exposed. Amemiya et al. succeeded in synthesizing {1 1 1} facetted Fe3O4 nanocrystals using wild magnetotactic bacteria [Citation7]. However, the same group found that mutant forms of the same bacteria synthesized Fe3O4 nanocrystals with high-index facets such as {2 1 0} and {3 1 1}, and that these crystals exhibited exceptional performance in radical polymerization [Citation8]. These results indicate that Fe3O4 particles with facets other than {1 1 1} can exhibit enhanced functionality.
For mass production, chemical synthesis is typically preferred to “biological” and “bioinspired” processes. Yu et al. and Zhao et al. reported that synthesis of Fe3O4 particles with low-index facets, including {1 0 0} and {1 1 1}, could be achieved by a solvothermal method, employing a chelating agent and an organic solvent such as hydrazine or ethylene glycol [Citation9,Citation10]. However, chemical synthesis of Fe3O4 crystals with high-index facets has not yet been achieved. Nevertheless, there have been several reports on hydrothermal synthesis of metal and other oxide crystals with different facets using organic additives that act as morphology control molecules [Citation11,Citation12]. In these studies, the morphology could be controlled by adsorption of additives onto specific surfaces, which led to changes in the surface energy. To date, there have been no reports on the synthesis of Fe3O4 with various crystal facets using only water, which is an environmentally benign solvent. Therefore, the hydrothermal method employing additives may also offer a simple, promising route to synthesize Fe3O4 crystals with diverse facets.
In the present study, an attempt was made to synthesize Fe3O4 particles with high-index facets by a hydrothermal method employing organic compounds as morphology control molecules.
2 Experimental
2.1 Synthesis
Ferrous chloride tetrahydrate (FeCl2·4H2O, >99.0%), picolinic acid (C6H5O2N, >98.0%), nicotinic acid (C6H5O2N, >98.0%), glycolic acid (C2H4O3, >98.0%), and hydrochloric acid (HCl, 35.0–37.0%) were purchased from Kanto Chemical Co., Inc. Pyridine (C5H5N, >99.5%), citric acid (C6H8O7, >98.0%), and sodium hydroxide (NaOH, >97.0%) were purchased from Wako Pure Chemical Industries, Ltd. All the reagents were used as supplied without further purification.
Magnetite crystals were prepared using the following hydrothermal reaction: 5 mmol of FeCl2·4H2O and 0–15 mmol of an organic additive (citric acid, glycolic acid, picolinic acid, nicotinic acid or pyridine) were dissolved in 20 ml of distilled water with stirring. The ratio of organic compound to Fe2+ is denoted by X ([organic compound]/[Fe2+] = X). Upon addition of picolinic acid, the solution turned red, while colorless solutions were obtained with all other additives. After the formation of a transparent solution, 10 ml of NaOH aq. (4 mol L−1) was immediately added to the solution and a suspension was formed. The prepared suspension (30 ml) was stirred vigorously, and then sealed in a Teflon-lined stainless steel autoclave. The autoclave was heated at 473 K for 6 h, and subsequently cooled to room temperature. Black precipitates were collected from the mixture by magnetic separation and sequentially rinsed three times, each time first with distilled water and then with ethanol. The final product was obtained after air drying at room temperature.
2.2 Characterization
The crystal phases present in the samples were characterized using powder X-ray diffraction (XRD; Bruker D2 Phaser, 40 kV and 30 mA) with CuKα radiation (λ = 1.5406 Å). Data were collected in the 2θ-θ scanning mode with a scan speed of 12° min−1 and a step size of 0.02°. The phase composition was confirmed by Raman spectroscopy (Jasco NRS-3300) in the backscattering geometry using an excitation laser with a wavelength of 732.2 nm and a power of 90 W. The incident beam was focused on the sample through a 100× microscopic objective lens to form a spot with a size of 2 μm. The surface morphology of the particles was observed using scanning electron microscopy (SEM, Hitachi SU1510) at an accelerating voltage of 5 kV. To identify the facets present, another SEM system equipped with an electron backscatter diffraction detector (SEM-EBSD, JEOL JSM-7100F) was used at an accelerating voltage of 20 kV. Samples for SEM-EBSD observation were prepared by dispersing the particles in ethanol and placing a drop of the suspension on a copper grid. Absorption spectra were measured using Fourier transform infrared (FT-IR) spectroscopy (Jasco, FT/IR-4200) in the wavenumber range 400–4000 cm−1. For the FT-IR measurements, all samples were diluted in KBr matrix pellets. Thermogravimetric-differential thermal analysis (TG-DTA, Shimadzu DTG-60H) was also performed. For this purpose, the sample was heated at a rate of 20 K min−1 under a 50 ml min−1 air flow up to a maximum temperature of 1273 K, with Al2O3 powder used as a reference.
3 Results and discussion
shows XRD patterns for samples synthesized by hydrothermal treatment of aqueous iron solutions in the absence or presence of 5 mmol of organic compounds (X = 1.0). The sample obtained in the absence of additives consisted of Fe3O4 with a trace amount of metallic iron ((a)). Splitting of the Kα1 and Kα2 peaks was clearly observed. This indicates that the Fe3O4 particles may have been larger than those previously reported using the hydrothermal method [Citation13,Citation14]. The XRD patterns for the samples synthesized using different organic compounds indicated that they were also composed of Fe3O4 and a trace amount of metallic iron ((b)–(f)). It is well known that Fe3O4 exhibits an XRD pattern similar to that for γ-Fe2O3 (maghemite). However, Raman spectroscopy (see Supporting Information Fig. S1) did not reveal the characteristic Raman shifts at 700 and 1160 cm−1 associated with γ-Fe2O3 [Citation15] for any of the samples. It was therefore concluded that single-phase Fe3O4 samples were obtained using the present technique, despite the presence of organic compounds capable of acting as morphology control molecules.
Fig. 1 XRD patterns for samples prepared by hydrothermal treatment of aqueous iron solutions in the absence and presence of additives (X = 1.0). Circles and triangles indicate, respectively, peaks due to metallic iron and Kβ peaks associated with {3 1 1} planes in Fe3O4.
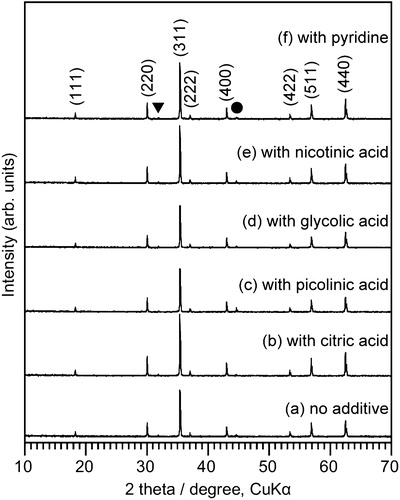
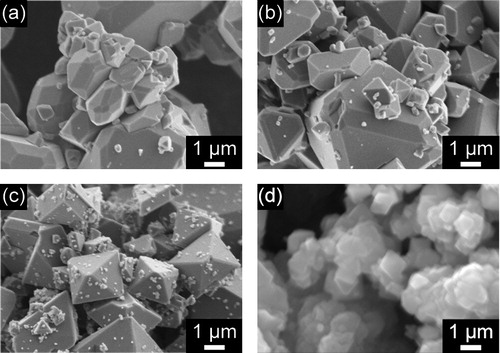
shows SEM images of samples obtained by hydrothermal treatment of aqueous iron solutions in the absence or presence of organic compounds (X = 1.0). As shown in (a), Fe3O4 particles synthesized in the absence of organic compounds had a quasi-octahedral structure, indicating the presence of {1 1 1} and {1 1 0} facets, which is consistent with the results of previous studies [Citation9,Citation10]. (b) shows that for the sample produced in the presence of citric acid, octahedral crystals with {1 1 1} facets were formed. In contrast, when picolinic acid or pyridine was used as the additive, polyhedral particles with triangular, hexagonal, and pentagonal shapes were obtained, along with a small fraction of quasi-octahedral crystals ((c) and (f)). These particles may have high-index facets, because the facet shapes could not be formed by only {1 1 1}, {1 0 0} and {1 1 0} planes. shows an SEM image and the results of an SEM-EBSD analysis of a sample obtained using equimolar amounts of picolinic acid and Fe. At first, a crystal orientation was determined for a multi-facetted particle by analysis of the Kikuchi lines observed by EBSD. Then, the angles formed by the facets were carefully measured, and the ideal angles were calculated. Finally, on the basis of the energy of each facet, it was concluded that the particle had at least {1 1 1}, {1 1 0}, {3 1 1} and {3 3 1} facets. Other high-index facets might also be present, because many crystal facets could be observed on other crystals ((c)). Further analysis is underway to determine the structure more precisely. Samples prepared using glycolic acid mainly contained octahedral particles with a small proportion of polyhedral particles ((d)). However, for the sample prepared in the presence of nicotinic acid, the Fe3O4 particles had only {1 1 0} and {1 1 1} facets, which is similar to the case for particles synthesized without an additive ((e)). Thus, the use of nicotinic acid was found to have no effect on the morphology. It should be noted that for all the experiments, the pH of the reaction solution (prior to the hydrothermal treatment) was between 13.5 and 13.8. Thus, it is unlikely that the Fe3O4 crystal morphology was related to the pH of the solution. In other words, the crystal facets can be controlled using organic additives alone.
Fig. 2 SEM images of Fe3O4 crystals obtained by hydrothermal treatment of aqueous iron solutions (a) in the absence of organic compounds, and in the presence of (b) citric acid, (c) picolinic acid, (d) glycolic acid, (e) nicotinic acid, and (f) pyridine. The amount of additives was equimolar to Fe (X = 1.0).
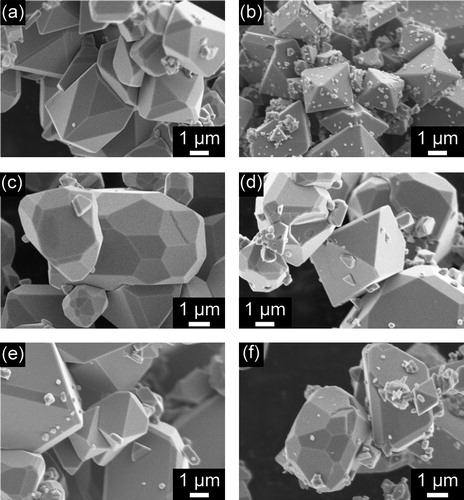
Fig. 3 SEM image and color map of facets on a Fe3O4 crystal obtained by hydrothermal treatment of an aqueous iron solution in the presence of equimolar amounts of picolinic acid and Fe (X = 1.0).
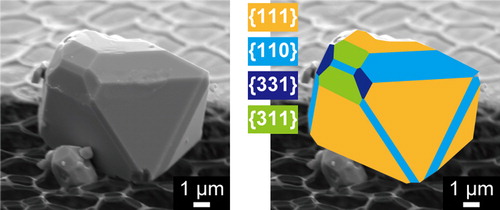
shows SEM images of Fe3O4 particles synthesized using different ratios of picolinic acid to iron (X = 0.1, 1.0, 2.0, and 3.0). When X was 0.1, polyhedral and quasi-octahedral particles were formed ((a)), while only quasi-octahedral particles were obtained in the sample produced without additives ((a)). This indicates that even a small amount of picolinic acid can affect the morphology. As X was increased from 1.0 to 2.0, the number of polyhedral particles increased ((b) and (c)). A larger number of quasi-octahedral particles were detected at X = 3.0 than at X = 2.0 ((d)). This revealed that there might be an optimal amount of picolinic acid for synthesizing polyhedral Fe3O4 with high-index facets.
Fig. 4 SEM images of Fe3O4 particles obtained by hydrothermal treatment of aqueous iron solutions in the presence of varying amounts of picolinic acid. (a) X = 0.1, (b) X = 1.0, (c) X = 2.0, (d) X = 3.0.
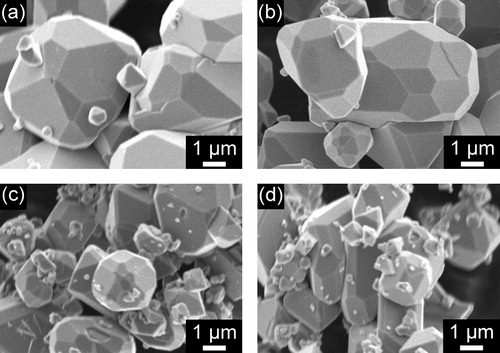
shows SEM images of Fe3O4 particles obtained by the hydrothermal treatment of aqueous iron solutions containing different amounts of citric acid (X = 0.1, 0.5, 1.0, and 2.0). For X = 0.1, only polyhedral particles were observed ((a)). For X = 0.5, both octahedral and polyhedral particles with uncommon facets were present ((b)). For X = 1.0, all particles were octahedral ((c)). This was also the case for X = 2.0 ((d)). It can be seen that the average particle size decreased with increasing citric acid content. These findings confirm that the synthesis of Fe3O4 particles with controlled morphologies could be accomplished using citric acid.
Fig. 5 SEM images of Fe3O4 particles obtained by hydrothermal treatment of aqueous iron solutions in the presence of varying amounts of citric acid. (a) X = 0.1, (b) X = 0.5, (c) X = 1.0, (d) X = 2.0.
FTIR and TG-DTA measurements were carried out on samples obtained in the presence of equimolar amounts of citric or picolinic acid and iron (X = 1.0) (Figs. S2 and S3). In the FTIR spectra of each sample, all of the peaks could be assigned to Fe3O4, and no peaks associated with adsorbed organic compounds were observed. TG-DTA analysis of the final products revealed no characteristic peaks due to water evaporation or organic compound decomposition/desorption. Thus, there were no residual organic compounds in the final products. The isoelectric point for Fe3O4 was reported to be pH = 7.9 ± 0.1 [Citation16]. The pH of the starting solution was measured to be 13.6 ± 0.2. In the present system, Fe3O4 particles were formed through partial oxidation of Fe(OH)2 and were presumably negatively charged. Under the conditions used, the additives are also expected to carry a negative charge considering their acid dissociation constants. Thus, it can be assumed that the negatively charged molecules were unable to coordinate with the final products owing to electrostatic repulsion. Nevertheless, during the formation of Fe3O4 from Fe(OH)2, the additives would have a direct effect on the surface energy of Fe3O4, resulting in the formation of Fe3O4 crystals with uncommon high-index facets. As discussed above, the use of picolinic acid, pyridine or citric acid was effective in yielding Fe3O4 crystals with high-index facets, while the addition of nicotinic acid had no effect. Thus, the adsorption of pyridine rings or carboxylic acids on the Fe3O4 particle surface may not lead to surface energies conducive to the formation of Fe3O4 crystals with various facets. Despite the dozens of experiments that were carried out, the details of the formation mechanism remain unclear. Further theoretical studies [Citation17], computer simulations [Citation18] and experiments are necessary to elucidate the overall mechanism involved in the present technique.
4 Conclusion
Synthesis and morphological control of Fe3O4 were achieved using a hydrothermal method with organic additives. The addition of picolinic acid, citric acid or pyridine effectively led to the formation of polyhedral Fe3O4 particles with high-index facets such as {3 1 1} and {3 3 1}, while quasi-octahedral Fe3O4 particles were obtained when no additives were used. Furthermore, the use of citric acid as an additive reduced the overall particle size and allowed exceptional control of the Fe3O4 particle morphology.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jascer.2014.05.008.
Hydrothermal synthesis of magnetite particles with uncommon crystal facets
Download MS Word (169 KB)Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas of “Fusion Materials: Creative Development of Materials and Exploration of Their Function through Molecular Control (Area No. 2206)” from MEXT, Japan.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- J.W.BulteM.D.CuyperMethods Enzymol.3732010175198
- T.HyeonChem. Commun.2003927934
- S.LiuF.LuR.XingJ.J.ZhuChem. Eur. J.172011620625
- M.A.LegodiD.D.WaalDyes Pig.742007161168
- B.DevouardM.PosfaiX.HuaD.A.BazylinskiR.B.FrankelP.R.BuseckAm. Miner.83199813871398
- Z.L.WangJ. Phys. Chem. B104200011531175
- Y.AmemiyaA.ArakakiS.S.StanilandT.TanakaT.MatsunagaBiomaterials28200753815389
- M.TanakaE.MazuyamaA.ArakakiT.MatsunagaJ. Biol. Chem.286201163866392
- X.YuK.ChenMater. Sci. Eng. B1762011750755
- L.ZhaoH.ZhangY.XingS.SongS.YuW.ShiX.GuoJ.YangY.LeiF.CaoChem. Mater.202008198204
- J.ZengY.ZhengM.RycengaJ.TaoZ.Y.LiQ.ZhangY.ZhuY.XiaJ. Am. Chem. Soc.132201085528553
- J.P.Y.TanH.R.TanC.BoothroydY.L.FooC.B.HeM.LinJ. Phys. Chem. C115201135443551
- X.M.LiuS.Y.FuH.M.XiaoMater. Lett.60200629792983
- X.F.QuQ.Z.YaoG.T.ZhouS.Q.FuJ.L.HuangJ. Phys. Chem. C114201087348740
- O.N.ShebanovaP.LazorJ. Raman Spectrosc.342003845852
- E.IllesE.TombaczColloids Surf. A230200399109
- N.NiekawaM.KitamuraCrystEngComm15201369326941
- H.NadaY.FurukawaPolym. J.442012690698
