Abstract
Silica nanoparticles containing covalently linked iodine and a near-infrared (NIR) fluorescence dye, namely porphyrin, have been synthesized through a one-pot sol–gel reaction. These particles are called iodinated silica/porphyrin hybrid nanoparticles (ISP HNPs). The ISP HNPs have both high X-ray absorption coefficient and NIR fluorescence. The ISP HNPs modified with folic acid (FA) and polyethylene glycol (PEG), denoted as FA-PEG-ISP HNPs, enabled the successful visualization of tumors in mice by both X-ray computed tomography (CT) and fluorescence imaging (FI). Thus, the FA-PEG-ISP HNPs are useful as contrast agents or probes for CT/FI dual-modal imaging.
Keywords:
1 Introduction
Multifunctional nanoparticles (NPs) are promising diagnostic agents that can be adapted to several diagnostic imaging units or modalities [Citation1–Citation6]. Imaging in which several modalities are combined is known as multimodal imaging. In this approach, the modalities that mutually complement one another and compensate for the weak points of each modality are selected. This method enables a rapid and accurate diagnosis. The combination of X-ray computed tomography (CT) and fluorescence imaging (FI), i.e., CT/FI dual-modal imaging, is one of the most effective combinations [Citation5,Citation6].
CT provides anatomical information such as size, location, and shape of tissues [Citation7], and is universally prevalent and inexpensive. However, it is difficult to distinguish a specific soft tissue from the surrounding soft tissues without using CT contrast agents because the CT contrast of soft tissues is inherently low. Iodine-containing molecules are currently used as CT contrast agents in clinical applications because iodine has a high X-ray absorption coefficient. However, the use of such compounds is limited to the imaging of angiography and urography because they non-specifically adhere to tissues. Therefore, CT contrast agents that enable the visualization of a specific soft tissue, such as a tumor, are strongly required.
FI presents images very rapidly and its operation is easy [Citation7]. However, the detection depth is limited to the penetration depth of light in the tissue. Light penetrates deeply into the body in the 600–900 nm wavelength range, which is known as the optical window or near-infrared (NIR) window for biological tissues, because the absorptions by hemoglobin, water, and melanin are minimized in this wavelength range. The development of fluorescent probes that have excitation (λex) and emission (λem) wavelengths in the optical window is required for in vivo FI.
We previously reported on iodinated silica/porphyrin hybrid nanoparticles (ISP HNPs) and demonstrated the synergetic effect of photodynamic therapy (PDT) and photothermal therapy (PTT) [Citation8] for the treatment of multiple myeloma, which remains an incurable malignancy [Citation9]. The 1O2 and heat generated by a light-emitting diode based on the effect of PDT combined with PTT effect were successfully utilized for the inhibition of tumor growth.
Herein, we report the one-pot synthesis of multifunctional NPs with high X-ray absorption coefficient, NIR fluorescence, and tumor specificity. Especially, this study focused on the in vivo CT/FI dual modal imaging of the ISP HNPs for a tumor derived from the RPMI 8226 multiple myeloma cell line using the multifunctional NPs.
2 Experimental
2.1 Materials
(3-Iodopropyl)trimethoxysilane (IPTMS) and aqueous ammonia (28%) were purchased from Sigma–Aldrich (MO, USA) and Kishida Chemical (Osaka, Japan), respectively. Tetrakis(4-carboxyphenyl)porphyrin (TCPP), tetraethyl orthosilicate (TEOS), 3-aminopropyltriethoxysilane (APTES), 3-mercaptopropyl(dimethoxy)methylsilane (MPDMMS), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDAC), N-hydroxysuccinimide (NHS), and N,N-dimethylformamide (DMF) were commercially available from Tokyo Chemical Industry (Tokyo, Japan). Folic acid and maleimide-heterobifunctionalized polyethylene glycol (FA-PEG-Mal, MW 3400) were purchased from Nanocs (NY, USA).
2.2 Synthesis of multifunctional silica NPs
Porphyrin-containing silicon alkoxide (PCSA) was prepared as the precursor. The preparation and structure of PCSA were described in our previous paper [Citation5,Citation6,Citation8,Citation10]. In brief, APTES (15 mM) and TCPP (3.75 mM) were dissolved in DMF; EDAC (15 mM) and NHS (15 mM) were added to this solution. The mixture was stirred for 24 h at room temperature to form PCSA via the amidation reaction between the amino group of APTES and the carboxylic acid groups of TCPP.
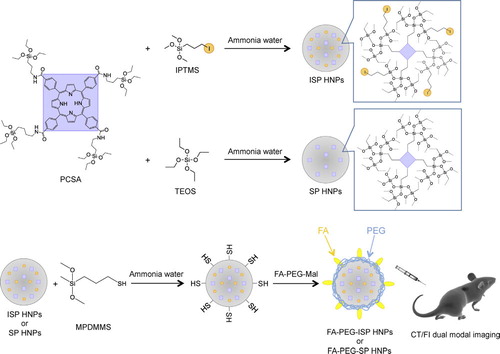
Iodinated silica NPs containing porphyrin were subsequently prepared via amide binding and the resulting materials were designated as iodinated silica/porphyrin hybrid NPs (ISP HNPs). The target compounds were prepared as follows: IPTMS (239 μmol) and PCSA (1.7 μmol) were dissolved in DMF (1 mL). Aqueous ammonia (0.8 mL) was added to the above solution and the mixture was stirred at 100 °C for 24 h. The ISP HNPs were collected by centrifugation (36,000 × g) of this dispersion and then redispersed in water. This sequence was repeated three times. In addition, as a control material, non-iodinated silica NPs containing porphyrin, i.e., silica/porphyrin (SP) HNPs, were produced by a similar method to that used for the synthesis of ISP HNPs by using TEOS instead of IPTMS.
In the final step, the ISP HNPs were modified with PEG and FA. The ISP HNPs (12.6 mg) were dispersed in ethanol (6.5 mL) and they were functionalized with thiol groups via the hydrolysis and condensation of MPDMMS (110 nmol) with aqueous ammonia (125 μL) at 50 °C for 24 h. The thiol-functionalized ISP HNPs were collected by centrifugation (36,000 × g) of this dispersion and they were then redispersed in water. This sequence was repeated three times. The ISP HNPs were subsequently modified with FA and PEG by adding FA-PEG-Mal (110 nmol) to the aqueous dispersion of thiol-functionalized ISP HNPs (20 mg/mL, 500 μL), with the mixture stirred at room temperature for 24 h.
2.3 Characterization
The absorption bands of TCPP, SP HNPs, and ISP HNPs were measured in phosphate buffered saline (PBS) using an ultraviolet–visible (UV–vis) spectrophotometer (U-3000, Hitachi). Fluorescence properties of TCPP, SP HNPs, and ISP HNPs were characterized in PBS using a fluorescence spectrophotometer (F-2500, Hitachi, Tokyo, Japan). The photoluminescence (PL) quantum yields of ISP HNPs, SP HNPs and TCPP were measured using an absolute PL quantum yields measurement system (C9920-03, Hamamatsu Photonics, Hamamatsu, Japan).
2.4 Cell line
The human myeloma cell line RPMI 8226 was obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The culture medium contained no folic acid. Prior to inoculation into mice, the cells were washed and resuspended in sterile PBS at a concentration of 1 × 108 cells/mL.
2.5 Ethics statement
The study protocol was approved by the Animal Care and Use Committee of the University of Tokushima (Tokushima, Japan).
2.6 Animals
Female CB17/Icr-Prkdc scid mice aged four weeks were purchased from Charles River Laboratories Inc. (Yokohama, Japan) and were maintained in a specific pathogen-free facility in our Animal Resources Center. All experiments were approved by the Committee on Animals of the University of Tokushima. In order to eradicate residual natural killer (NK) cells, the mice were injected intraperitoneally with 10 μL of rabbit anti-asialo GM1 antiserum (Wako Chemicals, Osaka, Japan) one day before the tumor inoculation. The inoculation of the human myeloma cells (5 × 106 cells) was accomplished by subcutaneous injection into the backs of mice.
2.7 FI
The fluorescence images were obtained using the IVIS Imaging System (Caliper Life Sciences, Hopkinton, MA, USA). The fluorescence intensities were estimated using the regions of interest (ROI) tool, which can determine the light flux. FA- and PEG-modified ISP HNPs (FA-PEG-ISP HNPs) were injected into the tumor-bearing mice. The in vivo fluorescence images of the mice were obtained using the IVIS Image System at λex = 640 nm and λem = 720 nm (n = 5).
2.8 CT
CT images of the ISP HNPs and SP HNPs in PBS, as well as in mice injected with FA- and PEG-modified ISP HNPs (FA-PEG-ISP HNPs), were obtained using the Aloka Latheta LCT-200 system (Hitachi, Tokyo, Japan; n = 5 per cohort). The imaging parameters were as follows: pixel size, 96; thickness, 192 μm; pitch, 192 μm. CT images were obtained using OsiriX (ver 3.8.1; 32 bit; OsiriX Foundation, Geneva, Switzerland). CT values were estimated using the ROI tool.
3 Results and discussion
3.1 Synthesis of iodinated silica/porphyrin hybrid nanoparticles (ISP HNPs) and silica/porphyrin hybrid nanoparticles (SP HNPs)
Silica NPs containing covalently attached iodine and porphyrin derivatives as NIR fluorescent dyes were synthesized to obtain particles with high X-ray absorption coefficient and NIR fluorescence. shows the processing schemes of the ISP HNPs, SP HNPs, and the modification of ISP HNPs for the in vivo experiment. Iodine was introduced from IPTMS to the ISP HNPS. Silicon alkoxide chemically bound with porphyrin (PCSA) was condensed with iodine-substituted silicon methoxide (IPTMS), yielding the ISP HNPs [Citation8]. Similarly, SP HNPs were synthesized from PCSA and TEOS. The structures were characterized using various spectroscopic methods in our previous study [Citation8]. In brief, the mean diameters of the ISP HNPs and SP HNPs, as estimated from the TEM images, are 47 ± 12 and 42 ± 10 nm, respectively. Thus, the ISP HNPs have about the same size as the SP HNPs. The ISP HNPs and SP HNPs consist of a siloxane network that incorporates porphyrin molecules through amide linkages, which were confirmed by solid-state 29Si and 13C NMR spectroscopy and FTIR spectroscopy. Furthermore, the ISP HNPs contain covalently attached iodine in addition to porphyrin molecules. According to XRF results, the ratio of iodine to silica is 1:4 in the ISP HNPs. On the other hand, the SP HNPs do not contain any iodine.
For in vivo experiments, the NPs were modified with polyethylene glycol (PEG) and folic acid (FA) to provide tumor specificity to the NPs. PEG prevents the uptake of NPs in the reticuloendothelial system (RES) and prolongs their circulation time in the blood, thus resulting in enhanced permeability and retention (EPR) effect [Citation11]. FA specifically binds the folate receptors that are overexpressed in the tumor cells [Citation12]. We have reported that the amount of FA grafted on the surface of FA-PEG-ISP HNPs was estimated to be 7.1 μmol/g [Citation8].
3.2 Optical properties
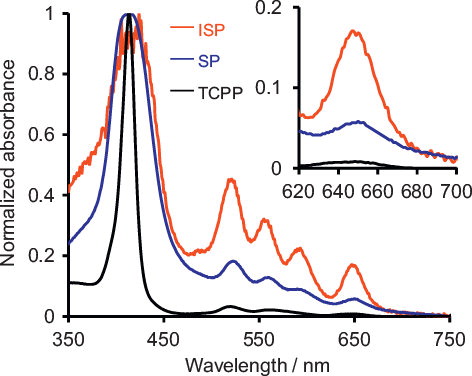
shows the UV–vis spectra of ISP HNPs, SP HNPs, and TCPP. TCPP and SP HNPs show quite weak Q band absorbances from 500 to 650 nm because Q bands are inherently spin-prohibited. On the other hands, as shown in the inset, the ISP HNPs show a much higher Q-band absorbance than SP HNPs and TCPP, probably because the external heavy atom suppresses the spin prohibition of Q bands in the ISP HNPs. The ratios of the absorbance at 650 nm (A650) to the absorbance of the Soret band at 420 nm (ASoret) for the ISP HNPs is 0.168, which is significantly higher than those of SP HNPs (0.058) and TCPP (0.008). The A650/ASoret ratio of the ISP HNPs to that of SP HNPs is 3. Therefore, the external heavy-atom effect increases the light absorption by approximately a factor of 3. In addition, since the A650/ASoret ratio for SP HNPs (0.058) is about 7 times higher than that of TCPP (0.008), the incorporation of porphyrin molecules into silica NPs contributes to the increase in light absorption. Furthermore, the molar absorptivities (ɛ650) of ISP HNPs, SP HNPs and TCPP were estimated by the Beer–Lambert law. The ɛ650 of ISP HNPs, SP HNPs and TCPP were 2100, 1800 and 110 L mol−1 cm−1, respectively. These results also demonstrate that the incorporation of porphyrin molecules into silica NPs and the external heavy-atom effect enhance the light absorption.
3.3 Fluorescence properties
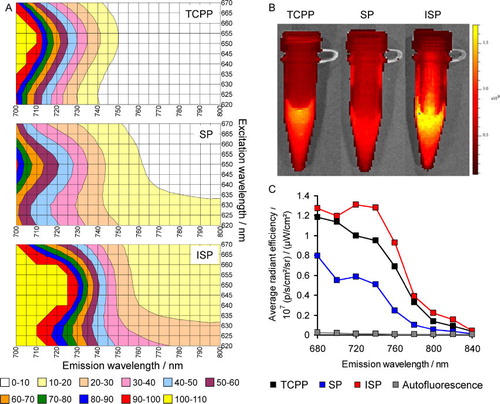
The fluorescence spectra of TCPP, SP HNPs, and ISP HNPs are shown in . It can be seen that enclosing the porphyrin within silica NPs shifts λem and λex to longer wavelengths. Furthermore, as evidenced by the fluorescence spectra and fluorescence images, the ISP HNPs emit stronger fluorescence than those emitted from SP HNPs and TCPP. Moreover, the fluorescence emitted from the ISP HNPs is significantly higher than the autofluorescence of mice over the entire NIR region. These benefits of the ISP HNPs are provided by the effect of the external heavy atom. In addition, the PL quantum yields of ISP HNPs, SP HNPs and TCPP were 1.7%, 1.5% and 0%, respectively (λex = 640 nm). These results demonstrate that the incorporation of porphyrin molecules into silica NPs contributes to the increase in PL quantum yield. This is because the porphyrin molecules form few aggregates and are uniformly distributed in the silica NPs. Furthermore, the external heavy-atom effect somewhat enhances the PL quantum yield.
3.4 CT in PBS
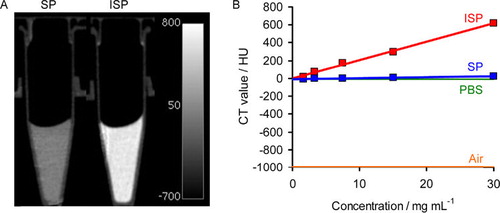
The CT images of the materials are shown in . It can be seen that the ISP HNPs enhance the CT contrast when compared to SP HNPs. Furthermore, the CT value of the ISP HNPs is significantly higher than that of SP HNPs. There is very little difference between SP HNPs and PBS in terms of the CT value. These results demonstrate that the iodine linked to the siloxane network certainly absorbs X-rays, thus leading to an enhancement in the CT contrast.
3.5 CT/FI dual-modal imaging of tumors
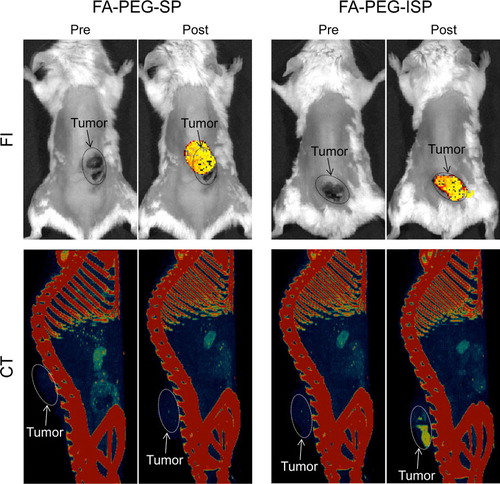
Using the methods reported in our previous study [Citation8], we confirmed that the ISP HNPs and SP HNPs were modified with FA and PEG. The amounts of FA and PEG on the surfaces of these materials were also determined [Citation8]. shows the CT/FI dual-modal imaging of a tumor using FA-PEG-ISP HNPs and FA-PEG-ISP HNPs as contrast agents or probes. Both FA-PEG-SP HNPs and FA-PEG-ISP HNPs clearly detect tumors by FI. Furthermore, FA-PEG-ISP HNPs successfully enable the tumor to be visualized by CT, whereas FA-PEG-SP HNPs cannot be used for this application because they do not strongly absorb X-rays. These results demonstrate that FA-PEG-ISP HNPs are useful contrast agents or probes for the CT/FI dual-mode imaging of tumors.
4 Conclusions
In the ISP HNPs, porphyrin and iodine are covalently bound to a siloxane network. The iodine in the ISP HNPs is responsible for the external heavy-atom effect, which shifts λem and λex to longer wavelengths and enhances the fluorescence intensity. Furthermore, the iodine present in the ISP HNPs allows the effective absorption of X-rays, thus resulting in the enhancement of the CT contrast. In addition, FA-PEG-ISP HNPs successfully allow tumors to be visualized by FI and CT. Therefore, these compounds are promising contrast agents or probes for CT/FI dual-modal imaging.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- M.K.YuJ.ParkS.JonTheranostics220123044
- J.U.MenonP.JadejaP.TambeK.VuB.YuanK.T.NguyenTheranostics32013152166
- Y.ZhangY.YangW.CaiTheranostics12011135148
- Y.XingJ.ZhaoP.S.ContiK.ChenTheranostics42014290306
- K.HayashiM.NakamuraK.IshimuraAdv. Healthcare Mater.22013756763
- K.HayashiM.NakamuraH.MikiS.OzakiM.AbeT.MatsumotoK.IshimuraChem. Commun.49201353345336
- N.G.AndersonA.P.ButlerContrast Media Mol. Imaging92014312
- K.HayashiM.NakamuraH.MikiS.OzakiM.AbeT.MatsumotoT.KoriK.IshimuraAdv. Funct. Mater.242014503513
- P.MoreauExpert Rev. Hematol.72014265290
- K.HayashiM.NakamuraH.MikiS.OzakiM.AbeT.MatsumotoK.IshimuraAdv. Funct. Mater.22201235393546
- H.MaedaJ.WuT.SawaY.MatsumuraK.HoriJ. Control. Release652000271284
- K.HayashiM.NakamuraW.SakamotoT.YogoH.MikiS.OzakiM.AbeT.MatsumotoK.IshimuraTheranostics32013366376