?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We report on the low-temperature synthesis of submicron-sized Cu3N powder produced from CuO and NaNH2 powder mixture by heating at 150–190 °C in a Teflon-sealed autoclave. The structure was the anti-RuO3 type with a lattice parameter of 0.3814(1) nm, and strong optical absorption was observed below ∼1.9 eV. This synthesis method has the potential of facile control of the reaction with less use of ammonia sources.
Cu3N has attracted attention for its potential uses in solar energy conversion, catalytic applications and electronic devices. It is a semiconductive nitride with a band gap of ∼1.4 eV, which is suitable for solar energy conversion [Citation1]. Incorporation of hydrogen and oxygen and its nonstoichiometry change its transport and optical properties [Citation2–Citation5]. Moreover, some recent studies focus on its catalytic properties, such as Huisgen cycloaddition [Citation6] and electrochemical oxygen reduction [Citation7]. Another interesting feature of Cu3N is its thermodynamic instability, which allows the formation of Cu metal at low temperature. This can afford the potential applications as recording media [Citation8,Citation9] and conductive ink [Citation10].
The synthesis of Cu3N powder has been reported by nitridation of Cu nitrate, chloride, fluoride, or complex using an ammonia flow [Citation6,Citation7,Citation10–Citation13]. These methods of synthesis partially rely on the purging of byproducts [Citation14] such as H2O or HF; thus, kinetic control is important using excess ammonia gas. The other method is sputtering of a Cu target in a nitrogen atmosphere [Citation8–Citation10,Citation15–Citation18], but large volume synthesis is difficult. In order to utilize the potential of Cu3N, a synthesis technique with less-toxic materials and facile control is highly desirable. Recently, we reported on the synthesis of InN, Mn6N5, and Fe3N by the nitridation of oxides using liquid NaNH2 at 240 °C in an autoclave [Citation19Citation[20]–Citation21]. These syntheses needed no ammonia flow, and facile control of the nitridation of oxides in a closed system was achieved. Herein, we report a new approach for the synthesis of Cu3N powder by the reaction between CuO and NaNH2 in an autoclave at 150–190 °C. These synthesis temperatures are lower than those of the nitridation of other oxides using NaNH2 [Citation14–Citation16] and below the melting point of NaNH2.
The method for synthesizing Cu3N was a slight modification to that in previous reports about the synthesis of other nitrides using NaNH2 [Citation19Citation[20]–Citation21]. First, 0.15 g of CuO (Kanto Kagaku) and 1.0 g of NaNH2 (Aldrich) were mixed using a mortar and pestle in a nitrogen-filled glovebox and put in a steel crucible. This steel crucible was set in a Teflon-lined autoclave, and then the autoclave was tightly closed. The autoclave was heated to 120, 150, 170, and 190 °C for 12–60 h. After it cooled down, the autoclave was opened under a normal atmosphere. The crucible was put in ethanol, and the product was obtained by filtration and further washing with ethanol and water. The crystal phase was examined by XRD (Rigaku; RINT-2000), and the morphology was observed using scanning electron microscopy (JEOL; JSM-6500). The specific surface area was measured by BELSORP-mini at 77 K. Surface analysis was performed by X-ray photoelectron spectroscopy (XPS: JEOL; JPS-9200) and the binding energies were corrected by reference to free carbon (284.6 eV). Thermal stability was investigated using a TG–DTA (Rigaku; Thermo plus EVOII) and optical reflectance was measured by a UV–vis spectrophotometer (Jasco; MSV-5200).
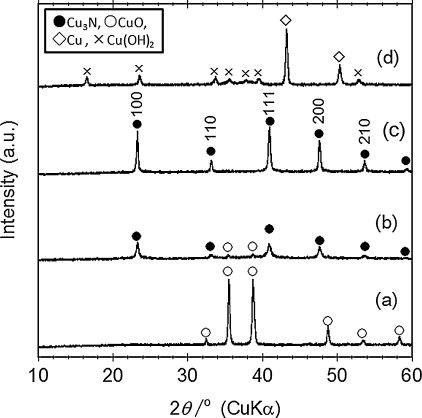
shows the XRD patterns after the reactions of CuO and NaNH2 at different temperatures for 60 h. At 120 °C, the peaks from unreacted CuO were found. The reactions at 150–170 °C produced the peaks of Cu3N with an anti-RuO3 structure. The lattice parameter of the synthesized powder at 170 °C was 0.3814(1) nm. This parameter is close to the reported value of stoichiometric Cu3N determined by neutron diffraction [Citation11]. Nonetheless, four kinds of stoichiometric Cu3N show slightly different lattice parameters [Citation11], which might be related to inclusion of hydrogen [Citation3]. Incorporation of oxygen does not significantly change the lattice parameter [Citation2]. Thus, we cannot deny the possibility of hydrogen and oxygen incorporation. The nitridation at 170 °C for less reaction time between 12 and 36 h still showed noticeable peaks of CuO phase, indicating that relatively long nitridation of 60 h was necessary. At 190 °C, the nitridation for 60 h gave Cu metal and Cu(OH)2 phases while that for 12 h produced CuO and Cu3N with slight Cu metal.
Fig. 1 XRD patterns of the products after the reaction of CuO with NaNH2at (a)120°C, (b) 150°C, (c) 170°C, and (d) 190°C for 60 h.
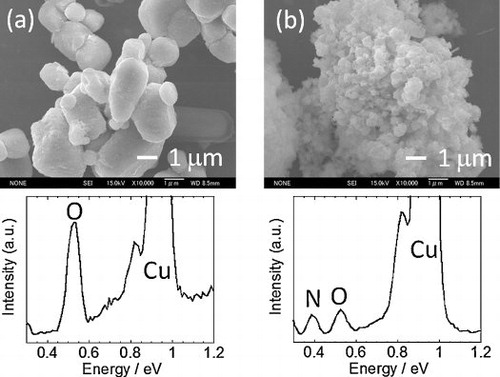
shows the SEM images of CuO powder before and after the nitridation at 170 °C. Round particles of a few microns in size changed into submicron-sized particles due to the nitridation. This nitridation increased its surface area from 2.2 m2 g−1 to 48 m2 g−1. The CuO showed signals for O and Cu, and the nitride showed peaks for N and Cu as well as a relatively weak peak for O when compared with that observed in CuO. This oxygen signal implies low-crystalline oxides/hydroxides phases though EDX is not very sensitive to the amounts of nitrogen and oxygen. No Na peak around 1.1 eV was detected.
Fig. 2 SEM images of (a) CuO and (b) Cu3N synthesized at 170°C for 60 h. TypicalEDX spectra are shown.
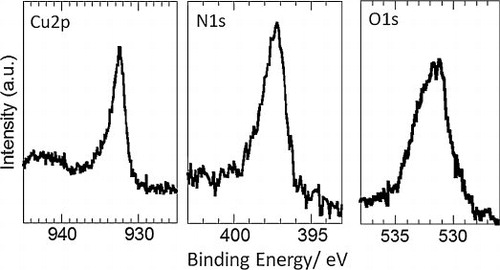
shows the XPS analysis of Cu 2p, N 1s and O 1s orbitals. The Cu 2p peak at binding energy of 932.4 eV was found with a shoulder around 934 eV. The former energy is close to the reported value of Cu3N thin film: 932.8 eV [Citation22]. This energy is slightly different from the energy of Cu metal (932.1 eV; not shown), and this slight difference between Cu and Cu3N agrees with close binding energies of Cu0 and Cu1+ [Citation22]. The shoulder at 934 eV can be assigned to Cu2+, such as CuO and Cu(OH)2 [Citation23]. The nitrogen profile shows the asymmetric peak at 397.2 eV with a shoulder toward high energy. This peak shape would be attributed to absorbed N on the surface at 397.7 eV and N in the bulk of Cu3N at 398.7 eV [Citation22]. The O 1s peak is relatively broad, and could be composed of oxygen in CuO and Cu(OH)2 at 531 eV and the other oxygen source such as OH, H2O and CO2 at 532.2 eV [Citation23].
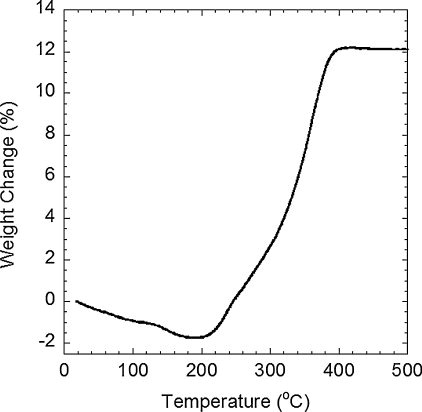
Thermal oxidation of Cu3N synthesized at 170 °C by heating under airflow is shown in . Mass loss of 1.1% below 140 °C would be due to absorbed species. The loss of 0.7% between 140 and 180 °C can be explained by decomposition of Cu(OH)2 [Citation24] and further heating above 200 °C causes the oxidation of Cu3N with a gain of 13.9%. Similar oxidation behavior has been reported in the literature [Citation10]. The product after the oxidation was found to be CuO by XRD. Theoretical mass change of Cu(OH)2 decomposition and Cu3N oxidation are −18.5% and +16.6%, respectively. This gives the estimate of 3.5 mass% of Cu(OH)2 and 83.7 mass% of Cu3N. Residual except absorbed species could account for 11.7 mass% of CuO. Therefore, Cu(OH)2 and CuO likely exist as low-crystallinity impurities. Relatively large amount of oxygen was found in many nitride powders with high surface area or small particle size [Citation20,Citation25,Citation26], and thus this may be a feature of such nitride powder after exposure to air and water.
Fig. 4 Weight change upon thermal oxidation of Cu3N synthesized at 170°C for 60 h: airflow of 100 mL min-1, heating rate of 10 K min-1.
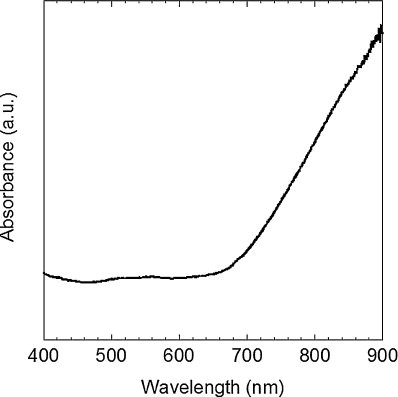
presents the optical absorption after the nitridation at 170 °C. Strong absorption is found above ∼660 nm, which corresponds to ∼1.9 eV. The synthesized powder was brown, which was consistent with this absorption. Brown powder and film have been also reported in the literature describing Cu3N [Citation10,Citation13,Citation16,Citation17]. However, this absorption is different from that in other reports (∼1.4 eV) [Citation1,Citation18]. This discrepancy could be related to the surface and/or oxygen incorporation in the product [Citation2].
The synthesis reaction can be formulated as follows:
NaNH2 does not melt at 170 °C (m.p. ∼210 °C). Since the reaction without mixing of CuO with NaNH2 yielded more unreacted CuO, solid–solid contact was quite important. Unreacted CuO phase after the nitridation at 170 °C with less reaction time suggests relatively low reaction rate of this solid–solid reaction. Further increasing temperature to 190 °C produced Cu metal and Cu(OH)2. This would be attributed to the low decomposition temperature of Cu3N, suggested by TG analysis. The nitridation mechanism would be different from the previously reported nitridation of Mn2O3 and Fe2O3 with melted NaNH2 at 240 °C, which complete the reaction in 36 h [Citation20]. Lower reaction temperature with well-mixed starting materials and relatively long reaction time would be necessary for this solid–solid reaction for Cu3N synthesis. Further investigation of pressure versus the amount of reactants is highly desired for scaling up this synthesis in safety.
The thermodynamic driving force is believed to be the formation of NaOH15 by considering the free formation energies of the components (298 K): CuO: −129.7 kJ/mol [Citation27], NaOH: −379.7 kJ/mol [Citation27], NaNH2: −64.0 kJ/mol [Citation27], NH3: −16.4 kJ/mol [Citation27], and N2: 0 kJ/mol [Citation27]. We could use the enthalpy as the estimated free energy of Cu3N, +74.5 kJ/mol [Citation28], assuming that the entropy affects only a few tens kJ/mol on the free energy. The energy of this nitridation is calculated to be −499.9 kJ; thus, this reaction would be thermodynamically favorable.
In summary, this low-temperature method using NaNH2 produced Cu3N from CuO in a closed system at 150–190 °C, which shows the potential of easy control of the nitridation with a low usage of ammonia source.
Acknowledgement
This work was partially supported by JSPS Grant-in-Aid for Young Scientists (No. 25820330).
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- A.ZakutayevC.M.CaskeyA.N.FiorettiD.S.GinleyJ.VidalV.StevanovicE.TeaS.LanyJ. Phys. Chem. Lett.5201411171125
- A.FallbergM.OttossonJ.-O.CarlssonJ. Cryst. Growth312201017791784
- J.WangJ.T.ChenB.B.MiaoF.ZhangP.X.YanJ. Appl. Phys.1002006103509
- M.G.Moreno-ArmentaG.SotoN.TakeuchiJ. Alloys Compd.509201114711476
- J.BlucherK.BangMater. Sci. Eng. A1171989L1L3
- B.S.LeeM.YiS.Y.ChuJ.Y.LeeH.R.KwonK.R.LeeD.KangW.S.KimH.B.LimJ.LeeH.J.YounD.Y.ChiN.H.HurChem. Commun.46201039353937
- H.WuW.ChenJ. Am. Chem. Soc.13320111523615239
- T.MaruyamaT.MorishitaAppl. Phys. Lett.691996890891
- A.MasaakiU.KazuoT.AkiraJpn. J. Appl. Phys.2919901985
- T.NakamuraH.HayashiT.A.HanaokaT.EbinaInorg. Chem.532014710715
- G.PaniconiZ.StoevaH.DobersteinR.I.SmithB.L.GallagherD.H.GregorySolid State Sci.92007907913
- D.WangY.LiChem. Commun.47201136043606
- R.DeshmukhU.SchubertJ. Mater. Chem.2120111853418536
- S.H.ElderF.J.DiSalvoL.ToporA.NavrotskyChem. Mater.5199315451553
- D.DorranianL.DejamG.MosayebianJ. Theor. Appl. Phys.6201219
- X.QianZ.HuangZ.CuiJ.GuoJ. Wuhan Univ. Technol., Mater. Sci. Ed.252010935937
- G.H.YueP.X.YanJ.Z.LiuM.X.WangM.LiX.M.YuanJ. Appl. Phys.982005103506
- K.J.KimJ.H.KimJ.H.H.KangJ. Cryst. Growth2222001767772
- A.MiuraT.TakeiN.KumadaCryst. Growth Des.12201245454547
- A.MiuraT.TakeiN.KumadaInorg. Chem.5220131178711791
- A.MiuraT.TakeiN.KumadaJ. Ceram. Soc. Jpn.12220148688
- C.NavíoM.CapitánJ.ÁlvarezF.YndurainR.MirandaPhys. Rev. B762007
- Z.AiL.ZhangS.LeeW.HoJ. Phys. Chem. C11320092089620902
- M.EndohA.DoiM.KawadaProc. Okayama Univ. Sci.8197297107
- M.YangM.J.MacLeodF.TessierF.J.DiSalvoJ. Am. Ceram. Soc.95201230843089
- A.MiuraM.LoweB.M.LeonardC.V.SubbanY.MasubuchiS.KikkawaR.DronskowskiR.G.HennigH.D.AbruñaF.J.DiSalvoJ. Solid State Chem.1842011711
- D.R.Lide83rd ed.CRC Handbook of Chemistry and Physicsvol. 832002CRC PressFlorida 5-5–5-50
- D.D.WagmanW.H.EvansV.B.ParkerR.H.SchummI.HalowS.M.BaileyK.L.ChurneyR.L.NutallJ. Phys. Chem. Ref. Data11Suppl. 219822156