?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this work is to valorize volcanic scoria by using them as starting material for geopolymers production. Nevertheless, volcanic scoria possesses low reactivity. Various amounts of metakaolin (5%, 10%, 15%, 20% and 25%) were added into two volcanic scoria (ZD and ZG) in order to improve their reactivity. Two alkaline solutions were used to activate the aluminosilicate materials. The starting materials were characterized by particle size distribution, specific surface area, chemical and mineralogical composition. The geopolymers were characterized by the setting time, XRD, FTIR, SEM and compressive strength. The results indicated that volcanic scoria have low specific surface area (2.3 m2/g for ZD, 15.7 m2/g for ZG), high average particle size (d50 = 13.08 μm and 10.68 μm for ZD and for ZG respectively) and low glass phase contents. Metakaolin have a smaller average particle size (d50 = 9.95 μm) and high specific surface (20.5 m2/g). The compressive strength of geopolymers increased in the ranges of 23–68 MPa and 39–64 MPa for geopolymers from ZD–MK and ZG–MK respectively. This study shows that despite the low reactivity of volcanic scoria it can still be used to synthesize geopolymers with good physical and mechanical properties.
1 Introduction
Geopolymers are a class of largely X-ray amorphous aluminosilicate materials, generally synthesized by activation of an aluminosilicate powder with a concentrated alkali metal silicate or hydroxide solution. Geopolymers have been the subject of a great deal of research interest, particularly during the last decade owing to their good properties and as they are low emission CO2 materials. It may be synthesized at an ambient or elevated temperature by alkaline activation of aluminosilicate obtained from industrial wastes [Citation1–Citation5], metakaolin [Citation6–Citation9], melt-quenched aluminosilicate [Citation10], natural minerals such as volcanic scoria [Citation11–Citation15], or mixtures of two or more of these materials [Citation16,Citation17]. Activation is achieved by addition of highly concentrated alkali metal hydroxide or silicate solutions. Tchakoute et al. [Citation17] reported that volcanic scoria contain low amount of alumina oxide (Al2O3), which can be compensated by addition of amorphous alumina. However, amorphous alumina is very expensive and the compressive strength of geopolymers synthesis at ambient temperature from a mixture of volcanic scoria and an excess (40% by weight) of amorphous alumina is less than 48 MPa, thus compensating partially the deficiency. This deficiency can be efficiently compensated by adding high reactive amorphous minerals such as metakaolin and fly ash because these amorphous aluminosilicate materials contain a good source of amorphous Al2O3 and SiO2.
A previous study [Citation18] has shown that geopolymer cements obtained only from volcanic scoria at ambient temperature exhibit long setting time, low compressive strength, and some volcanic scoria-based geopolymers swelled and presented cracks after demolding. Also, geopolymers synthesized from volcanic scoria collected in Djoungo could handle easily only after 14 days at ambient temperature; this is probably due to their low reactivity assessed by a low specific surface area (2.3 m2/g) and low glass phase content (34.8%) [Citation18].
The present work aims at altering the reactivity of volcanic scoria by adding 5%, 10%, 15%, 20% and 25% by weight of metakaolin (MK) in the volcanic scoria in order to compensate the deficiency of Al2O3 and to increase the amount of amorphous phase in the volcanic scoria, thus reducing the amount of metakaolin to be used for the synthesis of geopolymers and therefore reducing the cost of geopolymer cements. The effect of the addition of metakaolin on the geopolymerization reaction, structure and mechanical properties of the volcanic scoria-based geopolymer has been studied using setting time, X-ray diffraction, Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM) and compressive strength.
2 Materials and experimental methods
2.1 Materials
The volcanic scoria (ZD and ZG) and kaolin (MY3) used in this study were collected from Djoungo (Littoral Region-Cameroon), Galim and Mayouom (West Region-Cameroon). The starting materials, kaolin and volcanic scoria were previously studied by Njoya [Citation19] and Tchakoute et al. [Citation18] for use in ceramics production and geopolymers synthesis respectively. They reported that kaolin consists of approximately 79% kaolinite and 8% quartz. ZD and ZG content 34.8% and 64.8% of glass phase respectively. Taking into account the differences in density between quartz and kaolinite, MY3 was enriched with kaolinite by wet sieving at 100 μm. The volcanic scoria and the dried clay fraction () were crushed in a ball mill and mortar respectively and then sieved to 80 μm. The clay fraction was calcined in a programmable electric furnace (Nabertherm, Mod_LH 60/14) for 4 h at a heating rate of 5 °C/min at 700 °C to get high reactive metakaolin (MK). The temperature of 700 °C is chosen according to the conclusion of a previous work [Citation9].
The alkaline solution was a mixture of an aqueous solution of sodium hydroxide (12 M) and sodium silicate with bulk density of 1400 kg/m3. The sodium hydroxide solution was obtained by dissolving sodium hydroxide pellets of 99% purity in distilled water. The sodium silicate solution was made up of 28.7% SiO2, 8.9% Na2O and 62.4% H2O by weight. For the synthesis geopolymer pastes, alkaline activator solutions with two different SiO2/Na2O molar ratios (Ms = 1.1 and 1.4) were used. The alkaline activating solutions were stored at ambient temperature for at least 24 h before use to allow full silica dissolution and equilibration.
2.2 Experimental methods
The geopolymer cement pastes were obtained by first mixing volcanic scoria (ZD or ZG) with the designated percentage of metakaolin in Hobart mixer (M & O model N50-G) for 10 min. The alkaline solution was then slowly added to the mixture and mixed for another 10 min. In order to obtain a good workability, the liquid/solid mass ratios of 0.52 (ZG–MK) and 0.48 (ZD–MK) were used. One part of fresh geopolymer paste was used to determine the setting time and another were rapidly molded into cylindrical PVC molds (diameter 31 mm, height 62 mm and diameter 10 mm, height 20 mm). Once molded, the samples were vibrated for 5 min on an electrical vibrating table (M & O, type 202, No. 106) to remove entrapped air bubbles. During hardening of the geopolymer cement paste samples, the cylinders were covered with a thin film of polyethylene to avoid water evaporation and then kept for 24 h at ambient atmosphere (24 ± 3 °C) before demolding. The geopolymer cement pastes obtained with 5%, 10%, 15%, 20% and 25% of metakaolin are labeled ZD5; ZD10; ZD15; ZD20; ZD25 and ZG5; ZG10; ZG15; ZG20; ZG25 for geopolymers made from a mixture of ZD–MK and of ZG–MK respectively. The hardened geopolymer cylinder pastes stayed 28 days at ambient atmosphere in the laboratory and compressive strength measurements were carried out. Some fragments of hardened geopolymer cement pastes obtained after compressive strength tests were grounded and submitted to X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy. The geopolymer pastes which were molded in cylindrical PVC molds of 10 mm diameter and 20 mm height were used for scanning electron microscopy (SEM) analysis.
2.3 Methods of analysis of starting materials and geopolymer pastes
Particle size distribution of the grounded volcanic scoria (ZD and ZG) and metakaolin (MK) were measured using a laser diffraction granulometer by Sympatec equipped with the HELOS optical system and the WINDOX software for data acquisition.
The specific surface area of volcanic scoria (ZD and ZG) and metakaolin (MK) was determined by nitrogen adsorption using an automatic homemade apparatus Laboratoire Environnement et Mineralurgie (LEM, UMR 7569, Nancy-France).
Chemical analysis of volcanic scoria and metakaolin was carried out by ICP-AES (inductive coupled plasma-atomic emission spectrometry).
The crystalline phases of volcanic scoria (ZD and ZG), kaolin (MY3), metakaolin (MK) and selected geopolymers were determined by X-ray diffraction using a Philip PW 3050/60 diffractometer, operating by reflection of Kα1 radiation of Cooper. The crystalline phases were identified by using the PDF standard of ICDD (International Centre for Diffraction Data).
Fourier transform infrared (FTIR) spectroscopy was carried out on powders of raw materials (ZD, ZG and MK) and of the selected hardened geopolymer cement pastes using a Bruker alpha-p operating in absorbance mode from 4000 to 400 cm−1, a diamond ATR was used.
The setting time was measured from a fresh geopolymer pastes using the Vicat apparatus according to the EN 196-3 standard.
Compressive strength was determined for all hardened geopolymer cylinder paste samples aged 28 days using an electro-hydraulic press with capacity of 60 KN (M & O, type 11.50, No. 21). The specimens were subjected to a compressive force at average rate of 3 mm/min until the specimen failed (refer to ASTM C 109 standard test methods). Before compressive strength test, each specimen was polished with sand paper to obtain flat and parallel surfaces.
SEM was carried out on a Hitachi S-4800 scanning electron microscopy. Samples from hardened geopolymer pastes having 10 mm diameter and 20 mm height aged at least 28 days were sectioned and carbon coated prior to analysis.
3 Results and discussion
3.1 Characteristics of volcanic scoria and metakaolin
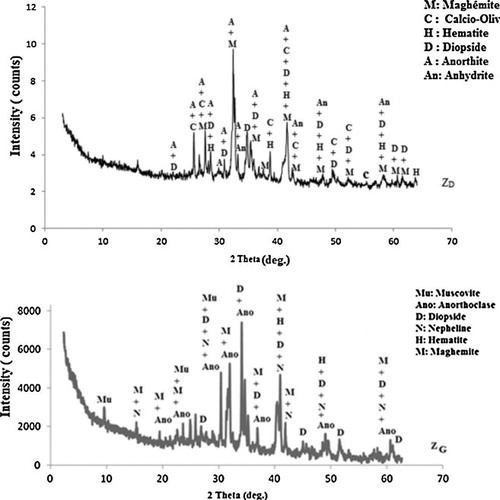
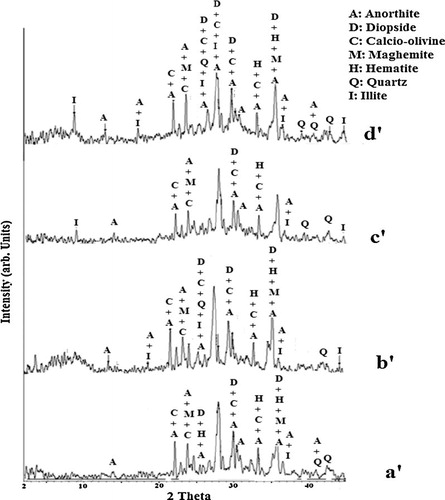
The chemical composition and the physical properties of the volcanic scoria (ZD and ZG) and metakaolin (MK) are summarized in . The SiO2/Al2O3 molar ratio is 4.90 for ZD, 4.55 for ZG and 1.42 for MK. The sum silica plus alumina is 59.4 wt% for ZD, 56.8 wt% for ZG and 88.79 wt%wt for MK. These are the basic ingredients of a geopolymeric reaction and are within the range of values reported by Davidovits [Citation20] and Palomo et al. [Citation3]. In addition to these oxides, the volcanic scoria also contains other oxides, such as iron oxide, calcium oxide, magnesium oxide and sodium oxide. The respective loss on ignition is 1.1 wt% (ZD), 9.3 wt% (ZG) and 2.43 wt% (MK) which may likely indicate the presence of higher organic matter or water and amorphous phase or higher mineral phases in ZG. XRD patterns of ZD, ZG, and MK show that in addition to maghemite, hematite and diopside that are common minerals to these volcanic scoria, ZD also contains calcio-olivine, anorthite and anhydrite and ZG contains anorthoclase, muscovite and nepheline (). XRD patterns of
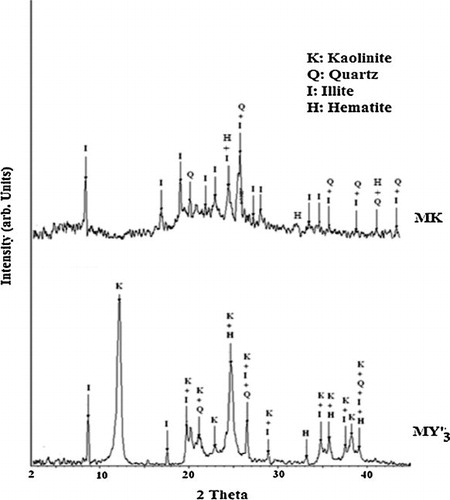
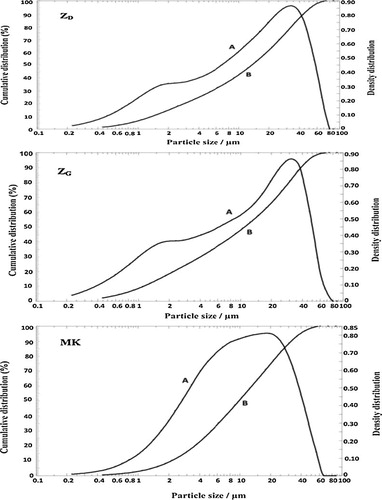
indicate the presence of kaolinite, quartz, hematite and illite while MK content quartz, hematite and illite (). Despite the crystalline phases, volcanic scoria and metakaolin show slightly diffused halo peak between 20° and 45° (2θ) for ZD and ZG () and a broad hump between 3–12° and 15–30° (2θ) for MK (). This hump indicates the presence of amorphous phase in the raw materials. The intensity of the diffused halo peak in MK is greater than that of volcanic scoria; hence MK has a large amount of amorphous phase. The particle size distribution of ZD and ZG samples ranges from 0.23 to 80 μm, the mode is around 35 μm for ZD and 32 μm for ZG and the average particle size (d50) is 13.01 and 10.68 μm for ZD and ZG respectively (). Whereas the particle size distribution of MK ranges from 0.23 to 66 μm, the mode is around 16 μm and the average particle size (d50) is 9.95 μm (). The density distribution of volcanic scoria (ZD and ZG) presents a bimodal distribution due to heterogeneity in their composition while the density distribution of metakaolin (MK) presents a unimodal distribution due to homogeneity in its composition (, curve A). The specific surface area is 2.3 m2/g for ZD, 15.7 m2/g for ZG and 20.5 m2/g for MK. These results indicate a good correlation between the particle size distribution and the specific surface area, viz. the finer the particles, the greater the specific surface area. This greater specific surface area exhibited by MK by comparison with ZG and ZD may result from the presence of more porous microstructure of MK particles. Particle size distribution is one of the most important physical parameters impacting on geopolymer synthesis of aluminosilicate as well as on the resulting products since a significant part of the reaction occurs at the particle–liquid interface [Citation21]. Thus for a given aluminosilicate raw material, the smaller and the more porous microstructure are the particles, the greater the specific surface area and the more activated is material in alkaline medium.
Table 1 Chemical composition and physical properties of volcanic scoriae (ZD, ZG) and metakaolin (MK).
3.2 Characteristics of geopolymers
3.2.1 X-ray diffraction
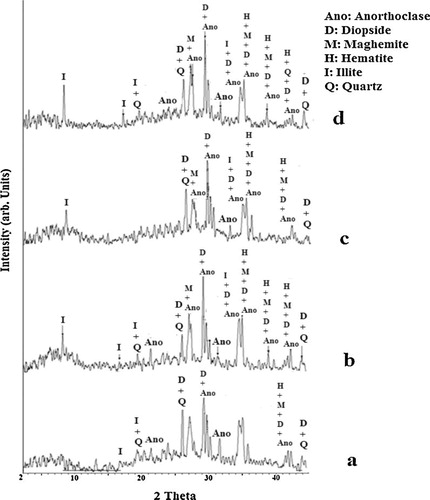
The XRD patterns of blend metakaolin and volcanic scoria-based geopolymers are presented in and . The major crystalline phases originally existing in ZD, ZG and MK are present in the synthesized geopolymers (except nepheline and muscovite which reacted during geopolymerization reaction) indicating the presence of undissolved metakaolin and volcanic scoria particles in the final geopolymer materials ( and ). These crystalline phases mentioned above are nearly inert to the geopolymerization reactions. The diffused halo peak registered between 20–45°, 3–12° and 15–30° (2θ) in the XRD diagram of volcanic scoria (ZD, ZG) and metakaolin (MK) respectively ( and ) has slightly shifted toward higher (2θ) values (5–13° and 18–38° (2θ)) in the XRD diagrams of the geopolymers. This is an indication of the partial dissolution of the glass phase of volcanic scoria (ZD, ZG) and amorphous phase of metakaolin (MK), and the formation of a new amorphous or glass phase in the matrix of geopolymer pastes [Citation22,Citation23]. The diffused halo peak at 3–12° (2θ) is only observed in the XRD patterns of ZG–MK based-geopolymers and ZD–MK based-geopolymers obtained with alkaline solution of which Ms = 1.4. This observation indicated that in the highly alkaline solution (SiO2/Na2O molar ratios increase in alkaline solution) geopolymer materials obtained have larger amount of geopolymer phases and subsequently a higher degree of geopolymerization.
3.2.2 FTIR spectra
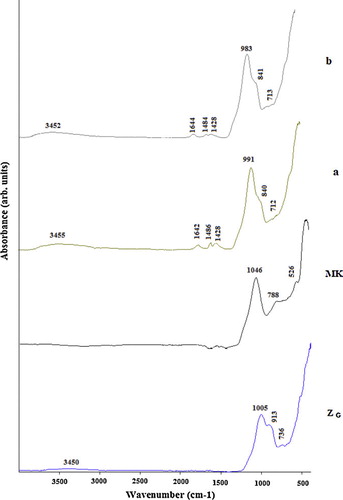
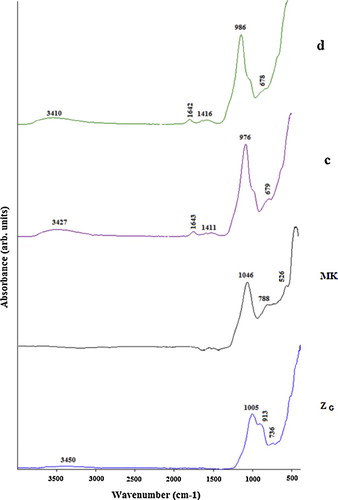
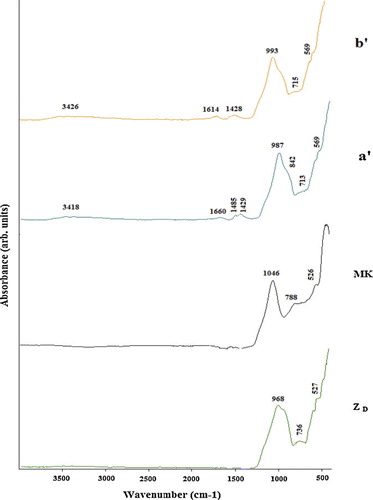
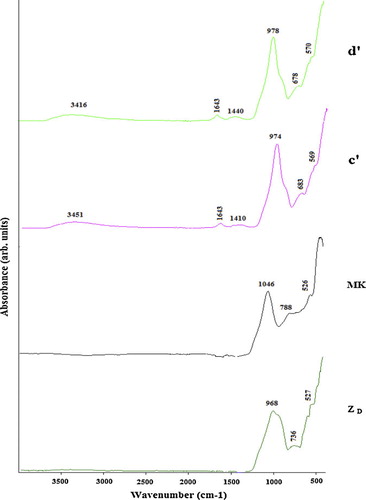
The FTIR spectra of volcanic scoria (ZD, ZG), metakaolin (MK) and the selected geopolymers (ZG5, ZG25, ZD5 and ZD25) are presented in –. Significant broad bands of O–H stretching and O–H bending were observed in the range of 3410–3455 and 1645 cm−1 in all FTIR spectra of selected geopolymers. These bands indicate the presence of water molecules which are surface absorbed or entrapped in the large cavities of the polymeric framework [Citation22,Citation24]. In all the FTIR spectra of geopolymers, the bands located between 1410 and 1484 cm−1 are attributed to stretching vibrations of O–C–O bond indicating the presence of sodium bicarbonate which is suggested to have occurred due to atmospheric carbonation [Citation22]. The most characteristic difference observed between the FTIR spectra of ZD, ZG, MK and FTIR spectra of the selected geopolymers concern the band attributed to the asymmetric stretching vibrations of Si–O–Si and Al–O–Si. The band that appeared as a broad band at around 968, 1005 and 1046 cm−1 in the FTIR spectra of ZD, ZG and MK respectively is shifted to lower wave number (974–993 cm−1) in the FTIR spectra of geopolymers indicating the formation of new amorphous aluminosilicate gel phases [Citation22]. These results were also confirmed by the XRD patterns of the selected geopolymer pastes ( and ). The intensity of these bands in ZD25 and ZG25 (c, d, c′ and d′) ( and ) is higher compared to ZD5 and ZG5 (a, b, a′ and b′) ( and ), indicating that ZD25 and ZG25 have the largest amount of geopolymer phases [Citation25], or that the degree of polycondensation reaction within the matrix of geopolymer pastes obtained from metakaolinin excess added in the volcanic scoria is complete. The weak band observed at 840–842 cm−1 on ZD5 and ZG5 spectra (a, b, a′ and b′) ( and ) is assigned to the bending vibration of Si–OH. The presence of Si–OH in the geopolymer products will lead to decrease the degree of polycondensation reaction, thus a reduction in mechanical strength [Citation26]. These bands are absent on the FTIR spectra of ZD25 and ZG25. The bands at 913, 736 and 788 cm−1on the FTIR spectra of ZD, ZG and MK respectively, related to the stretching vibration of 6-fold coordinated Al(VI)–OH and 6-fold coordinated Al(VI)–O [Citation26], have disappeared after polymerization. The band at 678–715 cm−1 only present on the FTIR spectra of geopolymers was ascribed to Si–O stretching symmetrically. The bands at 527–569 cm−1 were ascribed to symmetric stretching of Si–O–Si and Al–O–Si and are only present on the FTIR spectra of geopolymers [Citation23].
3.2.3 Setting time
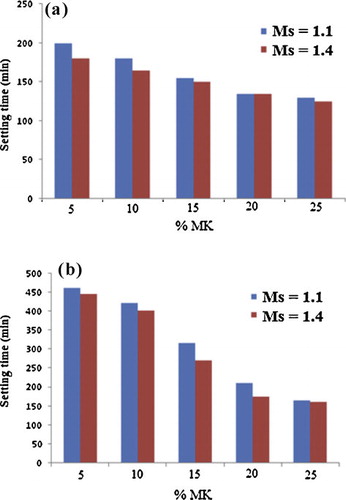
shows the effects on the initial setting time of the amount of metakaolin incorporated in the volcanic scoria and of SiO2/Na2O molar ratio in the alkaline solution of the geopolymer pastes. It is seen that the initial setting time decreases with an increase of the amount of metakaolin added an increase of the SiO2/Na2O molar ratio in the alkaline solution. This initial setting time is ranging from 500 to 160 min and between 220 and 125 min for ZD–MK-based geopolymers and ZG–MK-based geopolymers respectively. The initial setting time is directly related to the amount of metakaolin added and the sodium oxide (Na2O) content; it is found that the metakaolin added in the volcanic scoria and the SiO2/Na2O molar ratio in the alkaline solution are very important parameters for the setting time of volcanic scoria-based geopolymers. The greater the SiO2/Na2O molar ratio in the alkaline solution (Ms = 1.4), and the more added metakaolin (25%) in the volcanic scoria, the higher the viscosity of the geopolymer pastes; the faster the polycondensation phenomena and therefore, the faster the setting time of geopolymers.
3.2.4 Compressive strength
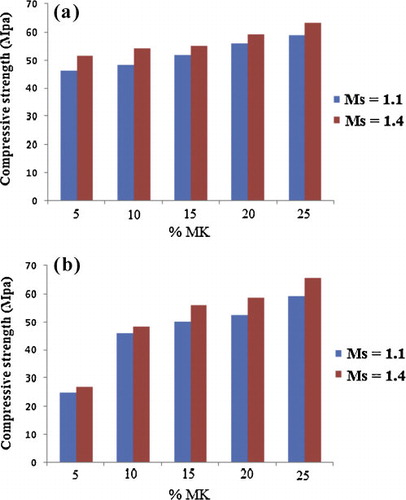
The compressive strength of geopolymer pastes increased with an increase of the amount of metakaolin added and also with an increase of the concentration of the alkaline solution (). These results indicate that a moderate addition of metakaolin in the volcanic scoria-based geopolymeric system will improve the mechanical property. After 28 days of casting, the highest compressive strengths are 64.3 MPa and 68.8 MPa for ZG25 and ZD25 respectively. In order to explain these results, it is important to note that metakaolin has smaller particle size than volcanic scoria (d50 is9.95 μm for MK, 13.01 μm for ZD and 10.68 μm for ZG), high specific surface area and high amorphous phase content than volcanic scoria (20.5 m2/g for MK and 2.3 m2/g for ZD, 15.7 m2/g for ZG). During the dissolution process the particle of metakaolin will react first and more than those of volcanic scoria, so the degree of geopolymerization and the amount geopolymer gel in the matrix rise. Thus the unreacted particle of volcanic scoria will act as coarse aggregate in the matrix and increase the compressive strength. The compressive strength of ZD25 is slightly higher than the one of ZG25. This is probably due to the sum of silica plus alumina in the volcanic scoria (59.30% for ZD against 56.80% for ZG). Then the addition of metakaolin increases the amount of these oxides in ZD and promotes high quantities of [SiO(OH)3]− and [Al(OH)4]− in the system during the dissolution process, producing a high degree of polycondensation reaction and therefore enhancing the compressive strength of ZD25. The compressive strengths of ZD25 and ZG25 are drastically higher than those of ZD5 and ZG5. This is justified by the presence of the band around 840 cm−1 on the ZD5 and ZG5 FTIR spectra which induce the decreasing of the degree of polycondensation reaction, thus a reduction in mechanical strength.
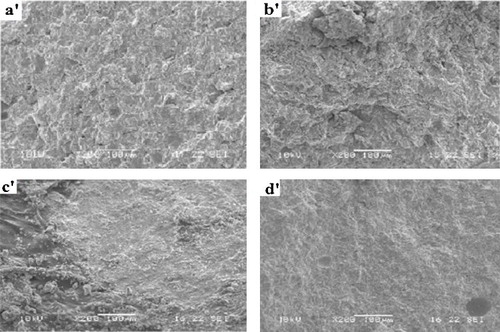
3.2.5 Microstructure
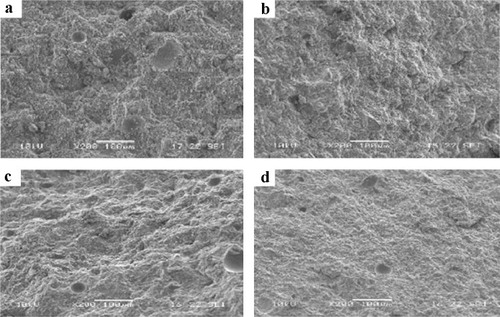
The microstructure of the selected geopolymer pastes (ZD5, ZD25, ZG5 and ZG25) is shown in and . It appears that the micrographs of ZD5 (a′, b′), ZD25 (c′), ZG5 (a, b) and ZG25 (c) are not homogeneous. This could be explained on one hand by the low SiO2/Na2O molar ratio in the alkaline solution and to the other hand by the heterogeneity of particles of ZD and ZG, so that the dispersion is not uniform. The geopolymer pastes ZD25 (d′) and ZG25 (d) show a more compacted structure due to the high SiO2/Na2O molar ratio in the alkaline solution and the more added metakaolin in the volcanic scoria, because with more the metakaolin is added, the amorphous phase increases in the system. This result corroborates with the one of compressive strength.
4 Conclusion
This study investigated the possibility of using a composite of metakaolin/volcanic scoria to produce geopolymers materials at ambient temperature. The properties of the synthesized products were dependent on the physico-chemistry properties of the aluminosilicate source used, the amount of metakaolin incorporated in the volcanic scoria and the SiO2/Na2O molar ratio in the alkaline solution. The initial setting time of the fresh geopolymers decreases with an increase of the amount of metakaolin added and also with an increase of the SiO2/Na2O molar ratio in the alkaline solution. The optimal geopolymer pastes reached a reasonably high 28-days compressive strength of 68.8 MPa for ZD25. The addition of metakaolin in the volcanic scoria increases the amount of amorphous SiO2 and Al2O3 in the system and therefore favors the dissolution of alumina and silica species, subsequently promoting the polycondensation phenomena and the formation of polymeric binder, resulting in an increase of the compressive strength and a decrease of the setting time of volcanic scoria-based geopolymers. This work showed that it was possible to alter the reactivity of volcanic scoria by adding amorphous aluminosilicate materials such as metakaolin to compensate the deficiency of amorphous SiO2 and Al2O3 in the volcanic scoria. It can be concluded that volcanic scoria could be used as an alternative source material for geopolymer synthesis at ambient temperature with high compressive strength and also be used to reduce the amount of metakaolin during the synthesis of geopolymer cements.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- P.V.KrivenkoProceedings of the First International Conference on Alkaline Cements and Concretes1994VIPOL Stock CompanyKiev, Ukraine11129
- J.G.S.van JaarsveldJ.S.J.van DeventerL.LorenzenMiner. Eng.101997659669
- A.PalomoM.W.GrutzeckM.T.BlancoCem. Concr. Res.29199913231329
- A.AllahverdiF.ŠkváraCeramics-Silikaty4520018188
- T.W.ChengJ.P.ChiuMiner. Eng.162003205210
- J. Davidovits, US Patent 4, 1982, pp. 349, 386.
- A.PalomoF.P.GlasserBrit. Ceram. Transl. J.911992107112
- H.RahierB.van MeleM.BiesemansJ.WastielsX.WuJ. Mater. Sci.3119967179
- A.ElimbiH.K.TchakouteD.NjopwouoConstr. Build. Mater.25201128052812
- J.P.HosP.G.McCormickL.T.ByrneJ. Mater. Sci.37200223112316
- A.AllahverdiK.MehrpourE.N.KaniJ. Eng. Sci.19200815
- D.BondarC.J.LynsdaleN.B.MilestoneN.HassaniA.A.RamezanianpourConstr. Build. Mater.25201129062910
- D.BondarC.J.LynsdaleN.B.MilestoneN.HassaniA.A.RamezanianpourConstr. Build. Mater.25201140654071
- H.K.TchakouteA.ElimbiB.B.D.KenneJ.A.MbeyD.NjopwouoCeram. Int.392013269276
- H.K.TchakouteJ.A.MbeyA.ElimbiB.B.D.KenneD.NjopwouoCeram. Int.39201316131621
- H.XuJ.S.J.van DeventerMiner. Eng.15200211311139
- H.K.TchakouteA.ElimbiJ.A.MbeyC.J.S.NgallyD.NjopwouoConstr. Build. Mater.352012960969
- H.K.TchakouteA.ElimbiE.YanneC.N.DjangangCem. Concr. Comp.3820137581
- D.NjoyaMinéralogie, propriétés physiques et mécaniques des céramiques des argiles de Mayouom (Cameroun) (Thèse de Doct 3eme cycle)2005Université de Yaoundé IYaoundé97
- J.DavidovitsJ. Therm. Anal. Calorim.37199116331656
- E.I.DiazE.N.AlloucheS.EklundFuel892010992996
- A.Fernandez-JimenezA.PalomoCem. Concr. Res.35200519841992
- D.PaniasI.GiannopoulouT.PerrakiColloids Surf. A: Physicochem. Eng. Aspects3012007246254
- J.C.SwanepoelC.A.StrydomAppl. Geochem.17200211431148
- U.RattanasakP.ChindaprasirtMiner. Eng.22200910731078
- Z.YunshengS.WeiL.ZongjinAppl. Clay Sci.472010271275