?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Co-Al and Ni-Al layered double hydroxides (LDHs) intercalated with three types of anionic molecules, dodecylsulfate (C12H25SO4−, DS), di-2-ethylsulfosuccinate ([COOC2H3EtBu]2C2H3SO3−, D2ES), and polytungstate (H2W12O4210−, HWO) were prepared by means of ion-exchange and co-precipitation processes. With the use of DS and D2ES as intercalation agents, high crystallinity was maintained after intercalation into the LDHs. In the case of HWO, the intercalated LDHs could be obtained by ion-exchange as well as co-precipitation with a decline in the crystallinity; however, unreacted LDH was detected in the ion-exchange samples, and some unwanted phases such as hydroxide and pyrochlore were generated by the co-precipitation process. The maximum specific surface area and pore volume of the Ni-Al sample with intercalated HWO, prepared by the ion-exchange process were 74 m2/g and 0.174 mL/g, respectively. The occupancies of DS, D2ES, and HWO within the interlayer space were approximately 0.3–0.4, 0.5–0.6, and 0.1–0.2, respectively, in the Co-Al and Ni-Al LDHs. Analysis of the catalytic activity demonstrated that the DS-intercalated Ni-Al LDH sample exhibited relatively good catalytic activity for conversion of cyclohexanol to cyclohexanone.
1 Introduction
Layered double hydroxides (LDHs) are generally composed of divalent cations (Mg, Ca, Zn, etc.) and trivalent cations (Al, Fe, Co, etc.), a hydroxide layer, and anions intercalated between the inorganic layers for charge compensation. LDHs are attractive candidates for use as adsorbents, photocatalysts, catalysts, anion exchangers, in electrochemical batteries, and so on. For use as an anion exchanger, the LDH must not be intercalated with CO32− as the anion because CO32− is the most stable anion within the interlayer space of the LDH [Citation1,Citation2]. For application as a photocatalyst or catalyst, active species must be included in the LDH component. For example, Silva et al. reported that the ZnCr LDH exhibited photocatalytic activity for decomposition of H2O using AgNO3 as a sacrificial agent [Citation3]. Katsumata et al. reported that the ZnCr LDH could be used for photocatalytic reduction of CO2 [Citation4]. As a catalyst, Jana et al. reported that the MgAl LDH with co-substituted polyoxometalate (POM) was active for conversion of cyclohexanol to cyclohexanone [Citation5]. Zhu et al. reported that a Cu-containing LDH was an effective oxidization catalyst for phenol [Citation6]. On the other hand, a Ni-containing LDH was examined for electrochemical application [Citation7,Citation8], where the Ni component acts as an electron receiver by variation of the valence of Ni. For all applications, the surface area accessible to gas or ion species is important. Exfoliation of LDHs has been reported as a means of expanding the LDH surface area [Citation9–Citation11]. Takei et al. reported the exfoliation of a transition metal-containing LDH and subsequent deposition by H2O containing CO32− ions to expand the surface area [Citation12]. Transition metals are generally expected to be good catalysts because of the presence of localized d-electrons; therefore, LDHs containing transition metals are promising materials as catalysts for hydrogenation, polymerization, oxidation, reduction reactions, etc., for the conversion of organic species.
LDHs exhibit some soft-chemical reactivities, such as intercalation, exfoliation, and ion-exchange. Ion exchange or thermal decomposition-reconstruction processes are generally utilized in the synthesis of intercalated LDHs [Citation13–Citation15]. The structure and catalytic properties of intercalated LDHs vary depending on the intercalated molecules and the chemical composition of the LDH. However, the relationships between the molecular structure and the porous and catalytic properties of intercalated LDHs are unclear. In this study, the effect of intercalated anions (some organics and POM) with different sizes and electronic densities on the porosity of transition metal-(Co and Ni)-containing LDHs is examined. In addition, the catalytic activity of the transition metal-containing LDHs with/without POM for the conversion of cyclohexanol to cyclohexanone is evaluated. Based on the catalytic behavior, the effects of both the transition metal in the LDHs and the POM are evaluated by using three molecules for intercalation.
2 Experimental
2.1 Preparation of LDHs containing Co and Ni
LDHs were prepared by a hydrothermal process. Divalent metal nitrate [Co(NO3)2·6H2O or Ni(NO3)2·6H2O] and trivalent metal nitrate [Al(NO3)3·9H2O] were used to supply the component cations of the brucite layer (hydroxide layer) of the LDH. These nitrates were combined in a 2:1 ratio and completely dissolved in distilled water (DIW) to give a final concentration of 0.25 mol/L for the divalent cations. The pH of the solutions was then adjusted to 7 ± 0.1 using NaOH and HNO3 and was measured after several minutes. The pH-adjusted solution was hydrothermally treated at 120 °C for 72 h to form the LDH phase. The obtained LDHs containing Co and Ni are designated as Co-Al X and Ni-Al X, where X is the name or chemical formula of the anion included within the interlayer space. For all of the as-prepared samples, X was expressed as CO32−.
Intercalation of the guest molecules into the LDH phase was carried out by either ion-exchange or co-precipitation during preparation of the LDHs. The typical process for preparation of the LDHs and intercalation by ion-exchange and co-precipitation are respectively explained in the two ensuing sections.
2.2 Ion-exchange process
LDHs should be decarbonated before intercalation of the guest. Decarbonation was carried out by the literature method [Citation1]. The LDH samples were stirred in acetate buffer solution containing NaCl for 24 h. The acetate buffer solution was composed of acetic acid, sodium acetate, and aqueous NaCl at a ratio of 1:9:250. After stirring, the treated samples were washed with N2-bubbled DIW and dried at room temperature (RT) using dried silica gel. Anions (X−) included in the decarbonated LDHs could thus be exchanged to Cl−.
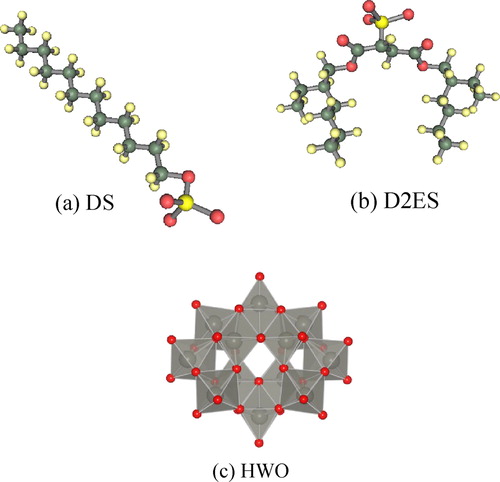
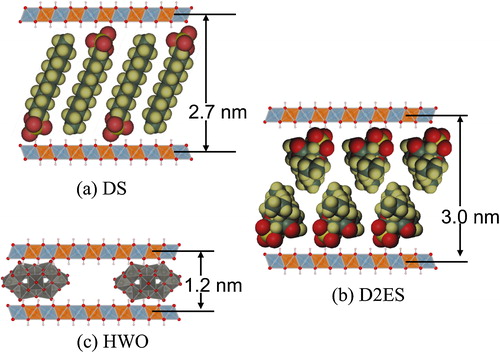
The decarbonated LDHs were then used for the intercalation. Three types of guest molecules were used, i.e., sodium dodecylsulfate (C12H25OSO3Na), sodium di-2-ethylsulfosuccinate ([COOC2H3EtBu]2C2H3SO3Na), and sodium polytungstate (Na10[H2W12O42]). These molecules readily dissociate into a sodium cation and relatively large anions with different charge density. These anionic molecules are designated as DS, D2ES, and HWO, respectively. Schematic illustrations of these anionic molecules are presented in , and the physical properties (surface area and volume) are listed in . The DS and D2ES anions are respectively single and dual-chain molecules that can expand the interlayer spacing easily. Consequently, intercalation of DS and/or D2ES may increase the accessible surface of the Co- or Ni-containing LDH, which may provide information on the catalytic activity of the LDH only. In the case of HWO, intercalation should produce a hybrid effect of the Co- or Ni-containing LDH and POM. These guests were first dissolved in DIW and the decarbonated LDH was placed into the solution containing an equivalent concentration of the guest (intercalation agent). The solutions were then shaken at 200 rpm at RT for 120 h. The resultant samples were washed with N2-bubbled DIW and dried with silica gel at RT. For the sample prepared by the ion-exchange process, ‘(IE)’ was appended to the sample name.
Table 1 Physical and electronic properties of the molecules intercalated within the interlayer space of LDH.
2.3 Co-precipitation during preparation of LDHs
The co-precipitation process was carried out as follows. The starting reagents for preparation of LDHs were the same as used in the typical preparation process mentioned above, except for addition of the guest molecules. Each of the three types of guest molecules was placed into the solution before adjustment of the pH. An amount of the guest molecule equivalent to the amount of the LDH was used. The pH of the solution was then adjusted and the solutions were hydrothermally treated at 120 °C for 72 h in the same manner described above. The resultant sample was washed with decarbonated DIW and dried at 50 °C. The abbreviation ‘(CP)’ appended to the sample name indicates that the sample was prepared by the co-precipitation process.
2.4 Characterization
The prepared intercalated LDH hybrids were identified by X-ray diffraction (XRD; RIGAKU RINT-2000) using monochromated CuKα radiation. The chemical compositions of the cations in the hybrid were measured via inductively coupled plasma (ICP; SII, SPS-1700) analysis. The amount of organic component in the hybrid was estimated by thermal gravimetric-differential thermal analysis (TG-DTA; RIGAKU, TG-8120). The microstructures were observed by means of field emission scanning electron microscopy (FE-SEM; JEOL, JEM-6500F). The porous texture was evaluated by using Brunauer–Emmett–Teller (BET) analysis and t-plot calculation for the specific surface area and micropore analysis using a microporosimeter (Nippon-BEL, BELSORP-mini). The conversion from cyclohexanol to cyclohexanone was measured via HPLC with an ODS column at 40 °C. The initial concentration of cyclohexanol was 0.1 mol/L and the concentration of the catalysts was 2 g/L. The reaction was carried out at 100 °C with stirring and the concentration was monitored at 20 min intervals.
3 Results and discussion
3.1 Ion-exchange in the interlayer space of LDH
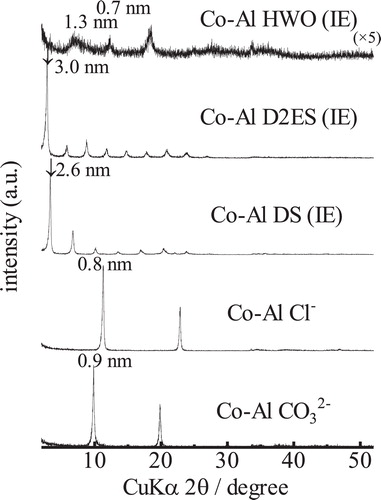
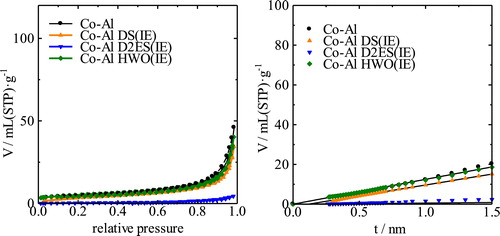
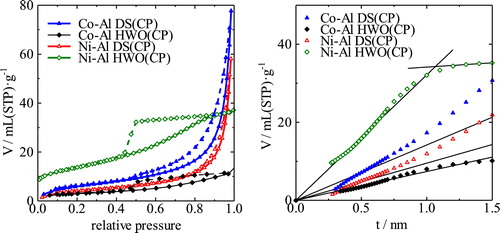
shows the XRD patterns of the Co-Al CO32− and Co-Al Cl− LDHs and their intercalated derivatives generated by the ion-exchange process. The interlayer spacing of the starting LDH was approximately 0.9 nm and decreased to ∼0.8 nm after ion-exchange from CO32− to Cl−. The interlayer spacing increased from the initial value to ∼2.6 nm with intercalation of DS, to ∼3.0 nm with D2ES, and to ∼1.3 nm with HWO. The diffraction peaks of the DS- and D2ES-intercalated LDH samples were very sharp whereas broad peaks were identified in the XRD pattern of the HWO-intercalated LDH, which are attributed to the intercalated phase. These broad peaks might result from the difficulty in forming the intercalated structure with HWO. The low crystallinity may result from the hardness of the anion. The DS and D2ES anions are primarily composed of alkyl chains that are very flexible, as shown in . On the other hand, the HWO anion can be regarded as a rigid large particle. Because the LDH nanosheet is composed of 2-dimensionally edge-shared M(OH)6 octahedra, the nanosheet is expected to be flexible and to bend easily. Therefore, the crystallinity of the sample might decrease with intercalation of HWO. In addition, a peak at 2θ = 18° can be observed in the patterns, which may be attributed to the hydroxide (Co(OH)2) phase. A small amount of the unreacted LDH phase is also observed in this sample. However, intercalation of HWO into the LDH can be definitively confirmed from the broad peak in the patterns. shows the isotherms and t-plots of the Co-Al derivatives. The adsorption volumes from the respective isotherms are very similar, except for the D2ES-intercalated LDH. The specific surface areas of these samples are almost the same at ∼15 m2/g. The N2 adsorption volume of the D2ES sample was very small. These isotherms confirm that the sample is non-porous and the particle size is very large for the ion-exchanged samples of Co-Al. In fact, this sample was obtained as a transparent bulk piece and the amount obtained was significantly smaller than the others.
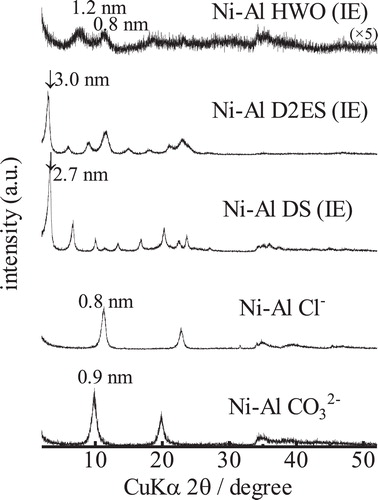
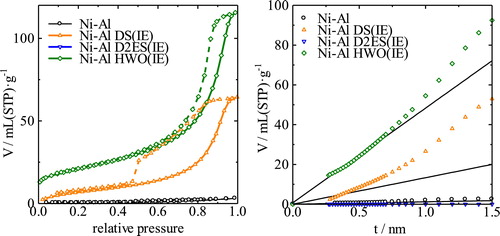
shows the XRD patterns of the Ni-Al CO32− and Ni-Al Cl− LDHs and their intercalated derivatives. The interlayer spacing increases from 0.8 nm to 2.7, 3.0, and 1.2 nm upon intercalation of DS, D2ES, and HWO, respectively. The XRD pattern of the sample with HWO is notably broadened. However, the hydroxide peak was not detectable. In the case of the D2ES sample, a small bulk piece was obtained, the appearance of which was similar to that of the Co-Al LDH. shows the isotherms and t-plots of the Ni-Al derivatives. From these isotherms, the adsorption volumes increased evidently due to intercalation. The specific surface areas of Ni-Al LDH, and the DS-intercalated and HWO-intercalated samples were 2, 28, and 74 m2/g, respectively. The surface area could not be determined for the sample with intercalated D2ES because the adsorption volume was small, similar to the case of the Co-Al LDH. Large hysteresis loops can be observed in the isotherms of DS and the HWO-intercalated LDHs. This hysteresis is attributed to a change of the hybridized texture. The micropore volumes could not be definitively determined from the t-plots of the samples because some capillary condensation seems to occur in the higher pressure range for certain samples.
3.2 Co-precipitation during preparation of LDHs by hydrothermal process
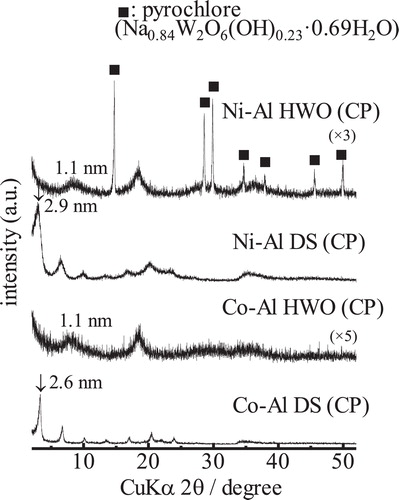
shows the XRD patterns of the co-precipitated Co-Al and Ni-Al samples respectively intercalated with DS and HWO during hydrothermal treatment. In the case of D2ES, no deposits could be obtained by the hydrothermal reaction. For the Co-Al samples, intercalation of DS resulted in relatively high crystallinity. The crystallinity of the LDH declines when HWO is intercalated during hydrothermal treatment. Similar to the LDH samples intercalated by means of ion-exchange, the interlayer spacings of the co-precipitated samples also increase. In the case of the Ni-Al LDH, the intercalation of DS proceeded giving rise to relatively low crystallinity of the LDH. In the case of the HWO-intercalated LDH, the pattern confirms the presence of both an intercalation phase with an interlayer space of ca. 1.1 nm and an undesired pyrochlore phase (Na0.84W2O6(OH)0.23·0.69H2O) with relatively high crystallinity. For both compositions (i.e., Co-Al and Ni-Al) some amount of the metal hydroxide was present in the HWO-intercalated LDHs. However, no unreacted LDH phase was observed in these patterns.
Fig. 6 XRD patterns of the hydrothermally prepared Co-Al and Ni-Al LDHs including anions within interlayer spaces.
shows the isotherms and t-plots of the intercalated LDHs obtained by hydrothermal treatment. From these isotherms, the Ni-Al LDH with intercalated HWO can be regarded as a porous material. The t-plots of these LDH derivatives confirm that the Ni-Al LDH with HWO has micropores of ca. 2 nm in size. The specific surface area of this sample is ca. 48 m2/g. The specific surface area of the pyrochlore is ca.10 m2/g based on estimation of the crystallite size from Scherrer's equation using the XRD patterns in . The smaller surface area relative to a similar sample prepared by shaking (74 m2/g) possibly results from the formation of the pyrochlore phase. It is difficult to determine whether micropores are present in the other samples based on the t-plot because capillary condensation occurs at higher pressure. From these results, the surface area of the Ni-Al LDH is generally larger than that of the Co-Al LDH. The presence of micropores can be confirmed for some samples; however, capillary condensation occurs at high pressure and occludes the detection of the micropores. Consequently, analysis of the hybrid texture based on theoretical consideration of the porous properties was required and is presented in the next section.
3.3 Pore structures of intercalated LDH
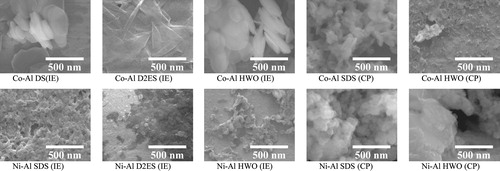
shows the FE-SEM micrographs of the intercalated Co-Al and Ni-Al LDHs. The particle size of the samples prepared by ion-exchange seems to be affected by the particle size of the starting LDHs. The samples with intercalated D2ES generally had a film-like morphology. The particle size of the co-precipitated samples was notably small. The co-anion may act as an inhibitor of the growth of the LDH particles. The particle size was especially small for the LDH with HWO prepared by co-precipitation, and the LDH-plate could not be observed. Despite the difficulty of evaluating the hybridization textures of the samples, it could be deduced that the primary particles of the LDH are smaller for Ni-Al than for Co-Al. Such small particles often tend to form pores between LDH primary particles, which affects the adsorption isotherms. Thus, the changes in the isotherms may result from these fine pores between the LDH primary particles.
Fig. 8 FE-SEM micrographs of the intercalated Co-Al and Ni-Al LDHs prepared by ion-exchange and co-precipitation processes.
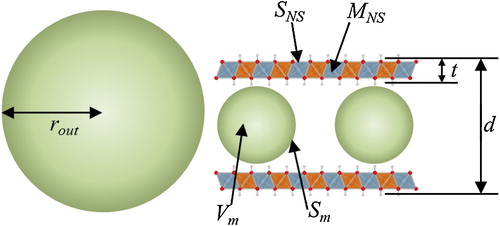
The physical morphology of the samples can be regarded as a mixture of intercalated hybrid and excess intercalation agent particles that cannot be intercalated, as shown in . For this model, the pore volume (Vp), specific surface area (Sp), and occupancy within the interlayer space (Oc) of the sample can be expressed as follows:(1)
(1)
(2)
(2)
(3)
(3) where d is the separation between the brucite layers, t is the thickness of the hydroxide nanosheet, and SNS and MNS are the crystallographic area and mass of a nanosheet inscribed in a unit cell (a = 0.3046 nm, hexagonal), respectively. The values of t and SNS used in this paper are 0.39 nm and 0.08 nm2. Vm, Sm, and Mm are the volume, surface area, and molecular weight of the intercalated anionic molecules, DS, D2ES, or HWO, as shown in .
,
and
are the volume, surface area, and molecular weight of the intercalated CO32−, respectively. The values of these parameters used herein are 0.042 nm3, 0.64 nm2, and 60 g mol−1, respectively. A and C are the amount of the intercalated anionic molecules and CO32− included in the sample, respectively, measured by TG-DTA and ICP analysis; k is the fraction of intercalated anionic molecules; rout is the particle size (radius) of the non-intercalated molecules that are present outside of the LDH particles. Vp and Sp are estimated from the N2 isotherms. Vp is determined at P/P0 = 0.962, which corresponds to 50 nm of the maximum mesopore size. In this model, the pore volume, Vp, is assumed to be generated only between the inorganic layers. The specific surface area (Sp) is assumed to be derived from a plane on the face of the inorganic layer. From these equations and this assumption, k and rout can be calculated. Oc indicates the volume occupied by the molecule within the interlayer space, i.e., the volumetric occupancy. The calculated parameters for assessing the porous morphological features are summarized in . In this model, the anionic molecules are assumed to exist both within the interlayer space and on the outer LDH surface, though CO32− is present only in the interlayer space. The general formula used in the model can be expressed as
. ICP results confirm that x ranges from 0.31 to 0.36 for Co-Al and Ni-Al.
Table 2 Physical properties and intercalation moieties of the molecule-intercalated LDH.
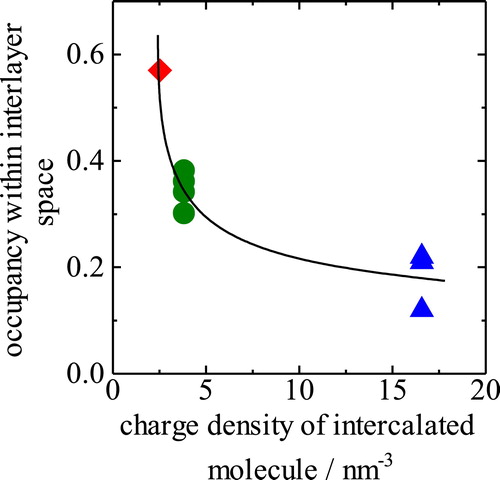
Based on the data in , most of the molecules placed into the solution are found to be intercalated within the interlayer space in all of the samples based on the minimum k values of 0.97. However, intercalation of HWO may be accompanied by intercalation of CO32−, which increases the CO32− content in the HWO-intercalated LDH. In addition, the outer particles comprising the intercalation agent are several nm in size in all of the samples. Therefore, the outside particles used can be ignored in this study. The volumetric occupancy within the interlayer space is 0.3–0.4 for DS, 0.5–0.6 for D2ES, and 0.2–0.3 for HWO. The volumetric occupancy may be determined by the charge density per unit volume of the molecules. In fact, the respective charge densities of the intercalated molecules are estimated to be 3.9, 2.5, and 16.6 nm−3 for DS, D2ES, and HWO, respectively, from the molecular volume and electronic charge in . Thus, a higher charge density of the molecule results in lower occupancy and a resultant large pore volume within the interlayer space. shows the relationship between the charge density of the intercalated molecules and the interlayer occupancy. From this plot, a higher charge density results in lower occupancy within the interlayer space. This tendency may be clear evidence of the intercalation of the three kinds of molecules into the interlayer space. shows a schematic illustration of the LDH derivatives. In the case of the DS and HWO-intercalated LDH, the molecules may be intercalated as a monolayer. A bilayer structure is plausible for the D2ES-intercalated LDH based on the XRD patterns, hybrid textural analysis, and other data presented above.
3.4 Catalytic activity for conversion of cyclohexanol
The catalytic activity of the DS- and HWO-intercalated LDH samples for cyclohexanol conversion was examined; however, unfortunately, only a small amount of the D2ES-intercalated LDH could be obtained from either hybridization process, thus precluding this analysis. shows the changes in the concentration of cyclohexanol and the generation of cyclohexanone in the presence of the hybrid catalysts from the co-precipitation process. The concentration of cyclohexanol decreased with the use of all samples. However, the amount of cyclohexanone increased only slightly, especially with the use of Ni-Al DS. Therefore, the general decrease in the cyclohexanol concentration probably results from adsorption of cyclohexanol. The intercalated compounds with HWO and DS seem to have a larger capacity for adsorption than the non-intercalated samples. Based on the compositional differences of the LDH samples, the decrease in the concentration of cyclohexanol appears to be similar for the non-intercalated samples and the HWO-intercalated hybrids. However, in the case of the DS-intercalated samples, the cyclohexanol vanished completely with the use of Ni-Al DS, where the cyclohexanone concentration increased in correspondence with the disappearance of cyclohexanol.
Fig. 12 Variation of concentration of cyclohexanol and generated cyclohexanone in the presence of the hybrid catalysts with DS and HWO.
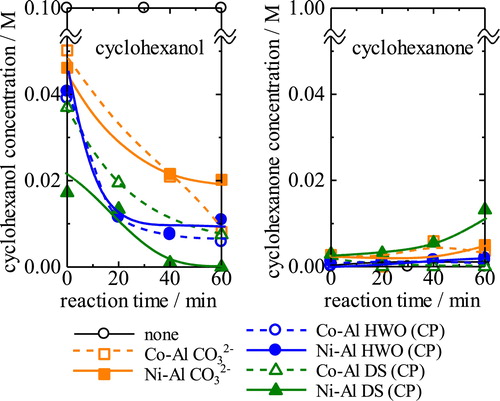
The catalytic behavior can be summarized as follows: cyclohexanol tends to be adsorbed on the hybrids or converted into other species. The cyclohexanol concentration decreased in the presence of the HWO-intercalated hybrids but was not converted into cyclohexanone. Of the DS-intercalated hybrids, Ni-Al DS is effective for conversion of cyclohexanol to cyclohexanone. A plausible reason for this conversion ability is the larger interlayer distance relative to the other materials. Because the maximum length of cyclohexanol is ca. 0.8 nm, it is difficult to insert cyclohexanol into the interlayer space of the hybrids, except for the DS samples. Thus, the Ni-Al LDH is catalytically active for the conversion of cyclohexanol to cyclohexanone.
4 Conclusions
Three types of molecules with different charge densities were intercalated into the Co-Al or Ni-Al LDH via ion-exchange or co-precipitation during synthesis of the LDH. The physical characteristics of the prepared hybrids were examined based on the XRD patterns and porous properties. The results can be summarized as follows:
| 1. | DS-, D2ES- and HWO-intercalated LDH hybrids were successfully prepared by the ion-exchange process, and the DS- and HWO-intercalated LDH hybrids were successfully prepared by co-precipitation during synthesis of the LDHs. | ||||
| 2. | The maximum specific surface area of 74 m2/g was obtained for the Ni-Al LDH intercalated with HWO by the ion-exchange process. | ||||
| 3. | Almost all intercalation agents are intercalated within the interlayer space. The residual agents are deposited on the outside of the LDH particles and are of the order of several nm in size. | ||||
| 4. | Nano-voids are formed between the primary particles of the Ni-Al samples because the primary particles of the Ni-Al LDH are much smaller (∼50 nm) than the primary Co-Al particles. | ||||
| 5. | The DS-intercalated Ni-Al LDH is catalytically active for conversion of cyclohexanol to cyclohexanone. | ||||
Acknowledgement
A part of this study was financially supported by the Grant-in Aid for Scientific Research (C) 24560822.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- N.IyiT.SasakiJ. Colloid Interface Sci.3222008237245
- N.IyiK.OkamotoY.KanekoT.MatsumotoChem. Lett.342005932933
- C.G.SilvaY.BouiziV.FornésH.GarcíaJ. Am. Chem. Soc.13120091383313839
- K.KatsumataK.SakaiK.IkedaG.CarjaN.MatsushitaK.OkadaMater. Lett.1072013138140
- S.K.JanaY.KubotaT.TatsumiJ. Catal.25520084047
- K.ZhuC.LiuX.YeY.WuAppl. Catal. A: Gen.1681998365372
- A.FaourC.MoustyV.PrevotB.DevouardA.De RoyP.BordetE.ElkaimC.Taviot-GuehoJ. Phys. Chem. C11620121564615659
- V.S.JamadadeV.J.FulariC.D.LokhandeJ. Alloys Compd.509201162576261
- T.HibinoM.KobayashiJ. Mater. Chem.152005653656
- L.LiR.MaY.EbinaN.IyiT.SasakiChem. Mater.17200543864391
- F.LerouxM.Adachi-PaganoM.IntissarS.Chauvie-ÁreC.ForanoJ.-P.BesseJ. Mater. Chem.112001105112
- T.TakeiA.MiuraN.KumadaK.OkadaJ. Porous Mater.202013777783
- Y.KameshimaH.SasakiT.IsobeA.NakajimaK.OkadaInt. J. Pharm.38120093439
- S.AisawaH.KudoT.HoshiS.TakahashiH.HiraharaY.UmetsuE.NaritaJ. Solid State Chem.177200439873994
- S.AisawaN.HigashiyamaS.TakahashiH.HiraharaD.IkematsuH.KondoH.NakayamaE.NaritaAppl. Clay Sci.352007146154