?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We report the structural analysis of Na+ and Cs+ in sodium cesium silicate glasses by using 23Na and 133Cs magic-angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy. In the NMR spectra of cesium silicate crystals, the peak position shifted to higher magnetic field for structures with larger Cs+ coordination numbers and to lower magnetic field for smaller Cs+ coordination numbers. The MAS NMR spectra of xNa2O-yCs2O-2SiO2 (x = 0, 0.2, 0.33, 0.5, 0.66, 0.8, 1.0; x + y = 1) glass reveal that the average coordination number of both the alkali cations decreases with increasing Cs+/(Na+ + Cs+) ratio. In addition, the coordination number of Na+ in xNa2O-yCs2O-2SiO2 glass is smaller than that of Cs+. This difference between the average coordination numbers of the alkali cations is considered to be one structural reason of the mixed alkali effect.
1 Introduction
Structural analysis of alkali ions in glass is an important topic in glass science because of the mixed alkali effect (MAE) that originates when the composition of a glass is altered by gradual substitution of one alkali for another [Citation1–Citation4]. More specifically, the MAE is characterized by the deviation from the linear additive properties of alkali silicate glasses, which can be attributed to structural, thermodynamic, electrodynamic, and other factors. It is well known that the electrical conductivity of alkali silicate glasses decreases when one type of alkali ion is replaced by another type [Citation1,Citation3]. Typically, the minimum electric conductivity is observed at an intermediate composition.
Thus far, various structural models have been proposed to explain the MAE on the basis of a homogeneous spatial cation distribution using nuclear magnetic resonance (NMR) spectroscopy [Citation5–Citation10], extended X-ray absorption fine structure (EXAFS) analysis [Citation11], and so on. For instance, Ingram et al. reported that the MAE is caused by the disturbance created when one type of alkali ion moves to a site that was previously occupied by another type of alkali ion [Citation12]. The transport of alkali ions in a glass network is hindered when the network contains a different type of alkali ions, mostly because of the energy mismatch between the sites. Several researchers have substantiated this assumption by performing ab initio molecular orbital calculations and XPS analysis [Citation13,Citation14]. In addition, the 29Si magic-angle spinning (MAS) NMR spectra of various types of single alkali silicates can be used to determine the relative amounts of different SiO4 units Qn, which designates tetrahedrally coordinated Si with n bridging oxygen atoms and (4 − n) non-bridging oxygen atoms. Each Qn value is established by the equilibrium of the following reaction: Qn ↔ Qn+1 + Qn−1 [Citation15]. This reaction proceeds to the right with increasing field strength (Z/r). In fact, Stebbins observed that replacing a high-field-strength ion with a low-field-strength ion (e.g., Li for Na) increases the mean number of non-bridging oxygen atoms associated with the high-field-strength ion.
Recently, we investigated the heterogeneous distribution of Na+ in mixed alkali silicate glasses (Na2O-K2O-SiO2 glass) by performing 23Na multiple-quantum magic-angle spinning (MQMAS) NMR studies [Citation16]. The results were also supported by the Na+ elution analysis, which showed that Na+ is extracted faster from more “aggregated” sites than from less aggregated sites. Moreover, the MQMAS results revealed that cations with high-field strengths are likely to cluster together in mixed silicate glasses.
Nevertheless, in the previous study, the local structure of K+ in Na2O-K2O-SiO2 glass could not be investigated, as the 39K sensitivity of NMR is extremely low [Citation16]. In order to understand the precise mechanism underlying the MAE, analyses of the local structure of both alkali ions are required. In this study, we focused on the Na2O-Cs2O-SiO2 glass system, which is known to show the MAE [Citation3], because 23Na and 133Cs NMR spectra can both be acquired.
Previously, in cesium sodium phosphate glasses, for a given cation species A, the average distance A–O increases when the cation A is replaced by another cation B with a smaller radius [Citation17]. This phenomenon is seemingly different from our previous results described above [Citation16]. To identify the reason for this discrepancy, in this study, we have analyzed Na2O-Cs2O-SiO2 glass from the view point of the heterogeneous distribution of the alkali sites by using 23Na and 133Cs MAS NMR. We have also qualitatively evaluated the average coordination number of each ion, which have not been previously reported.
2 Experimental
2.1 Preparation of glass samples
All glass samples were prepared using the melting method from SiO2, Na2CO3, and Cs2CO3 as starting materials. In the typical process, predetermined quantities of the starting materials were mixed and melted in a platinum crucible at 1350 °C for 1 h. Subsequently, the melts were quenched by immersing the bottom of the platinum crucible in water. The glasses thus obtained had a composition of xNa2O-yCs2O-2SiO2 (x = 0, 0.2, 0.33, 0.5, 0.66, 0.8, 1.0; x + y = 1). The actual compositions of the glasses were analyzed by X-ray fluorescence spectroscopy (XRF; Rigaku, ZSX 100e), as shown in . The difference between the batch and analyzed compositions was <7%. Therefore, the compositions of the glasses are given as the batch compositions in this report.
Table 1 Compositions of the xNa2O-yCs2O-2SiO2 glasses (y = 1.0, 0.8, 0.66, 0.5, 0.33, 0.2; x + y = 1) analyzed by using XRF. The amount of alkali oxides was calculated as SiO2 = 2.
2.2 Preparation of cesium silicate crystals
Cs2Si2O5 and Cs6Si10O23 crystals were synthesized to investigate the relationship between the coordination number and chemical shift of Cs+. In a typical process, Cs2Si2O5 crystals were prepared by mixing the starting materials in amounts corresponding to Cs2O-2SiO2 and melting them in a platinum crucible at 1350 °C for 1 h [Citation18]. The melt was slowly cooled at a rate of 0.1 °C/min. Similarly, the Cs6Si10O23 crystals were prepared using a glass heating process [Citation19]. Starting materials in amounts corresponding to Cs2O-4SiO2 were mixed and melted in a platinum crucible at 1350 °C for 1 h, then quenched by immersing the bottom of the platinum crucible in water. Finally, the quenched glasses were heated at 840 °C for 240 h to obtain Cs6Si10O23 crystals.
2.3 Characterization of crystal samples
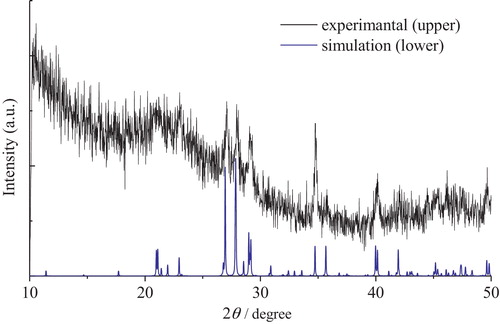
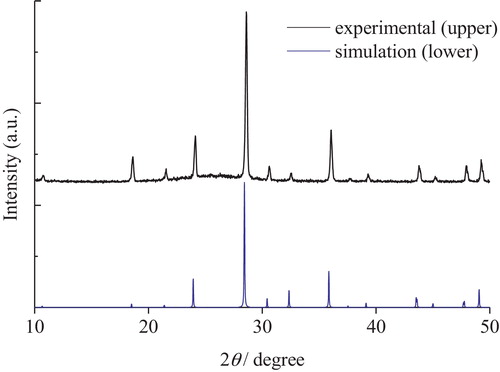
The crystal structure of the samples was identified by X-ray diffraction analysis (Rigaku, RINT-2100). As Cs2Si2O5 crystals are highly susceptible to the moisture absorbed from air, they were enclosed in a capillary tube made of borosilicate glass and fixed onto a jig during the measurements. In addition, the XRD patterns were also simulated from the crystal structure data [Citation18,Citation19] by using POWDER CELL [Citation20].
2.4 NMR spectroscopy
Solid-state 23Na and 133Cs NMR spectra of all the crystal and glass samples were acquired on a Chemagnetics CMX400 spectrometer using a commercial probe (4 mm). To avoid including water, the samples were crushed and sealed into sample holders in a glove box. After the measurements, the samples remained in a powder form, indicating that they absorbed no moisture. The rotation speed was set to 10 kHz with an accuracy of ±10 Hz. At an external field of 9.4 T, the resonance frequencies were near 103.7 and 53 MHz for 23Na and 133Cs, respectively. For each measurement, 90° pulses were set to 2.2 and 10 μs for 23Na and 133Cs, respectively. The spectra were obtained with a cycle time of 1 and 200 s for 23Na and 133Cs, respectively. The chemical shift references were 1 mol/L NaCl and 1 mol/L CsCl aqueous solutions, whose chemical shifts were set to 0 ppm. All the spectra were normalized, as the total areas are proportional to the content of the alkali that is observed by NMR. For example, the area of the 23Na MAS NMR spectrum of 0.8Na2O-0.2Cs2O-2SiO2 is 4 times larger than that of 0.2Na2O-0.8Cs2O-2SiO2. Accordingly, the areas of the spectra can be compared with each other.
3 Results
3.1 XRD analysis of cesium silicate crystals
The X-ray diffraction patterns of Cs2Si2O5 and Cs6Si10O23 crystals are shown in and , respectively. The XRD pattern of the Cs2Si2O5 crystal shows an amorphous broad peak at around 20°, corresponding to the borosilicate of the capillary tube. All other diffraction peaks could be indexed to the crystal phase of Cs2Si2O5, whose peaks were simulated from the crystal structure by using POWDER CELL. Similarly, the XRD pattern of the Cs6Si10O23 crystal also shows a trace of an amorphous peak at around 26°, corresponding to the glass remaining after the production process. All other diffraction peaks could be indexed to the crystal structure of Cs6Si10O23 [Citation19], whose peaks were simulated by using POWDER CELL like those of Cs2Si2O5. The XRD patterns of both cesium silicate crystals revealed a single phase without any impurities or mixed phases.
3.2 133Cs MAS NMR spectra of cesium silicate crystals
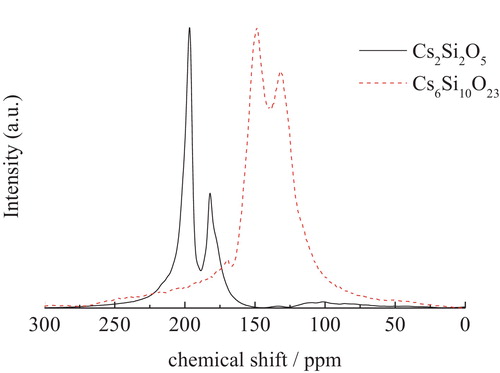
shows the 133Cs MAS NMR spectra of the Cs2Si2O5 and Cs6Si10O23 crystals prepared in this study. The NMR peaks were split by quadrupolar coupling, and thus were not isotropic chemical shifts. As is well known, performing measurements at multiple magnetic fields would provide the isotropic shifts for these spectra. However, such data were not available in this study. Therefore, the chemical shifts of the cesium silicate crystals were defined as the arithmetic averages of the splitting peaks. For example, the chemical shift for Cs2Si2O5 was calculated as (197 + 182)/2 ≈ 190 ppm. Although this treatment is insufficient for quantitative analysis, the spectra of the glass samples were broad and thus could be interpreted successfully. According to this method, the chemical shifts of Cs2Si2O5 and Cs6Si10O23 crystals were estimated as 190 and 140 ppm, respectively.
In addition, larger numbers of cesium silicate crystals are needed to evaluate the precise coordination numbers. However, only two kinds of crystals are available. We have tried to make additional single crystals using several different methods but failed to obtain single crystals.
3.3 23Na and 133Cs MAS NMR of xNa2O-yCs2O-2SiO2 glasses
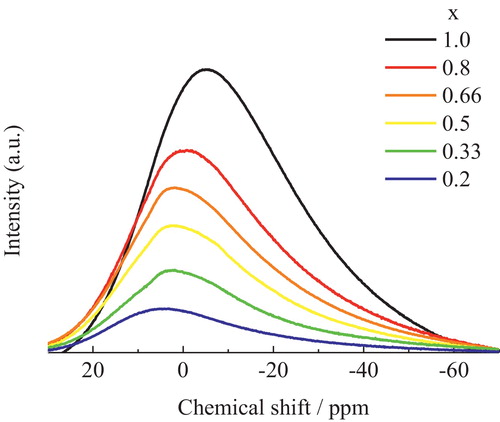
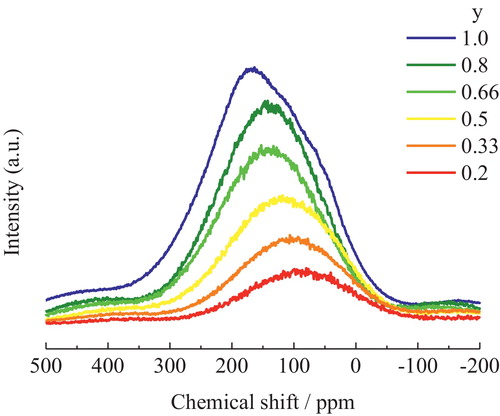
shows the 23Na MAS NMR spectra of the xNa2O-yCs2O-2SiO2 glasses prepared in this study. The spectra shifted to higher field with increasing Na2O content. The 133Cs MAS NMR spectra of xNa2O-yCs2O-2SiO2 glasses are shown in . When the Cs+ in the Cs2O-2SiO2 glass (y = 1.0) was replaced with Na+, the low-magnetic-field component of the NMR spectrum (at around 250 ppm) decreased in intensity, while the high-magnetic-field component (at around 100 ppm) became the main peak.
4 Discussion
4.1 Correlation between chemical shift and coordination number of Cs+ in silicate glasses
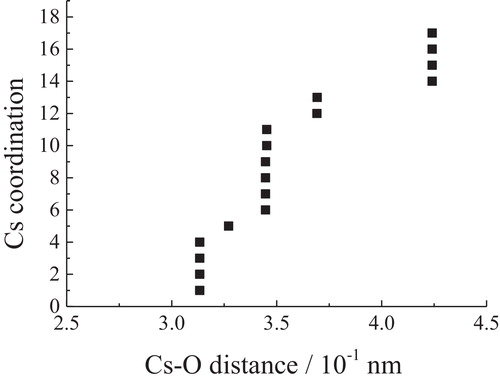
The coordination of the central atom in a crystal is defined as the number of nearest neighbors. By using POWDER CELL, the numbers of oxygen atoms were plotted as a function of the Cs–O distance in and for Cs2Si2O5 and Cs6Si10O23, respectively. As Xue and Stebbins pointed out [Citation21], there is a distance gap between the first coordination spheres containing oxygen and the outer sphere. In the present case, there is a gap at 0.350 nm for four kinds of cesium atoms in Cs2Si2O5. Thus, the Cs sites in Cs2Si2O5 are considered to be 8-coordinated. In Cs6Si10O23, there are 13 oxygen atoms at distances within 0.375 nm, and the more distant oxygen atoms are separated from this first group by about 0.1 nm. Therefore, the Cs sites in Cs6Si10O23 are considered to be 13-coordinated.
Fig. 6 Relationship between Cs–O distances and coordination numbers in Cs2Si2O5. There exist four kinds of cesium that are crystallographically independent.
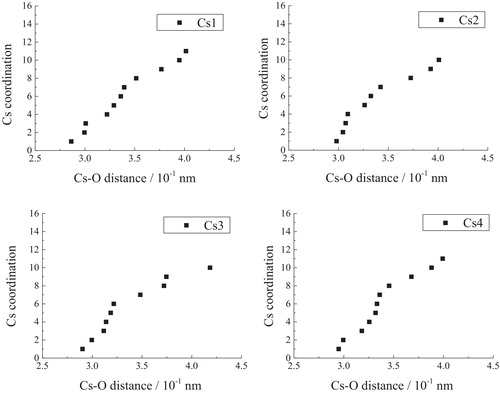
As can be seen from , the peak position shifted to higher magnetic field for larger coordination numbers of Cs+, while the peak positions shifted to lower magnetic fields for smaller coordination numbers of Cs+. In other terms, the Cs+ with the higher coordination number of 13 in Cs6Si10O23 showed a high-field shift (140 ppm), while that with the smaller coordination number of 8 in Cs2Si2O5 exhibited a low-field shift (190 ppm). This relationship is similar to that found in sodium silicate by Stebbins [Citation8,Citation21], wherein the crystal coordination number of Na+ ions is related to the 23Na crystal chemical shift. Even though there are only two kinds of crystals, we have nevertheless evaluated the relationship between the coordination number, Nc, and the chemical shift of Cs, σCs, as follows:(1)
(1) Using this equation, we have evaluated the coordination number of Cs+ in the glasses, which is shown in Section 4.4. We have also evaluated the relationship between the chemical shifts and the coordination number of Na+ in silicate glasses from reference data [Citation8].
4.2 23Na MAS NMR of xNa2O-yCs2O-2SiO2 glasses
Although there seems to be only one resonance in the 23Na MAS NMR spectra that shifted in response to composition changes, the broadness of the spectra is still assumed to be related to the sodium site distribution in the glasses and not just due to quadrupolar broadening [Citation16]. In particular, replacing Na+ in Na2O-2SiO2 glass (x = 1.0) with Cs+ decreases the intensity of the high-magnetic-field component in the NMR spectrum (at around −25 ppm), because their areas were proportional to the amount of Cs in the glasses. At the same time, the low-magnetic-field component (at around 5 ppm) became the main peak. According to the previous study [Citation8], the high-magnetic-field component in the 23Na NMR spectra of xNa2O-yCs2O-2SiO2 glass at around −25 ppm can be attributed to Na+ with a larger coordination number. On the other hand, the low-magnetic-field component in the 23Na NMR spectra at around 5 ppm can be attributed to Na+ with a smaller coordination number.
Upon replacing Na+ with Cs+, the 23Na MAS NMR intensity of Na+ with a larger coordination number (at around −25 ppm) decreased, while Na+ with a smaller coordination number (at around 5 ppm) became the main component. This behavior is similar to that of Na+ in Na-K silicate glass [Citation5]. Additionally, the FWHM of the 23Na NMR spectrum is a measure of the distribution of coordination numbers of Na+. When the Na+ in Na2O-2SiO2 glass (x = 1.0) was replaced with Cs+, the FWHM decreased from 37 ppm to 32.7 ppm. This observed reduction in the FWHM indicates that the coordination number of Na+ in Na-Cs silicate glasses tends to decrease when Na+ is replaced with Cs+.
4.3 133Cs MAS NMR of xNa2O-yCs2O-2SiO2 glasses
In the 133Cs NMR spectra of xNa2O-yCs2O-2SiO2 glass shown in , the high-magnetic-field component (at around 100 ppm) can be attributed to Cs+ with a larger coordination number, while the low-magnetic-field component (at around 250 ppm) can be attributed to Cs+ with a smaller coordination number. First, it was found that Cs+ also has a distribution of coordination numbers in xNa2O-yCs2O-2SiO2 glasses, like Na+. Second, when Cs+ is replaced with Na+, the MAS NMR signal for Cs+ with a smaller coordination number (the area around 250 ppm) decreases relatively, while the Cs+ with a larger coordination number (the area around 100 ppm) becomes the main component. Therefore, as the concentration of Na+ increases (decrease in Cs+ concentration), Cs+ with a larger coordination number becomes the main component. On the other hand, as the concentration of Na+ decreases (Cs+ concentration increases), Na+ with a smaller coordination number becomes the main component.
4.4 Changes in the coordination numbers of alkali ions in xNa2O-yCs2O-2SiO2 glasses
To compare the coordination numbers of Na+ and Cs+, the average coordination number of alkali cations in Na-Cs silicate glass and the chemical shift were calculated from their corresponding relationship in the crystal by using Eq. Equation(1)(1)
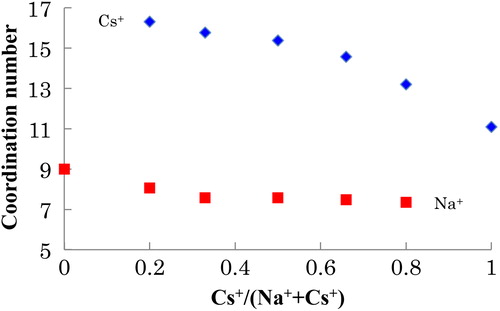
(1) . The results are shown in and . We note here that the coordination numbers were obtained by extrapolation, indicating that the values are only suitable for qualitative analysis. In Na-Cs silicate glasses, the average coordination numbers of both the alkali cations decreased (from 9 to 7 for Na+ and from 16 to 11 for Cs+) with increasing Cs+/(Na+ + Cs+). In this report, we cannot quantitatively discuss the larger change in the coordination number observed for Cs+, because such values were acquired by extrapolation and only provide qualitative information. A detailed discussion of this phenomenon will be presented elsewhere. The observed change in the average coordination number is attributed to the difference in basicity between Na2O and Cs2O. Since the basicity of Cs2O (1.18) is larger than that of Na2O (1.11), the basicity of the entire glass increases with increasing Cs+/(Na+ + Cs+). Consequently, the effective charge of O2− becomes larger [Citation22], and fewer O2− ions can compensate for the positive charge of the alkali cation. In brief, as the Na+ in the Na2O-2SiO2 glass (x = 1.0) was replaced with Cs+, the basicity of the entire glass increased, which was accompanied by a decrease in the coordination number of Na+ in the glass. As the Cs+ in the Cs2O-2SiO2 glass (x = 1.0) was replaced with Na+, the basicity of the entire glass decreased, and a corresponding increase in the coordination number of Cs+ was observed.
Table 2 Estimated coordination numbers of Na and Cs in xNa2O-yCs2O-2SiO2 glasses (x + y = 1) by using Eq. Equation(1)(1)
(1) . NNa and NCs are the coordination numbers of Na and Cs, respectively.
4.5 Structural reason of the mixed alkali effect
In Section 4.4, the effect of replacing one alkali with another was discussed from the viewpoint of the basicity of the alkali oxides. In this section, the coordination environment around each alkali ion is also discussed on the basis of ionic radii. This can be a more useful description, because the ionic radii provide a more straightforward explanation of the local environments around alkali ions.
According to , the coordination number of Na+ (around 7–9) is smaller than that of Cs+ (around 11–16) in xNa2O-yCs2O-2SiO2 glass. Since the ionic radius of Cs+ is larger than that of Na+, the bond distance between Cs+ and O2− is longer than that between Na+ and O2−. Such separation decreases the effective charge of O2−, and thus, a larger number of O2− ions can exist around Cs+ than around Na+. Accordingly, the observed difference in the average coordination number arises from the ionic radius.
The observed difference in the average coordination number contributes to the MAEs in Na-Cs silicate glasses. When Na+ at a site with a smaller coordination number is exchanged with Cs+ at a site with a larger coordination number, the exchange activation energy becomes larger. In addition, this exchange decreases the ionic conductivity, because the coordination environment around the alkali cation is different at the different sites. Therefore, the difference in the average coordination number of alkali cation can be considered a structural reason of the MAE.
5 Conclusion
In summary, we analyzed the local structure of the alkali ions 23Na and 133Cs in sodium cesium silicate glasses by using NMR. In the NMR spectra of the cesium silicate crystals, the Cs+ with the larger coordination number (Cs6Si10O23) showed a high-field shift, while that with the smaller coordination number (Cs2Si2O5) exhibited a low-field shift. In Na-Cs silicate glasses (xNa2O-yCs2O-2SiO2), it is found that (1) the coordination numbers of both alkali cations (Na+ and Cs+) become smaller as Cs+/(Na+ + Cs+) increases and (2) the average coordination number of Na+ is smaller (around 7–9) and that of Cs+ is larger (around 11–16) for xNa2O-yCs2O-2SiO2 glass. This difference in the average coordination number increases the exchange activation energy, which is considered to be a structural reason of the MAE.
Acknowledgments
This work was supported by the Collaborative Research Program of Institute for Chemical Research, Kyoto University (No. 2013-63, 2014-65) and Research Institute for Sustainable Humanosphere, Kyoto University.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- J.O.IsardJ. Non-Cryst. Solids11969235261
- P.MaassA.BundeM.D.IngramPhys. Rev. Lett.68199230643067
- R.TeraiJ. Non-Cryst. Solids61971121135
- D.E.DayJ. Non-Cryst. Solids211976343372
- R.DupreeD.HollandD.S.WilliamsJ. Non-Cryst. Solids811986185200
- B.GeeH.EckertBer Bunsen Phys. Chem.100199616101616
- J.F.StebbinsNature3301987465467
- J.F.StebbinsSolid State Ionics1121998137141
- A.AngelopoulouV.MontouilloutD.MassiotG.KordasJ. Non-Cryst. Solids3542008333340
- S.PrabakarK.T.MuellerJ. Non-Cryst. Solids34920048087
- H.C.BrookA.V.ChadwickJ.F.W.MosslemansG.N.GreavesRadiat. Eff. Defect Solids1501999403407
- A.BundeM.D.IngramP.MaassJ. Non-Cryst. Solids172199412221236
- T.UchinoT.SakkaY.OgataM.IwasakiJ. Phys. Chem.96199224552463
- T.UchinoT.YokoJ. Phys. Chem. B103199918541858
- H.MaekawaT.MaekawaK.KawamuraT.YokokawaJ. Phys. Chem.95199168226827
- Y.TokudaT.OkaM.TakahashiT.YokoJ. Ceram. Soc. Jpn.1192011909915
- J.SchneiderJ.TsuchidaH.EckertPhys. Chem. Chem. Phys.1520131432814339
- B.H.W.S.DejongP.G.G.SlaatsH.T.J.SuperN.VeldmanA.L.SpekJ. Non-Cryst. Solids1761994164171
- A.E.LapshinN.V.BorisovaV.M.UshakovY.F.ShepelevGlass Phys. Chem.332007250253
- W.KrausG.NolzeJ. Appl. Crystallogr.291996301303
- X.Y.XueJ.F.StebbinsPhys. Chem. Miner.201993297307
- J.A.DuffyJ. Phys. Chem. B10820041413714141