 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
B4C powders were synthesized by carbothermal reduction of ethylene glycol (EG) added borate citrate precursors, and effects of EG additions (0–50 mol% based on citric acid) on the morphologies and yields of synthesized B4C powders were investigated. The conditions most suitable for the preparation of precursor were optimized and optimum temperature for precursor formation was 650 °C. EG additions facilitated low-temperature synthesis of B4C at 1350 °C, which was around 100–300 °C lower temperature compared to that without EG additions. The lowering of synthesis temperature was ascribed to the enlargement of interfacial area caused by superior homogeneity and dispersibility of precursors enabling the diffusion of reacting species facile. The 20% EG addition was optimal with free residual carbon lowered to 4%. For smaller EG additions, the polyhedral and rod-like particles of synthesized product co-existed. With higher EG additions, the morphology of synthesized product was transformed into needle and blade-like structure.
1 Introduction
Boron carbide (B4C) has a leading role in numerous high performance applications owing to its extreme hardness, low density, high melting point, high Young's modulus, great resistance to chemical agents, excellent thermoelastic and thermoelectric properties and high corrosion and oxidation resistance. The combination of these properties renders B4C a promising material for numerous applications including body and vehicle armor, abrasive powder, nuclear applications and aerospace applications [Citation1–Citation4].
B4C is generally produced by carbothermal reduction of boron oxide at high temperatures according to reaction Equation(1)(1)
(1) and magnesiothermic reaction of boron oxide [Citation5–Citation10].
(1)
(1) However, this process has many associated problems, including, considerable amount of free carbon residue in the final product due to substantial loss of boron by volatilization of its oxides; difficulty and associated greater cost to grind the product into fine particle size for densification; requiring a high temperature furnace operation; contamination in the final product; and time consumption.
An alternative to above processes is the utilization of polymer precursors, e.g. sucrose and glucose, glycerin, polyols, and phenolic resin, as low-temperature synthetic route to B4C facilitated by improving the homogeneity and dispersibility of B2O3 and carbon [Citation11–Citation22]. Nevertheless, the disadvantage of using organic precursors is the presence of residual carbon in the products synthesized at lower temperatures. Citric acid (CA) has been employed to produce B4C with significant residual carbon at 1500–1900 °C [Citation15–Citation18]. Recently, it is indicated that the addition of tartaric acid to boric acid (BA)–glycerin ameliorates the dispersion of B2O3/C [Citation21]. It is concluded that the hydroxyl group can be easily condensed with BA, enhancing the esterification (B–O–C) and yielding the precursors with fine homogeneous dispersion.
Ethylene glycol (containing hydroxyl groups) is extensively utilized to synthesize a variety of compounds by esterification with metal citrate [Citation23,Citation24]. It is expected that EG addition to condensed borate citrate will modify the thermodynamics of carbothermal reaction by promoting the esterification reaction. In this work, the effects of EG additions on the synthesis of B4C from condensed BA-CA are investigated. The optimum starting compositions and optimum conditions for pyrolysis of precursor and preparation of products have been determined. FTIR, XRD, and SEM analyses are utilized to investigate the yield and morphology changes of precursors and synthesized B4C.
2 Experimental
Materials used were boric acid [(BA), Wako, 99.5%], ethylene glycol [(EG), Merck, 99.5%], and citric acid [(CA), Riedel-de Haen, 99.5%]. BA/CA of 2:1 was used and EG additions were 0–50 mol% (P0–P50) based on CA. The BA solution (2.5 M) in distilled water was prepared at 80 °C. CA was added slowly to the solution and held for 20 min until a yellowish solution was obtained. Subsequently, EG was added to the stirred solution. The solution was then slowly heated to 130 °C and maintained at this temperature to facilitate the polyesterification reaction. The condensed products changed from golden yellow to transparent light yellow with EG additions. The condensed products were pyrolyzed over the temperature range of 550–800 °C in air for 2 h. The obtained dark gray precursors were compacted and heated at 1050–1400 °C for 0–4 h in an Ar flow.
The B2O3 contents were determined by titration of mannitol–boric acid complex with standard NaOH solution (0.1 M). The residual carbon in the final products was determined by the method used in [Citation9,Citation22]. Fourier transform infrared (FTIR) spectra of starting materials and condensed products were recorded on Shimadzu Prestige 21 spectrometer. X-ray diffraction (XRD) patterns of pyrolyzed powder and products were obtained using powder X-ray diffractometer (Rigaku). The B4C peak intensity ratio of the products was estimated from the main peak intensities of each of the B4C, carbon, and B2O3. The morphology of pyrolyzed powder and products was examined using a scanning electron microscope (LEO 440). A Cilas 1064 laser particle size analyzer was utilized for determining the average particle size and particle size distribution. The BET specific surface area, pore size distribution by pore diameter, and pore volume were determined by Quanta chrome Autosorb 1C BET Surface Area & Pore Volume Analyzer. The surface areas and pore volumes (or pore size distribution) were determined from nitrogen adsorption–desorption isotherms by using the Brunauer–Emmet–Teller (BET) equation and Barret–Joyner–Halenda (BJH) methods.
3 Results and discussion
In order to comprehend the formation of borate ester, FTIR spectra of the starting materials and the condensed products, with and without addition of EG, are depicted in . (ii a and b) indicates the FTIR spectra of condensed products with and without the addition of EG. The peak observed at 2964 cm−1 is attributed to the asymmetric stretching of –CH2, –CH3 groups present in organic derivatives [Citation24,Citation25]. The appearance of the band at 1730 cm−1 for –COOH groups, the bands at 1585 and 1380 cm−1, respectively, for the asymmetric and symmetric stretching of C=O, and the bands at 1020 cm−1, 1080 cm−1, 1130 cm−1, and 1287 cm−1 for C–O and B–O–C [Citation13,Citation14,Citation21,Citation24,Citation25] strongly suggest that some –COOH groups in CA have reacted with ethylene glycol and boric acid, while some have not. This phenomenon is further strengthened by the fact that band at 1730 cm−1, for carbonyl groups (COO–), becomes weaker, and O–H stretching band becomes stronger with the addition of EG ((ii a)). The broad peak observed at 3230 cm−1 ((ii a)) is due to the presence of –OH groups in CA and ethylene glycol derivatives [Citation13–Citation17,Citation21,Citation23–Citation25], which is much weaker than that of starting citric acid and ethylene glycol. This result suggests the consumption of OH group by esterification and also the existence of unreacted OH groups. With the introduction of EG, a band at 1250 cm−1 also appears which is attributed to the C–O–C structure from ethylene glycol [Citation25]. Addition of EG also induces an additional B–O–C stretching vibration at 1287 cm−1 [Citation14,Citation21]. These results indicate the reaction of borate citrate with EG. Furthermore, the B–O–H bending band at 1190 cm−1 and O–H torsion band at 752 cm−1 [Citation14,Citation21] derived from BA disappear ((ii a and b)) in the condensed products. These results indicate that firstly, the borate citrate (B–O–C) is formed in the prepared condensed product by dehydration condensation of BA and citric acid ((ii b)). The borate citrate undergoes further esterification with ethylene glycol to grow into a polymer network. The polymeric structure is broken down and releases carbon and B2O3 in the firing process.
Fig. 1 FT-IR spectra of (i) (a) CA and (b) EG, and (ii) (a) P20 condensed product, (b) P0 condensed product, and (c) BA.
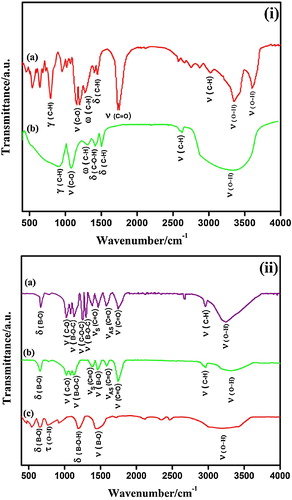
The C/B2O3 contents of the pyrolyzed samples, with and without the additions of ethylene glycol, at 550–750 °C for 2 h are depicted in . According to the stoichiometric carbothermal reaction between B2O3 and C to afford boron carbide, the C/B2O3 ratio in the pyrolyzed sample should be about 3.5. Moreover, the carbothermal reaction is also accompanied by a slight boric species loss owing to the formation of volatile boron suboxides above 1050 °C [Citation4,Citation6,Citation7,Citation9,Citation26–Citation28]. Therefore, it is necessary to control the C/B2O3 ratio to 3.5 for the synthesis of boron carbide powder with less free carbon. It is evident in that C/B2O3 contents of the pyrolyzed samples at 600 °C and 650 °C are close to 3.5, while these are exactly 3.5 for 20% EG additions at 650 °C. Consequently, heating at 650 °C should preferably be used as a suitable pyrolysis temperature. It is also obvious that the pyrolysis temperature, to achieve the C/B2O3 ratio in the range of 3.4–3.7, is lower (550 °C) for higher amounts of EG additions (40% and 50%). This phenomenon is ascribed to the low temperature decomposition of some unreacted isolated low-thermal-stability parts without ester bonds (B–O–C) forming with large amounts of EG additions.
Fig. 2 Change in C/B2O3 ratio of P0–P50 precursor powders prepared by pyrolysis at 550–750 °C for 2 h in air.
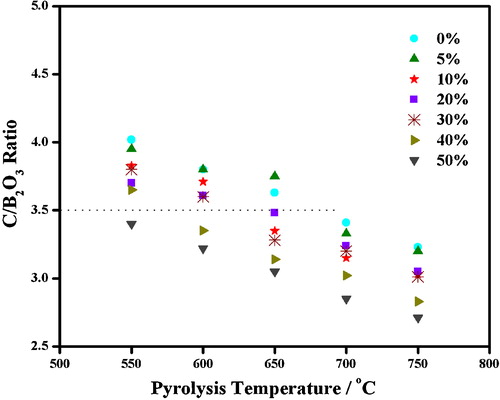
(a)–(d) depicts the morphology of pyrolyzed products of P0, P10, P20, and P30 precursors at 650 °C for 2 h. It is evidenced by XRD analysis (will be discussed later) that the precursors obtained by the pyrolysis of the condensed products are composed of fine carbon and B2O3 particles. (e) exhibits the fine carbon particles obtained by washing pyrolyzed P20 precursor powder in hot water. These results reveal that B2O3 nanoparticles disperse in fine carbon matrix and this dispersion is efficient for P20 sample.
Fig. 3 SEM images of (a) P0, (b) P10, (c) P20, (d) P30 precursors, and (e) corresponding carbon structure of P20 precursor obtained by removing B2O3.
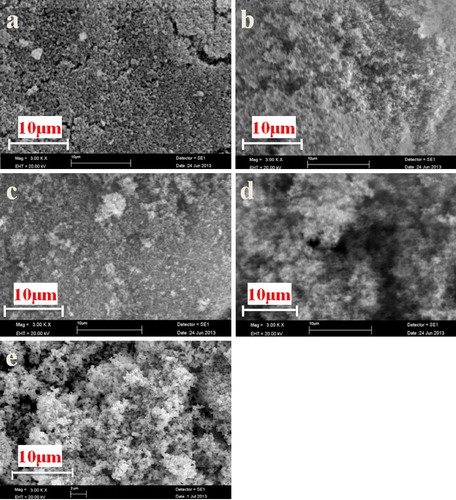
The XRD patterns of the products obtained after heat treatment of precursors P0, P10, P20, P30 and P40 at 1350 °C for 4 h are shown in . The presence of only one peak corresponding to B4C in the XRD pattern of the P0 sample, heated at 1350 °C, suggests that the B4C formation, according to reaction Equation(1)(1)
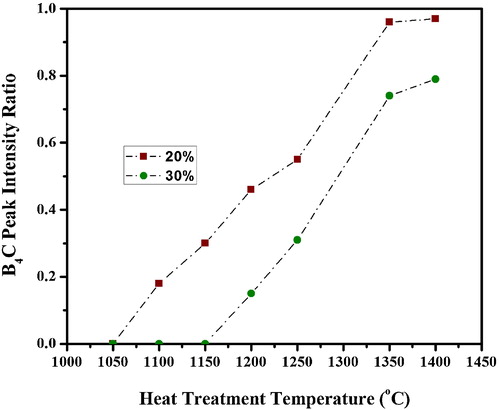
(1) , is not significant at this temperature for sample without EG addition. also indicates that the formation of B4C is accomplished at 1350 °C for samples with EG additions, which is around 100–300 °C, a lower temperature than that reported for B4C synthesis using a condensed product with CA [Citation15–Citation18]. indicates that the B4C peak intensity ratio increases rapidly up to 20% addition and B4C with less free carbon is obtained at 1350 °C for P20 sample. Calculations indicate that the amount of residual carbon for P10, P20, P30, and P40 are 11%, 5%, 18%, and 30%, respectively. The higher residual carbon contents in P10, P30, and P40 samples may be ascribed to a lower interaction between the reactive species, i.e. CA-EG derived carbon and BA derived boron sub oxides, with respect to P20 composition, in which the formation of a polymeric gel results in a highly dispersed structure leading to an enlarged surface-active area between the B2O3 and carbon components with superior reactivity, enabling the low-temperature synthesis of B4C. Moreover, along with residual carbon, some unreacted B2O3 has also been observed in the XRD patterns of the products prepared from P30 and P40. Hence, the variation in B4C formation behavior is assigned to the differences in C/B2O3 of precursor as well as the homogeneity and dispersibility of the B2O3 and carbon components in the precursor. The homogeneity and dispersibility are superior for P20, enabling the diffusion of reacting species facile, resulting in a higher yield of B4C powder at lower temperature.
Fig. 4 XRD patterns of the products obtained by heat treatment of P0–P40 precursors at 1350 °C for 4 h.
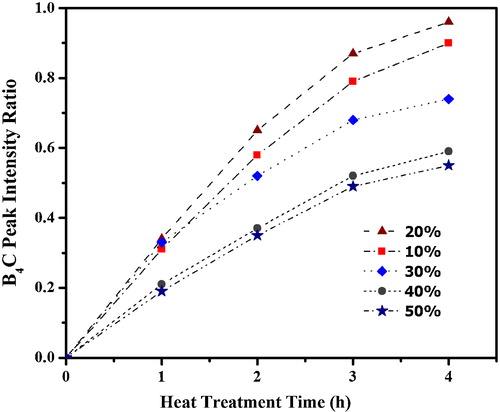
Fig. 5 Change in B4C peak intensity ratio for the products obtained by heat treatment of P0–P50 precursors at 1350 °C for 4 h.
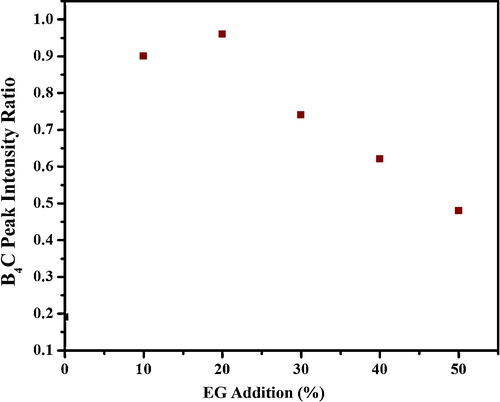
(i) exhibits the XRD spectra of the products prepared from precursor P20 at different heat treatment temperatures of 1050–1400 °C for 4 h in an Ar flow. It is obvious that the peaks pertaining to rhombohedral B4C crystal were observed at 1100 °C and above, implying the inception of B4C formation at 1100 °C. Moreover, the crystalline B4C powder with less free carbon is obtained at 1350 °C, which is a significantly lower temperature than that reported for B4C synthesis using citric acid alone as well as other organic precursors. (ii) indicates that the critical formation temperature of B4C for precursor P30 is 1200 °C, which is around 100 °C higher than that for Precursor P20 (1100 °C), indicating that the dispersion size of B2O3 particles is closely related to the lowering of the synthesis temperature of B4C. summarizes the changes in the B4C peak intensity ratio of the products prepared from Precursors P20 and P30 at different heat treatment temperatures of 1050–1400 °C for 4 h in an Ar flow. It is obvious for 20% EG addition that the B4C peak intensity ratio increases rapidly with increasing temperature, and B4C with less free carbon is obtained at 1350 °C. In contrast, the increase is gentle above 1150 °C, and residual carbon and B2O3 are still remained at 1350 °C for 30% EG addition sample. It is speculated that the efficient dispersion of nanosize B2O3 particles results in an enhancement in the interfacial area between carbon and B2O3 components for Precursor P20. Since the synthesis of B4C is accelerated at the interface in order to facilitate the diffusion of reacting species, therefore, the lowering of synthesis temperature may be ascribed to the enlarged interfacial area.
Fig. 6 XRD patterns of the products obtained by heat treatment of (i) P20 precursor at 1050–1350 °C for 4 h and (ii) P30 precursor at 1150–1400 °C for 4 h.
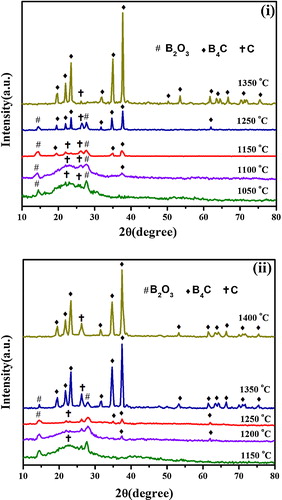
Fig. 7 Change in B4C peak intensity ratio for the products obtained by heat treatment of P20 and P30 precursors at 1050–1400 °C for 4 h.
displays the change in the XRD patterns with holding time for the products obtained by the heat treatment of P20 precursor at 1350 °C for 1–4 h in an Ar flow. The peaks corresponding to B4C crystal are obvious at 1 h and longer. Only P20 and P10 samples have exhibited the B4C peaks with less free carbon at 4 h. In contrast, unreacted carbon and B2O3 peaks are observed for all other samples (not shown) at 1–4 h. The time dependence of the B4C peak intensity ratio of the products prepared from P10–P50 samples at 1350 °C for 1–4 h is shown in . The B4C formation is the fastest for P20 and P10 samples. The B4C peak intensity ratio increases rapidly with increasing heat treatment time for the homogeneous precursors P20 and P10 at 1350 °C, and the formation of B4C is saturated at a heat treatment time of 4 h for precursor P20. In contrast, the increase in the B4C peak intensity ratio is much lower for P30, P40, and P50 precursors, indicating that the synthesis reaction is heterogeneous for these precursors. This variation in B4C formation behavior is caused by the differences in the homogeneity and dispersibility of the B2O3 and carbon components in the precursor. Therefore, the formation of B4C takes place within a short time throughout the entire homogeneous precursors P20 and P10. On the contrary, widely spaced B2O3 and carbon reactive species may exist in the heterogeneous precursors P30, P40, and P50. This structural heterogeneity can retard the formation of B4C for P30, P40, and P50 samples.
Fig. 8 XRD patterns of the products obtained by heat treatment of P20 precursor at 1350 °C for 1–4 h.
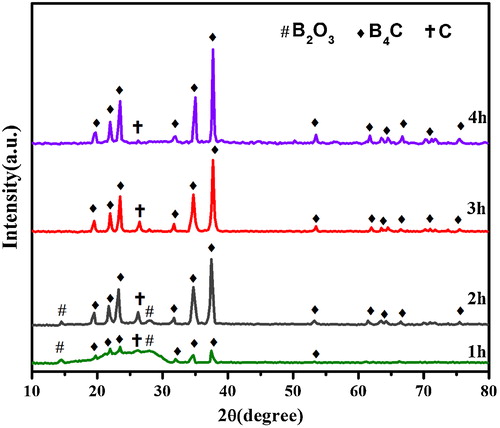
Fig. 9 Change in B4C peak intensity ratio for the products obtained by heat treatment of P10–P50 precursors at 1350 °C for 1–4 h.
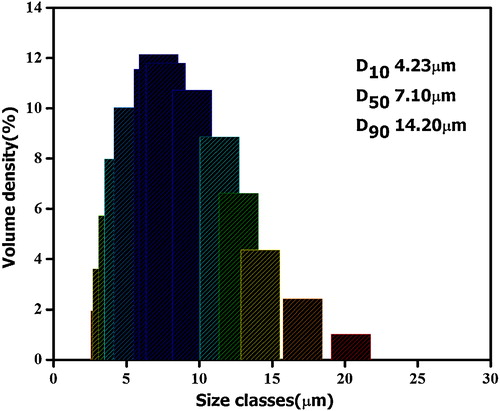
(a)–(e) displays the SEM images of B4C powders synthesized at 1350 °C. P10 sample has exhibited a variety of irregular shaped particles ranging from polyhedral particles, such as truncated octahedral and twinned octahedral, to rod-like particles. The morphology for P20 sample is a mixture of various polyhedral with most of the particles are rounded off. In the case of P30 composition, morphology is mainly needle and rod-like with an aggregate of fine particles. With higher EG additions (P40 sample), morphology is totally transformed into needle and blade-like. These morphology changes, depending on C/B2O3, are associated with B2O3 volatilization losses. With higher EG additions (lower C/B2O3 for P30 and P40), morphology is transformed from polyhedral to needle and blade-like. This needle and blade-like morphology is facilitated by vapor–liquid–solid interfacial reactions [Citation4,Citation6,Citation7,Citation9,Citation22,Citation29,Citation30], testifying the volatilization of B2O3 for B2O3-rich composition. Moreover, it is obvious that the particle size of the powder decreased slightly with increasing EG additions. The particle size distribution of the B4C powder obtained by heat treatment of P20 precursor is shown in . The powder is composed of irregular particles with a narrow size distribution; the median size is 7.10 μm, whereas D90 is less than 14.20 μm. In order to further elucidate the particle size and structure of synthesized powders, BET specific surface areas, total pore volumes, and pore sizes of B4C powders synthesized from P10, P20, P30, and P40 precursors are recapitulated in . The BET specific surface area (SSA) values of powders obtained from P10 and P20 samples are very close, whereas the total pore volume is remarkably higher for the samples with higher additions of ethylene glycol. Moreover, the larger pores obtained for P30 and P40 samples () are in agreement with their low SSA values and high pore volume fraction. It is obvious that P20 sample exhibits the highest specific surface area with smallest pore volume and pore size. It implies again that the powder synthesized from P20 precursor has a larger contact area of the B2O3 and carbon components, resulting in the formation of more nucleation sites.
Table 1 Surface area and porosity parameters of synthesized B4C powders.
Fig. 10 SEM images of products obtained by heat treatment of (a) P0, (b) P10, (c) P20, (d) P30, and (e) P40 precursors at 1350 °C for 4 h.
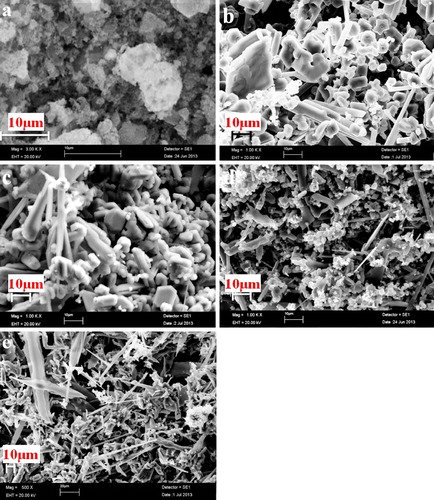
Fig. 11 Particle size distribution of the B4C obtained by heat treatment of P20 precursor at 1350 °C for 4 h.
It is well known that the hydroxyl group can be easily condensed with BA to directly form B–O–C. Also, the carboxyl group decomposes BA by virtue of the esterification reaction between EG and CA. Therefore, the EG (with two hydroxyl groups) addition enables the formation of more B–O–C bonds with high dispersibility. Such a condensed product yields the precursors with fine homogeneous dispersion of B2O3/C. Such a fine dispersed structure provides a large active surface area between B2O3 and carbon with superior reactivity facilitating the low-temperature synthesis of B4C. Moreover, EG induces a decrease in the solution viscosity resulting in the homogenous dispersion of B2O3/C sources and thus increasing the interfacial area. Since, B4C synthesis reaction is favored at the interface to accommodate the diffusion of reacting components, therefore, the lowering of synthesis temperature is ascribed to the enhanced interface area.
4 Conclusions
The effects of EG additions on synthesis of B4C from condensed borate citrate precursor were investigated. EG addition induced the formation of more B–O–C bonds in condensed product yielding precursors with much homogeneous and finely dispersed B2O3/C, resulting in large interfacial area between reactive species facilitating the low-temperature synthesis of B4C. B4C contents of products increased with EG additions up to 20% and residual carbon in final product was lowered to 4% for P20. P20 was found to be optimum composition with suitable pyrolyzed temperature was 650 °C. The B4C peak intensity ratio increased rapidly with increasing heat treatment time for the homogeneous precursors P20 and P10 but the B4C formation was found to be retarded for the heterogenous precursors containing higher amounts of EG additions. The results had indicated that the compatibility of the composition, the dispersibility, and the homogeneity of the B2O3 and carbon components in the precursor led to the low-temperature synthesis of crystalline B4C powder. The morphology of synthesized product was transformed from polyhedral into needle and blade-like with an increase in EG additions from 10% to 40%.
Acknowledgements
This work is financially supported by Materials Division, PINSTECH, Islamabad, Pakistan. Authors also thank the School of Materials Science & Engineering, USTB, Beijing, China for providing the technical support in this project.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- F.ThevenotJ. Eur. Ceram. Soc.61990205225
- P.A.MedwickH.E.FischerR.O.PohlJ. Alloys Compd.20319946775
- E.M.SharifiF.KarimzadehM.H.EnayatiPowder Technol.2282012334338
- A.K.SuriC.SubramanianJ.K.SonberT.S.R.Ch.MurthyInt. Mater. Rev.552010440
- C.-H.JungM.-J.LeeC.-J.KimMater. Lett.582004609614
- R.M.MohantyK.BalasubramanianS.K.SeshadriJ. Alloys Compd.44120078593
- T.KobayashiK.YoshidaT.YanoCeram. Int.392013597607
- E.M.SharifiF.KarimzadehM.H.EnayatiAdv. Powder Technol.222011354358
- A.AlizadehE.T.NassajN.EhsaniJ. Eur. Ceram. Soc.24200432273234
- F.DengH.-Y.XieL.WangMater. Lett.60200617711773
- A.NajafiF.Golestani-FardH.R.RezaieN.EhsaniJ. Alloys Compd.509201191649170
- T.R.PilladiK.AnanthansivanS.AnthonysamyPowder Technol.2462013247251
- M.KakiageN.TaharaI.YanaseH.KobayashiMater. Lett.65201118391841
- I.YanaseR.OgawaraH.KobayashiMater. Lett.6320099193
- S.CorradettiS.CarturanL.BiasettoA.AndrighettoP.ColomboJ. Nucl. Mater.4322013212221
- A.M.HadianJ.A.BigdelooJ. Mater. Eng. Perform.1720084449
- A.SinhaT.MahataB.P.SharmaJ. Nucl. Mater.3012002165169
- D.J.KosanovicL.J.MilovaovicS.MilovanovicA.SaponjicMater. Sci. Forum5552007255260
- T.R.PilladiK.AnanthansivanS.AnthonysamyV.GanesanJ. Mater. Sci.47201217101718
- M.KakiageY.TominagI.YanaseH.KobayashiPowder Technol.2212012257263
- N.TaharaM.KakiageI.YanaseH.KobayashiJ. Alloys Compd.57320135864
- J.DuQ.LiY.XiaX.ChengY.GanH.HuangW.ZhangX.TaoJ. Alloys Compd.5812013128132
- T.ZakiK.I.KabelH.HassanCeram. Int.38201220212026
- W.CaoY.XuS.WangP.LuG.HuangC.XuMater. Lett.59200519141918
- K.KrishnanR.S.KrishnanProc. Indian Acad. Sci. Sect. A641966111122
- A.W.WeimerW.G.MooreR.P.RoachJ.E.HittR.S.DixitS.E.PratsinisJ. Am. Ceram. Soc.75199225092514
- M.G.InghramR.F.PorterW.A.ChupkaJ. Chem. Phys.251956498501
- V.D.DerkachS.P.GordienkoB.V.FenochkaA.P.EpikN.F.KolesnikPowder Metall. Met. Ceram.101971301303
- J.A.BigdelooA.M.HadianInt. J. Recent Trends Eng.12009176180
- R.MaY.BandoChem. Phys. Lett.3642002314317
