?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Phase-pure multiferroic bismuth ferrite (BFO) nanoparticles were synthesized by energy efficient, simple and low temperature sol–gel followed by auto-combustion route. Highly crystalline and well-shaped BFO nanoparticles of size about 50 nm were observed in TEM. Thermal analysis was used to optimize the calcination temperature as 500 °C. An endothermic peak at 834 °C has been detected in the DTA curve, representing the Curie temperature. The dielectric anomaly around Neel temperature (TN) was observed signifying the magnetoelectric coupling. The BFO nanoparticles were found to be highly resistive (ρ ∼ 3 × 109 Ω-cm) and had very low leakage current of the order of μA/cm2, which resulted from phase purity. A significantly enhanced weak ferromagnetism was observed due to smaller particles size and remnant magnetization and coercive field were 0.067 emu/g and 185 Oe, respectively. P–E loop confirmed the ferroelectric behavior of BFO nanoparticles. The direct band gap energy was calculated to be 2.2 eV from UV–vis studies.
1 Introduction
Ferroic materials that exhibit spontaneous and switchable internal ordering have great importance due to a wide range of functional applications and their interesting fundamental physics [Citation1]. Multiferroic combines two or more primary ferroic orderings out of ferroelectricity, ferromagnetism and ferroelasticity [Citation1–Citation4]. The most interesting thing about these materials is their magneto-electric coupling, as a result of which electric polarization can be swapped by magnetic field and vice versa. This coupling multiplies the degrees of freedom, due to which multiferroics can be used in the field of highly dense non-volatile memory devices, spintronics, telecommunication and sensors [Citation4–Citation8].
Mostly studied single-phase ABO3 type perovskite multiferroics are BiFeO3 (BFO), BiMnO3 (BMO) and some rare earth manganites like YMnO3 [Citation9]. Out of these, BFO is the only promising candidate that retains ferroelectric (TC ∼ 825 °C) along with G-type antiferromagnetic (Neel temperature, TN ∼ 360 °C) orderings fairly above room temperature. It possesses rhombohedrally distorted perovskite structure with space group R3c, which can also be described by hexagonal system of basis with lattice parameters ahex = 5.58 Å and chex = 13.90 Å [Citation5]. It shows small residual magnetization as a result of canting of spins from their perfect antiparallel direction, which is greatly enriched in nanoparticles of size less than period of cycloid modulation, i.e. 62 nm [Citation10]. Also, it has a small band gap lying in visible region, so it finds potential application as a visible light photo-catalyst [Citation11].
Although BFO is mostly investigated multiferroic, its practical applications are limited on account of high leakage current, which is caused by defects and compositional instability [Citation12]. It is synthesized in various forms like single crystals, ceramics, nanoparticles/crystals and thin films [Citation13–Citation15]. However, stoichiometric phase-pure synthesis is a challenging task. Conventionally, it is synthesized by solid-state reaction of Bi2O3 and Fe2O3 at high temperature, but reaction kinematics suggests that the temperature range for stability is very small and deviation from this range leads to impurity phases like Bi2Fe4O9 and Bi25FeO39 [Citation16]. Furthermore, bulk form of BFO offers insignificant electric and magnetic moments. So, it is beneficial to synthesize in the form of BFO nanoparticles and thin films.
Various low temperature wet chemical methods, like hydrothermal, co-precipitation, micro-emulsion technique, modified Pechini's method, mechanochemical, sol–gel and solution combustion route, have been reported for the synthesis of BFO nanoparticles/ceramics [Citation17–Citation22]. Besides, several literature describe auto-combustion route but it is rare to find collective studies for structural, electrical, magnetic and optical properties of BFO nanoparticles [Citation23Citation[24]–Citation25]. It is known that the phase purity is needed for obtaining better magneto-electric properties of BFO. Therefore, the objective of this work is to synthesize stoichiometric phase-pure BFO nanoparticles at low temperature via auto-combustion route which is a simple and energy efficient method, and to investigate structural, electrical, magnetic and optical properties while maintaining nanoparticle nature of BFO intact. In the process, we have got enhanced properties than previous reports, particularly in case of temperature-dependent dielectric study in which an anomaly nearby TN signifying the magnetoelectric coupling is observed.
2 Experimental
2.1 Sample synthesis
Bismuth ferrite (BFO) nanoparticles were synthesized by low temperature sol–gel followed by auto-combustion route, which was based on modified Pechini's method. In this method, Bi(NO3)3·6H2O and Fe(NO3)3·9H2O (Sigma Aldrich, purity >99%) were used as starting materials according to the stoichiometry. First, bismuth nitrate was dissolved in 1 N nitric acid at 60 °C, and then iron nitrate was added to it while maintaining the heating and stirring to make a homogeneous solution. Citric acid as a chelating agent was added in 2:1 molar ratio with metal ions and the solution was stirred vigorously for another 2 h. As-obtained light brownish solution was kept on hot plate till it became viscous resin. While continuous heating, dried resin auto-ignited and released large amounts of gases and became a dark brownish powder, which was further ground and calcined at 500 °C for 3 h to get pure phase of BFO.
2.2 Characterization
The dried resin was subjected to TG-DTA analysis with Netzsch-409STA apparatus at a heating rate of 10 °C/min under nitrogen flow to obtain an optimum temperature range for the calcination. Structure and phase study of BFO nanoparticles were carried out by XRD with PANanalytical Diffractometer (Model: X’Pert Pro; Cu-Kα1, λ = 1.5405 Å) at room temperature. Morphology of nano-sized particles was studied by TEM (Tecnai 300 kV Ultra twin; FEI Company). J–E characteristic for leakage current analysis was obtained by applying a DC field and using a computer controlled Keithley Source Meter (Series 2400). Ferroelectric hysteresis loop was traced by an indigenously built Sawyer–Tower circuit (Marine India) driven by a lock-in amplifier. Dielectric measurement was carried out by the four-point probe system on Agilent E4980A LCR meter in the temperatures range of 30–460 °C and at frequencies ranging from 20 Hz to 2 MHz. These all characterizations were performed on 0.5 mm thick pellet of BFO nanoparticles, annealed at 500 °C for 2 h on which high-grade silver paste was coated uniformly to serve as electrodes. The magnetic hysteresis loops of the powder samples within a field range of ±2.2 T were measured at room temperature using a vibrating sample magnetometer (JDAW-2000D VSM). The UV–vis absorption spectra of the BFO nanoparticles were measured on Varion Cary-100 UV–vis spectrophotometer.
3 Results and discussion
3.1 Thermal analysis
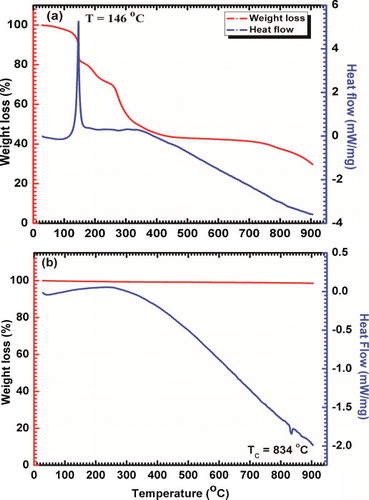
Thermal analysis (TG-DTA) was used to get appropriate temperature range of calcination for obtaining pure phase of bismuth ferrite. For this, as-obtained precursor was heated up to a temperature of 900 °C with heating rate of 10 °C/min in a continuous flow of N2 gas. TG curve (a) showing weight of dried resin decreased in a step-like manner up to temperature of 450 °C. There is a sudden dip at temperature of 146 °C, accompanied with a large exothermic peak in DTA, which indicates the starting of combustion of metal citrate complex that formed during reaction and other organic materials. So, large amount of energy and gases like CO2, NO2, etc. were released. Then, there is a plateau region from 450 °C to 650 °C, which is the most suitable range of temperature for calcination. This auto-combustion route of BFO synthesis is based on principles of propellant chemistry [Citation25], where a high-energy redox reaction takes place. As formation of oxide requires enormous energy, a high temperature is required for the reaction to begin. However, in this method, energy is supplied at molecule level by the combustion of complex carbonized ions, which get used for formation of oxide without attaining a high temperature.
b shows the TG-DTA curve of BFO nanoparticles calcined at 500 °C, where straight TG curve (no weight loss) confirms the quite stability on heating of BFO nanoparticles throughout the temperature range. An endothermic peak at ∼834 °C in DTA curve may be due to change in the crystal structure of BFO which clearly indicates the Curie temperature (TC).
3.2 Structural analysis
The room temperature powder X-ray diffraction pattern of BFO nanoparticles calcined at 500 °C is shown in . All the diffraction peaks are indexed with various (h k l) planes according to hexagonal system of basis. The position of each peak was found to be in good agreement with the perovskite BiFeO3 [JCPDS Card 86-1518]. Further, hexagonal crystal structure of the BFO was confirmed by the clear splitting of peaks (1 0 4) and (1 1 0). Sharp and high intensity XRD peaks support the good crystallinity of the as-synthesized BFO nanoparticles. Traces of some very low intensity peaks corresponding to secondary phases like Bi2Fe4O9, Bi25FeO39, etc. were identified [Citation22]. But, overall there had been a good control over the non-perovskite phases which confirms the phase purity of BFO nanoparticles. Thus, loss of bismuth was minimized due to low temperature synthesis in this method.
Fig. 2 XRD pattern of BFO nanoparticles calcined at 500 °C; peaks were indexed according to space group R3c (* and #: some traces of secondary phases) and Reitveld refined pattern with refinement parameters (inset).
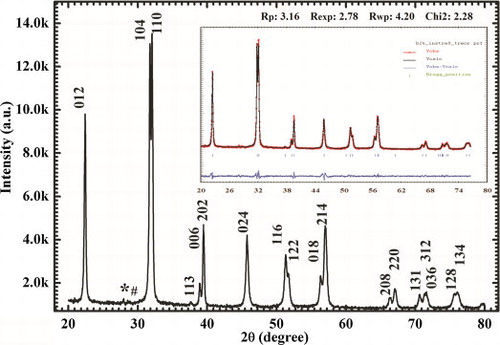
The Rietveld refinement was performed using Fullprof software package with starting parameters for hexagonal system of basis and space group R3c. The refined unit cell parameters ahex, chex and Vhex are 5.5820 Å, 13.8758 Å and 374.429 Å3, respectively and have resemblance with that reported previously [Citation19]. There is also good agreement between observed and calculated XRD patterns [ (inset)]. In our case, the refinement goodness parameters like χ2 (2.28) and R-factors are very low compared with some previous reports [Citation19,Citation23]. The obtained peaks of the powder XRD were found to be broad which is due to the nano-crystallite size of BFO. The crystallite size of the BFO nanoparticles was calculated using Scherrer formula,(1)
(1)
The average crystallite size of the diffraction pattern was found to be about 50 nm which is less than the period of spiral modulated spin order, 62 nm [Citation24].
3.3 Transmission electron microscopy (TEM) analysis
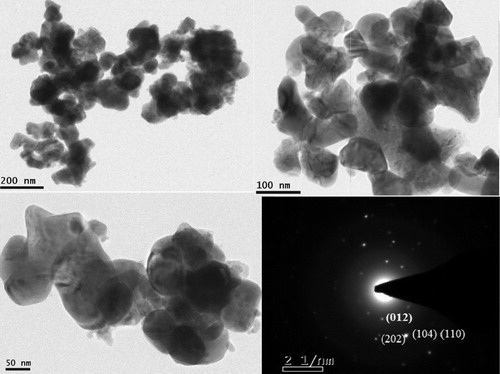
TEM micrographs () show the typical morphology of the low temperature synthesized BFO nanoparticles. For TEM sample preparation, BFO powder was dispersed in methanol by sonicator and dropped on carbon coated copper grid. Uniformly shaped nanoparticles can be observed in the TEM images. The diameter of the spherical BFO particles was measured to be about 50 nm, which is in good agreement with that calculated from powder XRD using Scherrer's formula. The selected area electron diffraction (SAED) shows bright diffraction spots, which confirms the formation of a high-quality well-crystalline structure of perovskite BiFeO3 nanoparticles. X-ray energy dispersive spectroscopy (EDS) attached to TEM was used to find elemental composition of prepared sample, which fairly agreed with expected stoichiometry of the compound.
3.4 J–E analysis
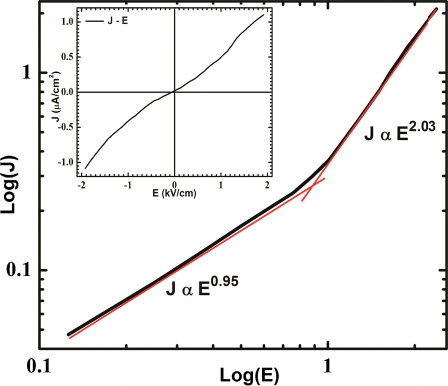
The conductivity analysis is one of the important tools for inspecting the feasibility of any material to be multiferroic. For this purpose, leakage current density versus electric field (J–E) measurement of BFO nanoparticle was carried out at room temperature. The leakage current was measured for both polarities of the bias voltage and no remarkable difference was obtained on reversing the bias voltage, which can be clearly seen from the inset of . Thus, metal-insulator (MI) contacts were ohmic in nature and conduction process was mainly bulk limited. shows relationship between log(J) and log(E) and indicates power law dependence, J ∝ En where ‘n’ is indices. In the low field region, ‘n’ is close to unity and it exhibits ohmic nature of conduction, whereas, in high field region, ‘n’ is nearly 2 and follows the space-charge limited conduction (SCLC) mechanism [Citation26]. In this mechanism, injected charge carriers dominate over volume generated free charge carriers. This shows that defects mediated conductivity is relatively less dominated, so oxygen vacancies, which are caused by volatility of bismuth (Bi), may be suppressed due to low temperature synthesis. Leakage current density was found to be in the range of μA/cm2 and resistivity (ρ) of sample was found to be about 3 × 109 Ω-cm, which was decreased to about 2 × 109 Ω-cm at higher field. Conduction mechanism of BFO is also explained on the basis of Poole–Frenkel (PF) emission [Citation27], where field-assisted hopping of charge between Fe2+ and Fe3+ occurs. But, this type of defects activated conductivity was not observed in our sample, which may be due to low applied field and less defect centers.
3.5 Dielectric analysis
The dielectric measurement of BFO nanoparticles was performed over a wide range of frequency 20 Hz to 2 MHz and temperature 30–460 °C. a shows the variation of real dielectric constant (ɛ′) and loss factor (tan δ) with log of frequency at room temperature. The value of real dielectric constant was found to be about 65 for 1 kHz and loss factor (tan δ) in the range of 0.8–0.02 at room temperature. In lower frequency region, the dielectric constant abruptly decreased following a maxima (ωp) in dielectric loss and then attained nearly constant value (ɛ∝ ∼ 40) at higher frequencies. This dielectric behavior is expected from every ferroelectric material and is known as low frequency space-charge relaxation. At lower frequencies, various polarizations viz. space charges, ionic defects, permanent dipoles, induced atomic and ionic dipoles, contribute toward dielectric resulting in high values. As frequency increases, different participating mechanisms for dielectric constant decrease gradually and subsequently dielectric constant decreases [Citation28,Citation29]. Also, each polarization mechanism is accompanied with relaxation frequency (fr) for which a maximum phase shifts between polarization (P) and applied electric field (E), causing maximum dielectric loss. The high value of dielectric constant may also be attributed to smaller particle size [Citation30].
Fig. 5 (a) Real dielectric constant and loss (tan δ) with log frequencies at room temperature and (b) real dielectric constant as a function of temperature at various frequencies.
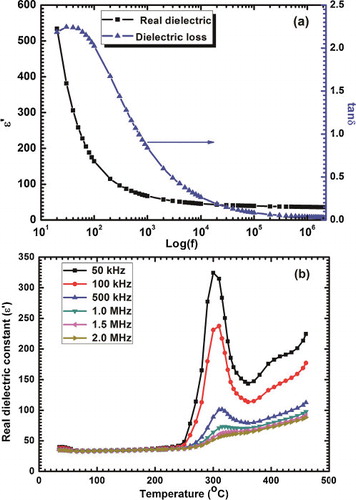
b illustrates the variation of dielectric constant as a function of temperature at various frequencies. The dielectric constant shows a fairly constant behavior up to 230 °C for all the frequencies. Above 230 °C dielectric constant gradually increases and a broad peak is observed around Neel temperature (TN). This broad peak near TN is frequency dependent and shifts to a higher temperature and decreases in intensity with an increase in frequency, which is a typical characteristic of relaxor ferroelectrics [Citation28,Citation31]. This type of dielectric anomaly may be a direct consequence of magnetoelectric coupling and Huang et al. have also observed this for YMnO3 [Citation32,Citation33]. As for a magnetic transition, only modifications in spin structure take place but it is coupled with ferroelectric ordering, on account of which, there is a little change in structural parameters, thus an anomaly in dielectric behavior may be observed.
3.6 P–E loop measurement
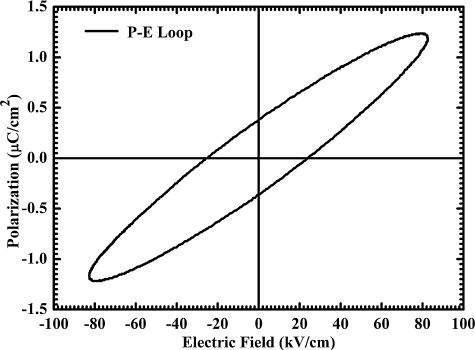
Ferroelectric hysteresis (P–E) loop for BFO nanoparticles was traced using a standard Sawyer–Tower circuit. An external AC field of frequency 50 Hz and strength 85 kV/cm was applied to measure P–E loop. shows the relationship between polarization and applied field at room temperature. As sample was able to bear an electric field as high as 85 kV/cm without breaking down, which was because of minimized leakage current, it is observed that even for such a high electric field, the P–E loop could not be fully saturated. The remnant polarization (Pr) and coercive field (Ec) of this unsaturated hysteresis loop were obtained to be 0.4 μC/cm2 and 25 kV/cm, respectively. However, in the case of thin films, a well-saturated P–E loop with very large remnant polarization has been reported for BFO [Citation13,Citation34]. The high coercive field and leakage current may be responsible causes for unsaturated PE loop and low value of polarization in BFO [Citation14].
3.7 M–H loop measurement
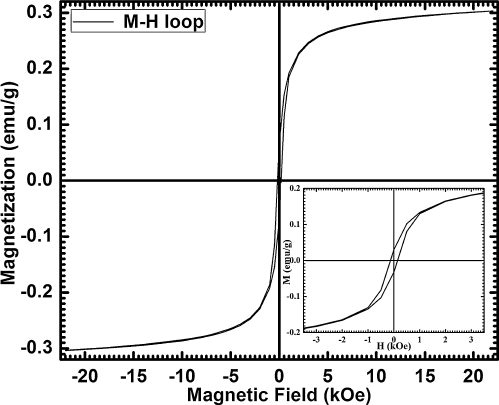
The M–H hysteresis loop has been measured at room temperature to study magnetic properties of BFO nanoparticles, which is shown in . It confirms that as-synthesized BFO nanoparticles exhibit weak ferromagnetic ordering with residual magnetic moment of 0.067 emu/g and coercive field of 185 Oe on applying a magnetic field of 22 kOe. The observed remnant magnetization is higher than many other previous reports [Citation18], which may be due to smaller size and uniform shape of nanoparticles. The particle size of our as-synthesized BFO nanoparticles is about 50 nm, which is less than the wavelength 62 nm of cycloid structure of canted spin. Thus, residual magnetic moment may be a result of uncompensated spins on the surface and broken order of cycloid spin structure [Citation11]. Other possibilities for this magnetization may be ruled out on the basis of phase purity and high resistivity of the sample. Saturation in hysteresis loop was not attained, which may be expected from G-type antiferromagnetic nature of BFO.
3.8 UV–vis spectra
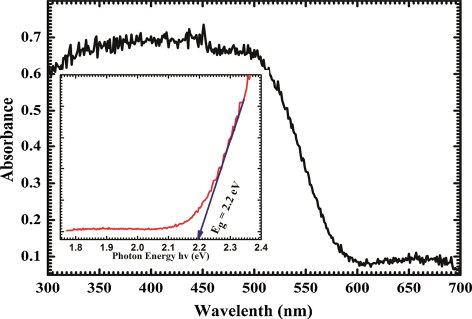
The UV–vis absorption spectrum of as-synthesized BFO nanoparticles is shown in . This is seen from the spectrum that BFO nanoparticles have strong absorption in UV–vis region 300–550 nm and slight absorption in 550–700 nm. This absorption characteristic can be used in the photo-catalytic activity in decomposition of organic compounds. The absorption curve gives an absorption edge at 510 nm. The inset of shows (αhυ)2–hυ plot for the direct band gap calculation. For any semiconductor, optical absorption near band edge obeys following equation,(2)
(2) where α, h, υ and Eg are the optical absorption coefficient, Plank's constant, light frequency and band gap energy, respectively. For the direct band gap, the variable ‘n’ in the equation is equal to 1/2. As BFO is a direct band gap semiconductor, the extrapolated line intercept at horizontal axis gives the value of direct energy band gap as 2.2 eV. The band gap value is comparable with the earlier reported values [Citation12,Citation17]. Also, the band gap of BFO at bulk level is reported to be 2.6 eV, which decreases to 2.2 eV for nanoparticles due to the size effect. Thus, BFO nanoparticles may be used for photo-catalysis in degradation applications.
4 Conclusion
In conclusion, phase-pure BFO nanoparticles have been synthesized using low temperature sol–gel followed by auto-combustion route. Phase purity and crystallite size of the BFO nanoparticles were confirmed by XRD and TEM studies. TG-DTA analysis revealed the optimum temperature range of calcination and showed an endothermic peak at 834 °C corresponding to ferroelectric phase transition (TC) of BFO. Temperature and frequency-dependent dielectric study confirmed relaxor behavior, whereas a dielectric anomaly near Neel temperature has provided a direct proof of magnetoelectric coupling. Particles size (∼50 nm) was found to be smaller than the period of spin cycloid which resulted in weak ferromagnetism and exhibited net magnetic moments of Mr = 0.067 emu/g. High resistivity of ρ = 3 × 109 Ω-cm and reduced leakage current have been achieved which is a direct consequence of suppression of oxygen vacancies. P–E loop confirmed the ferroelectric behavior of BFO nanoparticles and observed values of Pr and Ec were 0.4 μC/cm2 and 25 kV/cm, respectively. Also, small band gap of Eg = 2.2 eV makes it suitable candidate for photo-catalysis applications. Hence, this method can be useful for synthesizing highly resistive and uniform-sized BFO nanoparticles for various applications.
Acknowledgements
We are thankful for the financial support received through UGC project (Sanction No. 41-914/2012: SR) and DST project (Sanction No. SR/S2/CMP-0068/2010). Mr. Sanjay Godara and Ms. Geeta Ray are thankful to UGC and CSIR (India) for Junior Research Fellowships.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- N.A.SpaldinS.-W.CheongR.RameshPhys. Today63201038
- W.EerensteinN.D.MathurJ.F.ScottNature4422006759765
- N.A.SpaldinM.FiebigScience3092005391392
- S.-W.CheongM.MostovoyNat. Mater.620071320
- G.CatalanJ.F.ScottAdv. Mater.21200924632485
- J.F.ScottNat. Mater.62007256257
- M.BibesNat. Mater.112012354357
- H.BéaM.GajekM.BibesA.BarthélémyJ. Phys.: Condens. Matter202008434221
- C.N.R.RaoC.R.SerraoJ. Mater. Chem.1720074931
- T.ParkG.C.PapaefthymiouA.J.ViescasA.R.MoodenbaughS.S.WongNano Lett.72007766772
- F.GaoX.Y.ChenK.B.YinS.DongZ.F.RenF.YuanAdv. Mater.19200728892892
- Y.P.WangL.ZhouM.F.ZhangX.Y.ChenJ.-M.M.LiuZ.G.LiuAppl. Phys. Lett.8420041731
- J.WuJ.WangD.XiaoJ.ZhuACS Appl. Mater. Interfaces420121182
- R.PalaiH.SchmidJ.F.ScottR.S.KatiyarPhys. Rev. B812010064110
- J.WangJ.B.NeatonH.ZhengV.NagarajanS.B.OgaleB.LiuScience29920031719
- J.L.MukherjeeF.F.Y.WangJ. Am. Ceram. Soc.5419713134
- S.LiY.LinB.ZhangY.WangC.NanJ. Phys. Chem. C114201029032908
- S.K.SrivastavN.S.GajbhiyeJ. Am. Ceram. Soc.95201236783682
- S.HoK.SunH.GiH.LeeH.KangJ.SeogCeram. Int.36201013651372
- N.DasR.MajumdarA.SenH.S.MaitiMater. Lett.61200721002104
- I.SzafraniakM.PołomskaB.HilczerA.PietraszkoL.KępińskiJ. Eur. Ceram. Soc.27200743994402
- G.Rojas-GeorgeJ.SilvaR.CastañedaD.LardizábalO.A.GraeveL.FuentesMater. Chem. Phys.146201473
- E.C.AguiarM.A.RamirezF.MouraJ.A.VarelaE.LongoA.Z.SimõesCeram. Int.3920131320
- J.XuH.KeD.JiaW.WangY.ZhouJ. Alloys Compd.4722009473477
- J.YangX.LiJ.ZhouY.TangY.ZhangY.LiJ. Alloys Compd.509201192719277
- S.K.PradhanB.K.RoulPhys. B: Condens. Matter407201225272532
- G.W.PabstL.W.MartinY.-H.ChuR.RameshAppl. Phys. Lett.902007072902
- C.ElissaldeJ.RavezJ. Mater. Chem.11200119571967
- G.RayN.SinhaB.KumarMater. Chem. Phys.1422013619625
- M.K.GuptaB.KumarJ. Alloys Compd.5092011L208
- M.G.MasudA.GhoshJ.SannigrahiB.K.ChaudhuriJ. Phys.: Condens. Matter242012295902
- Z.J.HuangY.CaoY.Y.SunY.Y.XueC.W.ChuPhys. Rev. B56199726232626
- A.SinghV.PandeyR.K.KotnalaD.PandeyPhys. Rev. Lett.1012008247602
- J.WuS.QiaoJ.WangD.XiaoJ.ZhuAppl. Phys. Lett.1022013052904