?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A new class of conventional and low-cement ferrochrome slag-based castables were prepared from 40 wt.% ferrochrome slag and 45 wt.% calcined bauxite. Rest fraction varied between high alumina cement (HAC) acting as hydraulic binder and calcined alumina as pore filling additive. Standard ASTM size briquettes were prepared for crushing and bending strengths evaluation, and the samples were then subjected to firing at 800, 1100 and 1300 °C for a soaking period of 3 h. The microstructure and refractory properties of the prepared castables have been investigated using X-ray diffraction (XRD), scanning electron microscopy (SEM), cold crushing strength, modulus of rupture and permanent linear changes (PLCs) test. Castables show good volume stability (linear change <0.7%) at 1300 °C. The outcomes of these investigations were efficacious and in accordance with previously reported data of similar compositions. High thermo-mechanical and physico-chemical properties were attained pointing out an outstanding potential to increase the refractory lining working life of non-recovery coke oven and reheating furnaces.
1 Introduction
The development of refractory castables is important due to their increasing applications in the cement, non-recovery coke ovens, chemical, metallurgical industries, ceramics, chemicals, oil/petrochemicals, etc. [Citation1,Citation2]. Unshaped monolithic refractories have been increasingly used instead of the shaped refractory bricks of the same class due to their easier replacement, lower cost, more efficient installation and lower material consumption, especially in steel-making applications such as the production of steel ladles and the linings of tundishes [Citation2,Citation3]. The addition of fine calcined alumina in castables improves the refractory properties, has a high melting point (2072 °C) and has good mechanical properties that are suitable for high-temperature applications [Citation4,Citation5]. High alumina cement (HAC), one of the most widely used as refractory binders in monolithic refractories, promotes initial hardening and mechanical strength before firing [Citation6–Citation10].
Every year, more than 300 million tons of industrial solid wastes are being produced by various industries in India and government is seeking ways to reduce the dual problems of disposal and health hazards from the accumulation of by-products. In recent years, the use of waste materials in the construction industry has become an important option, as it offers cost reductions, energy savings, and reduced CO2 emissions from the production of Portland cement, as well as reduced environmental impacts of construction materials. The predominant industrial byproducts that can be effectively used include chemical gypsum, fly ash, lime sludge and ferrochrome slag [Citation11–Citation14].
Out of the different waste materials being generated, the use of byproduct ferrochrome slag is significant in the production of monolithic refractories. Ferrochrome slag is a waste material obtained from the manufacturing of high carbon ferrochromium alloy, which is usually dumped. Global ferrochrome production is totaled around 8.9 million tons in the year 2011 [Citation15]. Utilization of dumped ferrochrome slag in refractory castables reduces the cost of the product and is friendly to the atmosphere. This material is being used for brick manufacturing, and recently has been tried in cement industry and base layer material of road pavements because of its excellent technical properties as well as chemical stability [Citation13–Citation15].
This slag is formed as a liquid at 1700 °C and its main components are SiO2, Al2O3 and MgO. Additionally it consists of chrome, ferrous/ferric oxides and CaO [Citation16,Citation17]. The smelted products obtained from the smelting furnaces are ferrochrome alloy and slag, and the slag production is 1.1–1.6 ton/ton ferrochrome alloy [Citation17]. Binary compound (magnesium-aluminate spinel) formed by interaction of MgO and Al2O3 present in the slag offers good mechanical, chemical and thermal properties both at ambient and elevated temperatures [Citation18–Citation20]. MA spinel is now extensively applied in high performance refractory castables [Citation21,Citation22].
Evaluated effects of fine calcined alumina addition in castables reduce the permanent linear change. Mullite (3Al2O3–2SiO2), which is another binary component of this slag-based system, is formed by reactions of silica glass melt and alumina present in bauxite. It is a thermodynamically most stable product in the high-temperature solid-state reaction between silica and bauxite [Citation23,Citation24]. In situ formed, elongated needle-like mullite crystals grow with increasing temperature and lock the structure to create a strong refractory bond system improving the mechanical properties of the castable [Citation25,Citation26].
The aim of this work is to prepare conventional castable and low cement castables by utilizing this waste ferrochrome slag, calcined bauxite and fine calcined alumina. Use of secondary resources makes it possible to solve problems of materials availability; it reduces cost for their extraction, processing and their industrial discharge in the atmosphere thereby providing economic and financial benefits to the country as well.
2 Materials and experimental procedure
2.1 Materials
Calcined bauxite (Shiva Minerals Pvt. Ltd., Rourkela) along with ferrochrome slag (byproduct of TATA Ferroalloy, India) was used as aggregate. Details of particle grading and chemical composition are included in . High alumina cement CA-270 (Almatis Kolkata, India) is introduced as a hydraulic binder. Fine calcined alumina (Shiva Minerals Pvt. Ltd., Rourkela, India) was used as superfine additive. The percentage of aggregates was kept constant at 85% throughout the study. The fine calcined alumina content with respect to high alumina cement (HAC) varied from 0 to 15 wt.%.
Table 1 Particle size and chemical composition of the raw materials.
2.2 Preparation of castables
Conventional and low cement refractory castables are generally prepared using approximately 15–10 and 3–5 wt.% HAC, respectively. The ferrochrome slag and calcined bauxite were used in castable formulation in the present study with small additions of fine calcined alumina. The formulation () shows the detailed composition with their names. In the first step for castable formulation, ferrochrome slag was oven dried, crushed and ground for grading into three groups of coarse (6–2 mm), medium (1–0.5 mm) and fine (<0.5 mm). The jar and grinding media were of titanium-coated stainless steel material. At one time 250 g of ferrochrome slag material was taken in the jar and ground in a high-energy ball mill for 30 min at 400 rpm. Similarly, it was processed to complete the grinding of complete material. The ground material was then kept in various selected sieves and set up on the motorized vibro-sieving equipment for grading. The same technique was used for grinding and grading calcined bauxite material. The particle size distribution has an important role in the properties of refractory castable. Incorrect particle size distribution may cause militancy or the excess water required by the castables. The particle size distribution of the fine fraction is generally a representation of the flow characteristics. The trials of aggregate proportions were taken in a 1000 cm3 flask filled up to 250 cm3 and vibrated for 30 s and the packing density calculations were carried out for each trial. Aggregate having highest packing density was chosen for further analysis. The materials were dry mixed in a plastic container for 10 min with a spatula and then were taken for sample preparation. Generally, conventional and LCCs require less than 12 and 5 wt.% of water respectively to achieve the desired rheology; therefore, water was added in two steps. The casting was done by adding the first two-thirds proportion of water at a time. Then, one-third of water was added slowly to get a homogeneous mixing. The wet mixing was performed for up to 5–6 min to achieve proper flow. Immediately after wet mixing, the castable mix was filled into a cubic mold (50 mm) made of hard steel. The mold was placed on the vibrating table filled with the wet mixed castable and the mixes were vibrated for 10 min, showing better compactness. For each composition, several samples were prepared for laboratory test. The samples were cured in a moisture-saturated environment (95% RH) in a humidity chamber at room temperature for different time periods. For firing the samples, they were first oven dried at 110 °C for 24 h. shows the pictorial representation of the ferrochrome slag castables. The test samples were fired at 1100 and 1300 °C with dwell time of 3 h, using an electric furnace at a heating rate of 5 °C/min. The furnace was equipped with SiC heating element and a programmer PID528, manufactured by Selectron Process Controls Pvt. Ltd, India. The programmer has the temperature control accuracy of ±1 °C.
Table 2 Batch composition of ferrochrome slag-based castables.
2.3 Characterizations of prepared castables
Preparation of 50 mm cubic and 25 mm × 25 mm × 150 mm castable briquettes had been the same as published in our previous contributions [Citation27,Citation28]. They were cured in a humid atmosphere (RH > 95%) at 27 °C, dried at 110 °C and then heat-treated at 800, 1100 and 1300 °C. The apparent porosity (AP), bulk density (BD), cold crushing strength (CCS) and cold modulus of rupture (CMOR) of all the castables were determined according to the ASTM C20-00 [Citation29] and ASTM C133-97 [Citation30]. Furthermore, permanent linear change (PLC) of heat-treated castables was determined according to the ASTM C113-02 [Citation31]. Thermal shock resistance (TSR) of castables was determined by ASTM C1171-05 standard. The strength loss is measured by the difference in modulus of rupture (MOR) between uncycled specimens and the specimens subjected to thermal cycling [Citation32]. Consequently, samples were analyzed by XRD for investigation of phases present in the matrix of the samples after sintering at different temperatures. X-ray diffraction patterns were observed using a portable XRD machine (Rigaku, Tokyo, Japan) using Ni filtered Cu Kα radiation operating at 30 mA and 40 kV. Sintered castables were polished and etched thermally at 1200 °C. Micrographs were recorded with the help of a scanning electron microscope (SEM) (INSPECT 50 FEI).
3 Results
3.1 Phase analysis of the castables at different firing temperatures
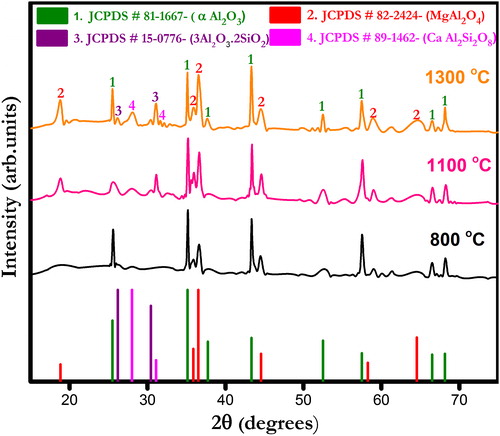
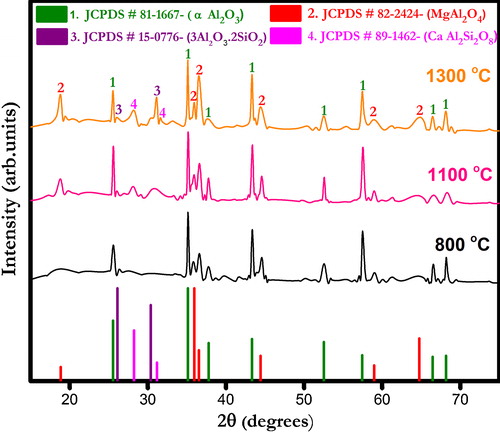
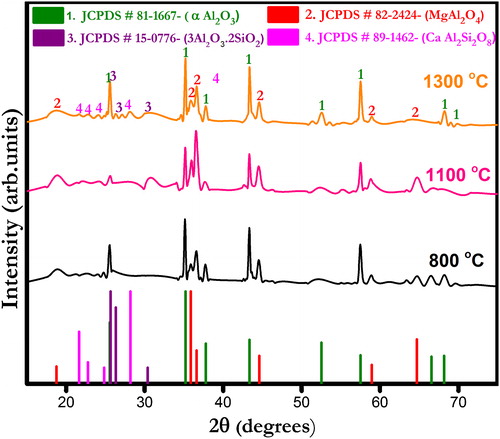
show X-ray diffraction (XRD) patterns of ferrochrome slag- and bauxite-based castables fired at different temperatures (800, 1100 and 1300 °C). The corundum (α-Al2O3) and spinel (MgAl2O4) phases appear as major crystalline peaks in all the castables. Other phases include anorthite (CaAl2SiO8) and mullite (3Al2O3·2SiO2) which are present in small quantities. Formation of anorthite phase seemingly increases with increasing temperature in all the castables. At low firing temperatures viz. 800 and 1100 °C only parental phases are detected which indicates no ceramic reaction bonding occurrence. At 1300 °C, the appearance of anorthite phase can be described by the reactions taking place between alumina, silica and cement [Citation30]. Furthermore, a certain amount of silica diffused into the solution together with fluxing oxides helped the formation of other metastable silica bearing compounds that were similar to anorthite [Citation31]. Mullite phase is also found in all castables, and its formation is promoted due to the presence of silica impurities in bauxite, which reacts with fine alumina added. The presence of different impurities in bauxite also helps the densification process by liquid phase sintering reactions [Citation33].
Fig. 2 X-ray diffraction pattern of ferrochrome slag-based CA 0 castables fired at different temperatures.
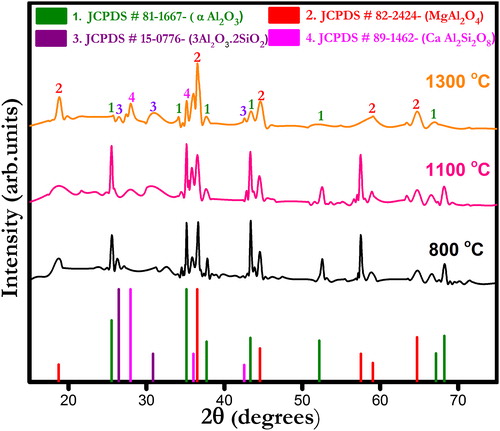
Fig. 3 X-ray diffraction pattern of ferrochrome slag-based CA 5 castables fired at different temperatures.
3.2 Microstructural analysis of prepared castables
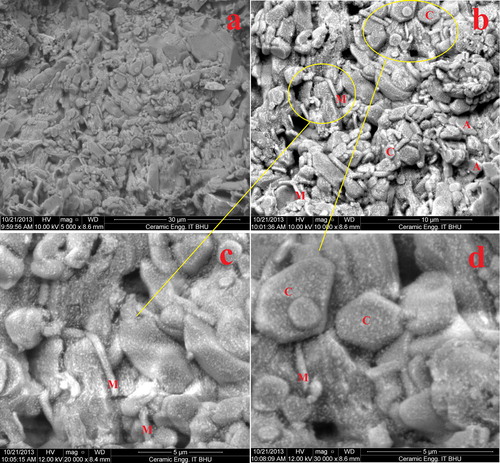
The microstructural evolution of castables CA 10 and CA 12 fired at 1300 °C is recorded using the scanning electron microscope of cut specimens. shows the micrograph of CA 10 castable and portrays microplots of CA 12. Here a, b, c and d represent different magnification and scan area of same sample. Microstructure of the castable shows homogeneously embedded corundum grains in the matrix which are free from micro-cracks. This is due to transformation of bauxite minerals to corundum. Some lenticular anorthite and needle-shaped mullite crystals are also distributed from place to place which have been marked by A and M respectively. The presence of acicular mullite creates an interlocking structure in the castables. This matrix, according to previously established facts, increases the density, thermal shock and CCS of the castables [Citation34Citation[35]–Citation36]. The matrix also has spinel and some liquid phase uniformly present which is in conformity with the XRD patterns of the ferrochrome slag used.
3.3 Bulk density (BD) and apparent porosity (AP)
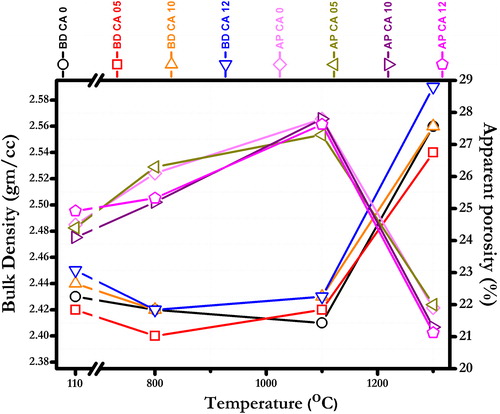
The BD and AP of the present castables measured at different temperatures (110, 800, 1100 and 1300 °C) are shown in . At 110 °C distribution of fine calcined alumina powder acting as pore filling agent improves the packing density of the castables. As can be seen, the BD decreases slightly after firing, from 2.45 gm/cm3 at 110 °C to 2.42 g/cm3 at 1100 °C. This is accounted for disappearance of water of crystallization and abrupt ending of cement hydraulic reactions in the temperature range (800–1100 °C) which creates porosity in castable body structure, while no ceramic bond formation occurs. At 1300 °C anorthite liquid phase is formed resulting from the reaction between alumina, slag and cement which fill the interspaces among the constituents and helps densification. Formation of high-density mullite phase (2.56 g/cm3) also decreases the AP (from 21.1 to 21.3%) in castables CA 12 and CA 10. This leads to a highly dense structure as compared to castables sintered at low temperatures.
3.4 Cold crushing strength (CCS) and cold modulus of rupture (CMOR)
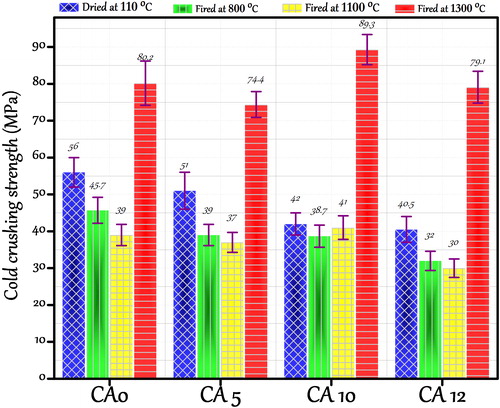
and show the CCS and CMOR of castables dried at 110 °C for 24 h and fired at 800, 1100 and 1300 °C for 3 h. Castable CA 0 containing 15 wt.% cement dried at 110 °C for 24 h shows the highest CCS and CMOR which is ascribed to higher amount of cement hydrated phases CAH10 and AH3. These phases play the essential role of hydraulic bonding. Decreasing the cement content up to 10 wt.% slightly affects the crushing strength in castable CA 05 (from 56 to 51 MPa). Decrease of the cement content down to 5 wt.% and 3 wt.% (castables CA 10 and CA 12) causes reduced strength (from 42 to 40.5 MPa). This is due to low amount of cement available for good bonding.
Fig. 10 CMOR of different ferrochrome slag-based castable samples fired at different firing temperatures.
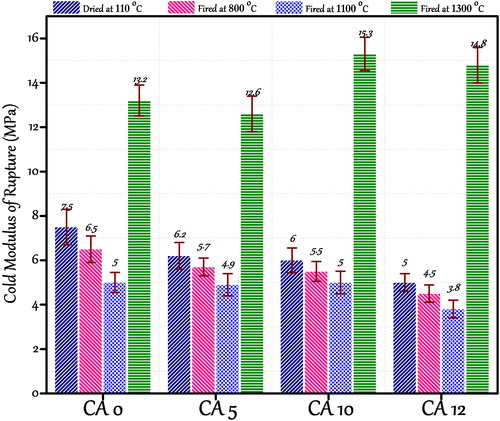
At 800 and 1100 °C strength degradation is observed in prepared castables. Castables have low CCS and CMOR as no ceramic bond formation could take place. It is perceptible that the general trend of strength degradation among all the castables prepared in the temperature range (800–1100 °C) is same. This is also a well-established fact that high alumina cement bonded castables degrade within this temperature range due to complete dehydration of cement. Beyond 1200 °C the properties have been enhanced, possibly due to the progressive densification in castable with ceramic bond formation and liquid phase sintering. Castables CA 10 and CA 12 show the high CCS and CMOR value when compared with other three castables, due to the presence of a relatively higher amount of mullite and anorthite phase formed. Mullite forms a network of needle-like structure, which strengthens the structure at high temperatures [Citation37]. Present crushing and bending strength also draws an analogy to apparent porosity values which shows the maximum attained strength when a substantial decrease in porosity at 1300 °C is observed.
3.5 Thermal shock resistance (TSR) and permanent linear change (PLC)
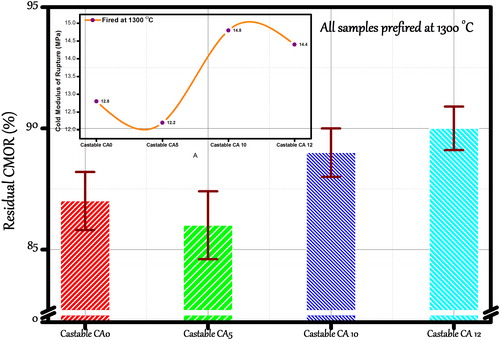
Thermal shock resistance properties enlighten pre-firing conditions since strength loss behavior can be highly dependent on the firing temperature. Thermal shock resistance is evaluated on castables heated to a determined temperature (T) followed by subsequent quenching in water (Twater). After 20 min of water cooling, the residual cold modulus of rupture (CMORr) is measured and compared to the original strength (CCS0). A percentage residual strength loss is calculated as:(1)
(1) shows the residual strength of the fired castables (1300 °C). Thermal shock resistance of materials not only depend on intrinsic factors, such as structure of materials, grain size, shape and distribution of internal defect, but also depended on the macroscopic physical indexes, such as strength, modulus of elasticity, thermal conductivity, thermal expansion coefficient and Poisson's ratio of materials. Good thermal shock resistance is obtained for the castables CA 10 and CA 12 fired at 1300 °C. Strength loss for CA 10 and CA 12 castables is 11 and 10%, respectively. This is dedicated to alumina content and good distribution of the mullite phase formed.
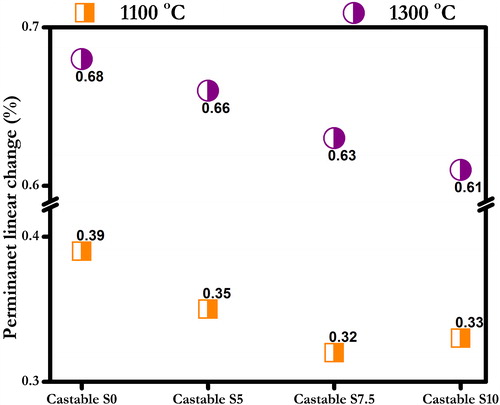
Permanent linear change (PLC) reports the determination of the linear dimensional change of refractories when heated under prescribed conditions. PLC was calculated as follows:(2)
(2) shows that prepared castables had very limited dimensional change when fired at 800 °C, 1100 °C and 1300 °C. All the fired castables have less than 0.7% shrinkage.
4 Discussion
The nature of crystallization of phases in all compositions was strongly dependent on sintering temperature which was reflected in the obtained data of X-ray diffraction patterns. In all samples, layered alumino-silicate anorthite (CaAl2Si2O8) phase is observed at high temperatures. When Al substitutes for Si in the network, the appropriate ratio for determining the structure will be O/(A1 + Si) ratio. In case of anorthite the ratio O/(A1 + Si) is 2; and a three-dimensional structure is observed. As a result of this three-dimensionality, its melting point is high (1550 °C). This result is in good agreement with high mechanical strengths of formulated castables. The observed porosity of these materials may be explained by sintering theory. According to this theory, densification and subsequent shrinkage of a compacted powder are achieved by the transport of material from the contact area between grains through a thin liquid film to the off-contact neck region in final state sintering [Citation38]. This process results in a continuous reduction in pore size and a continuous change in grain shape that should become increasingly anhedral until the pore is sharply reduced. High-temperature tensile ductility in fine-grained pure Al2O3 is an extensively researched phenomena which is enriched by small amounts of secondary phases. For enhancement of this phenomenon, suppression of alumina grain growth and co-dispersion of spinel, magnesia or zirconia particle is required [Citation39]. The pre-formed spinel, as observed through XRD patterns (–;), here acts as dispersive agent in fine calcined alumina. Due to this superplastic behavior, rise in the bending strengths (CMOR 15.3 MPa) is observed when alumina is added incrementally. Abnormal grain growth is suppressed as is seen by microstructural plots even though densification is not hampered (BD 2.59 g/cm3). The potential advantages of acicular growth of mullite include more effective crack deflection and superior load-bearing capacity, which is quite evident from the mechanical strength plots ( and ). Other possible toughening mechanisms may include crack bridging and pullout. The strengthening of castables is attributed to the load transfer to the mullite needles and lenticular anorthite growth as seen in (b). The mechanical strength draws a close analogy to porosity and bulk density plots, where suppressed porosity causes a substantial improvement in crushing and bending values. The maximum in situ mullite formation enables the castable CA 12 to achieve highest residual bending strength amongst all other formulations. Mullite has very low thermal expansion coefficient (4.5 × 10−6 °C−1). Thermal expansion of solid materials is stimulated by thermal vibration of atom. Equilibrium spacing in crystal is determined by potential energy of atom space, and vibration of atom is intensified with the increasing temperatures. Thermal expansion appears on macroscopic scale because of extending atomic distance. More energy is needed when lattice vibrates because of strong bond strength. This formulation is close to alumina–silica–calcia eutectic system where anorthite melts incongruently leaving some residual silica melt, hibonite and a solid solution of mullite. This residual silica melt makes enough room to accommodate thermal strains leaving the whole structure intact. Quenching does not allow time to nucleate sufficient anorthite in the melt and therefore, added fine calcined alumina reacted with silica derived melt crystallizing into mullite. This is the reason that addition of fine calcined alumina has positive role on improved resistance against explosion or crack generation by thermal shock. This composition shows high mechanical strength in wide temperature range and is suitable for lining to which excellent resistance against abrasion and spalling is required. Improvement in mechanical strengths of this brittle ceramic is very important if these materials are to be used for load-bearing applications such as coke oven batteries. Present data and assertion are also supported by a successful implementation of a working lining in a coke oven door at VISA Steel Ltd., Jajpur K Road, India.
5 Conclusions
Conventional and low cement castables were prepared from ferrochrome slag, calcined bauxite, high alumina cement and calcined alumina. They exhibit good physico-mechanical and refractory properties. The high CCS and CMOR value of CA 10 and CA 12 castables is due to higher amount of mullite phase formed. Prepared castables exhibited characteristic low PLC (shrinkage) and good volume stability. These excellent properties of ferrochrome slag-based castables enable their use in various refractory applications such as non-recovery coke ovens, boilers and reheating furnaces. Ferrochromium slag may be used as an aggregate in castables, proposing a more economical benefit than the present industry standards. After industrial implementation it is found that using approximately 50 wt.% ferrochrome slag as an aggregate in castables saves around the 45% refractory cost per ton.
Acknowledgement
The authors gratefully acknowledge the financial support of DST [(TDT Division), Reference No. DST P 07/535/SSTP/UP/197(G) 2012], Ministry of Science & Technology, New Delhi, India.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- Z.NingshengProceedings of the First International Conference on Refractories2004148154
- J.BerjonneauP.PrigentJ.PoirierCeram. Int.352009623635
- K.MurakamiT.YamatoY.UshijimaK.AsanoK.H.CorpRefract. Appl. News8520031213
- S.MartinovicM.VlahovicInt. J. Appl. Ceram. Technol.8201111151124
- M.A.SerryR.TelleJ. Am. Ceram. Soc. Bull.7920007175
- F.A.CardosoM.D.M.InnocentiniM.M.AkiyoshiV.C.PandolfelliJ. Eur. Ceram. Soc.24200420732078
- F.A.CardosoD.M.M.InnocentiniM.F.S.MirandaA.O.F.ValenzuelaV.C.PandolfelliJ. Eur. Ceram. Soc.242004797802
- R.SalomaoV.C.PandolfelliCeram. Int.35200931173124
- V.KumarV.K.SinghA.SrivastavaJ. Am. Ceram. Soc.96201321242131
- V.KumarV.K.SinghA.SrivastavaG.N.AgrawalJ. Am. Ceram. Soc.95201237693775
- Ideas 1st Research is registered trademark of ideas 1st information service limited (www.ifisresearch.com).
- B.B.LindA.M.FaellmanL.B.LarssonWaste Manage.212001255264
- D.S.RaoS.I.AngadiS.D.MuduliB.D.NayakAt. Miner. Process. English Edition5120105
- A.YılmazM.KarasahinMater. Struct.432010309317
- J.ZelicCem. Concr. Res.35200523402349
- A.YılmazandM.K.AhinJ. ASTM Int.92011112
- P.C.HayesTransformation through TechnologyProceedings of Tenth International Ferroalloys CongressCape Town, South Africa20040-9584663-5-1
- L.HolappaY.XiaoJ. S. Afr. Inst. Min. Metall.August2004429438
- M.ErdemH.S.AltundoganM.D.TuranF.TumenJ. Hazard. Mater. B1262005176182
- G.M.BiggarO.M.J.HaraJ. Am. Ceram. Soc.531970534538
- P.GehreC.G.AnezirisD.VeresC.ParrH.FrydaM.NeurothJ. Eur. Ceram. Soc.33201310771086
- L.A.DiazR.TorrecillasA.H.De AzaP.PenaS.De AzaJ. Eur. Ceram. Soc.25200514991506
- F.C.ZhangH.H.LuoS.G.RobertsJ. Mater. Sci.42200767986802
- M.HamidoucheN.BouaouadjaH.OsmaniR.TorrecilliasG.FantozzJ. Eur. Ceram. Soc.161996441445
- M.HamidoucheN.BouaouadjaC.OlagnonG.FantozziCeram. Int.292003599609
- X.ChengS.KeQ.WangH.WangA.ShuiP.LiuCeram. Int.38201232273235
- P.H.KumarA.SrivastavaV.KumarM.R.MajhiV.K.SinghJ. Asian Ceram. Soc.22014169175
- A.SrivastavaV.K.SinghV.KumarP.H.KumarH.TripathiA.ChaudharyK.AsiwalR.PandeyS.K.SumanJ. Alloys Compd.5862014581587
- ASTM, Standard test methods for B.D. and A.P., ASTM C20-00 (2005).
- ASTM, Standard test methods for cold crushing strength and modulus of rupture of refractories, ASTM C133-97 (2008).
- ASTM, Standard test methods for permanent leaner change, ASTM C113-02 (2008).
- ASTM, Standard test methods for quantitatively measuring the effect of thermal shock and thermal cycling on refractories, ASTM C1171-05 (2011).
- T.N.SenS.K.M.H.HassanP.PalCeramics Silikaty49200597103
- A.M.HundereB.MyhrePresented at UNITECR 97Nov. 4–8, New Orleans, USA199730
- S.KumarS.K.DasP.K.DaspoddarCeram. Int.292003139144
- M.Nouri KhezrabadiF.ArianpourR.NaghizadehA.NassiriP.AssadollahpourS.Kh.MirhosseiniRefract. Ind. Ceram.5320123338
- S.MukhopadhyayS.GhoshM.MahapatraR.MazumderP.BarickS.GuptaS.ChakrabortyCeram. Int.282002719729
- B.N.KimK.HiragaK.MoritaY.SakkaActa Mater.492001887895
- A.SrivastavaV.K.SinghV.KumarP.H.KumarCeram. Int.409 Part A20141406114072