?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Blends of a naturally occurring clay (0–30 wt.%) and phosphate sludge were heated at different temperatures and times and their microstructures were investigated using impedance spectroscopy, dilatometry, X-ray diffraction and scanning electron microscope. The weights of the effects of the change of temperature, soaking time and clay addition on some physical ceramic properties (shrinkage, water absorption and compressive strength) were assessed. For the latter purpose, the response surface methodology was used. The results showed that the sintering process was effective between 750 and 1000 °C and occurred by melt flow. It was accompanied with low activation energy for ionic conduction (0.20–0.35 eV). Due to the quantitative formation of gehlenite (the unique neoformed phase), the ionic conduction regressed and the melt formation was limited. Also, it was shown that the effects of the experimental factors on physical properties of the blends were well described with the adopted polynomial models, and the weights of the effects of the factors followed the order: temperature > clay content > soaking time. The effects of the interactions between the factors on the properties studied were evaluated and discussed in relation to the microstructure change.
1 Introduction
Phosphate sludge is a by-product commonly composed of fluorapatite, clays, quartz and carbonates [Citation1]. Because of the presence of plastic materials, filler and flux, it could be suitable for ceramics manufacturing. However, because of shortage of alumina and silica, it should be enriched with aluminosilicates such as clays [Citation1].
The physical properties of ceramics are tightly related to the microstructure, which in turn depends on, among others, the firing cycle parameters [Citation2], the shaping method [Citation3], the mineralogical and the chemical compositions of the starting materials [Citation4,Citation5]. To evaluate the effect of these parameters, the microstructure changes are monitored for instance by thermal analysis, dilatometry and examined by electron microscopes. Recently, it has been reported that impedance spectroscopy (IS) is a practical technique to characterize the microstructure of ceramics [Citation6–Citation8]. By the help of this technique, changes due to grains and defects (grain boundaries, pores, etc.) could be distinguished [Citation9–Citation11].
Much less attention has been paid to the potential use of phosphate waste for industrial ceramics manufacturing. Moreover, to the best of our knowledge, no study has been carried out on the sintering process of phosphate waste-based ceramics using impedance spectroscopy.
The aim of this work was to monitor the microstructure of heated clay-phosphate waste blends using, among others, impedance spectroscopy. Furthermore, the effects of the firing cycle parameters and the clay content on some physical ceramic properties were investigated. For the latter goal, the response surface methodology was applied.
2 Materials and methods
2.1 Materials
The phosphate sludge (PS) was from the beneficiation plants of the phosphate rocks of Gantour (Youssoufia, Morocco). The alumina- and silica-bearing material was a swelling raw clay (SC) from the phosphate basin area. The chemical and mineralogical compositions of both materials are given in . In this respect, it may be noted that the amounts of the identified minerals were estimated by the Rietveld method, and the chemical compositions were determined with inductively coupled plasma atomic emission spectroscopy (ICP-AES), using a Perkin Elmer Optima 3100 RL apparatus. Some physical characteristics of PS and SC are given elsewhere [Citation1].
Table 1 Chemical and mineralogical compositions (in wt.%) of the phosphate sludge (PS) and the raw clay (SC).
2.2 Experimental procedures
Blends of the phosphate sludge and the clay (up to 30 wt.%) were damped (water/solid = 40%) and kneaded for 30 min. Pellets (<2 cm diameter) were shaped from the paste and dried at ambient temperature, then at 105 °C. The samples were heated at 900–1100 °C for up to 4 h.
The impedance spectroscopy measurements were carried out at 650–1100 °C using a Solartron SI 1260 impedance analyzer. The voltage used was 2000 mV and the frequency varied from 0.1 to 106 Hz.
Phase identification was performed by means of X-ray diffraction (XRD) and scanning electron microscope (SEM). The XRD patterns were recorded with a Philips X’Pert MPD diffractometer equipped with a copper anode (Kα = 1.5418 Å). The SEM examinations were carried out with a JEOL JMS 5500 apparatus equipped with an EDAX Falcon spectrophotometer. For this purpose, the samples were coated with a thin layer of gold.
Shrinkage was measured by following the sample dimensions before and after heating. For water absorption measurement, the weights of the heated sample were determined before and after immersion in boiled water for 2 h. The compressive strength of the heated samples was measured with an Instron 3369 apparatus. The load and loading speed were 50 kN and 0.1 mm/min, respectively.
2.3 Experiments design
The effects of the clay content (τ), sintering temperature (T) and soaking time (t) on a physical property (Y) of the sample were evaluated by using the following equation [Citation12,Citation13]:X1, X2 and X3 are the coded variables related to the real factors (τ, T and t) as follows:
τ0, T0 and t0 are the clay content, sintering temperature and soaking time at the centers of the experimental domains (τ0 = 17.5 wt.%, T0 = 1050 °C and t0 = 2 h). Δτ, ΔT and Δt are the variation steps of the natural variables (Δτ = 12.5 wt.%, ΔT = 150 °C and Δt = 2 h).a0 is a constant, and a1, a2 and a3 are the weights of the effects of τ, T and t, respectively. aii is considered as a curve shape parameter, and aij represents the weight of the effect of interactions between i and j factors. The coefficients were determined by least-squares regression using the software: New Efficient Methodology for Research using Optimal Design (Nemrod) [Citation14]. For this goal, 16 experiments, planned according to the Doehlert matrix design, were realized. The planned experiments and the experimental values of the corresponding properties are given in .
Table 2 Experimental design matrix (Doehlert matrix) and the measured values of the studied properties (Y1: firing shrinkage (%); Y2: water absorption (%); Y3: compressive strength (MPa)).
The validity of the above model was verified by, among others, the analysis of variance (ANOVA) [Citation15].
3 Results and discussion
3.1 Impedance measurements and dilatometry analysis
The Nyquist plots of the heated blends displayed a single hemicircle, such as seen in . The diameter of the hemicircles decreased with increasing temperature. Seeing that the diameter can be taken as the bulk resistance [Citation16], the electrical resistance of the samples diminished as temperature increased. In fact, the increase of temperature has a marked effect on the reduction of blocking defects of the microstructure such as pores and grains boundaries [Citation17].
Fig. 1 Nyquist plots related to the pellets of phosphate sludge and phosphate sludge-clay (30 wt.%). (a and b) Ordinary plot; (a′ and b′) Magnified plot.
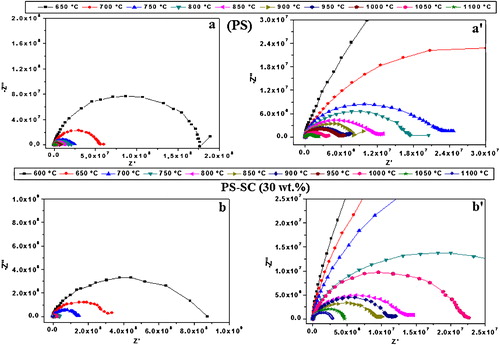
The impedance spectrum of ceramic material is regularly composed of two adjacent arcs: one is linked to the grains and the other to the grain boundaries [Citation18]. The occurrence of a single semicircle () lets to think that the signal corresponded to the effect of the bulk [Citation16,Citation19,Citation20]. The unresolved effects of grains and grain boundaries could be linked to the presence of fast conduction paths along the surface of grains. Such a phenomenon prevails in the case where the microstructure consists of grains with large surface area together with the presence of less retained ions [Citation16].
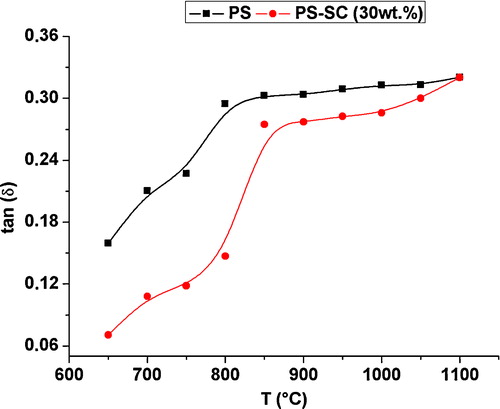
The plot of ln σ = f(1/T) (σ is the conductivity of the samples; σ = σ0 exp(−Ea/kT), σ0 is a pre-exponential term, k is a constant of Boltzmann and Ea is the energy of activation) displayed three distinguished branches (). Thus, the sintering process involved three different phenomena, depending on the range of temperature. The energies of activation in the discriminated ranges 650–750 °C, 750–1000 °C and T > 1000 °C are reported in . It is worth noting that Ea was relatively low in the intermediate interval of temperature, which is considered as the main domain of sintering. The change of the microstructure beyond about 800 °C was accompanied with a marked increase in the dielectric loss (). This could be due to the presence of melt, which played a chief role in the sintering process [Citation21,Citation22]. In fact, the melt formation was expected since the samples comprised fluxing oxides ().
Fig. 2 Arrhenius-type plots of the conductivity of the phosphate sludge and the phosphate sludge-clay (30 wt.%) blend.
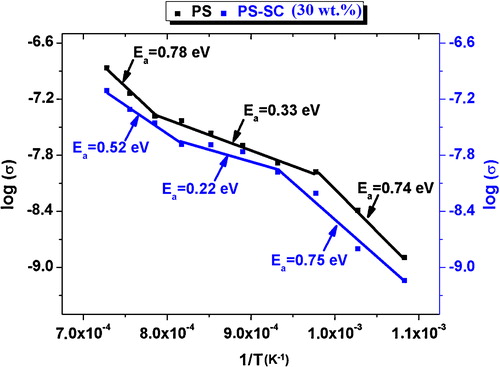
Fig. 3 Loss tangent obtained from impedance measurement for the phosphate sludge and the phosphate sludge-clay (30 wt.%) as a function of the sintering temperature.
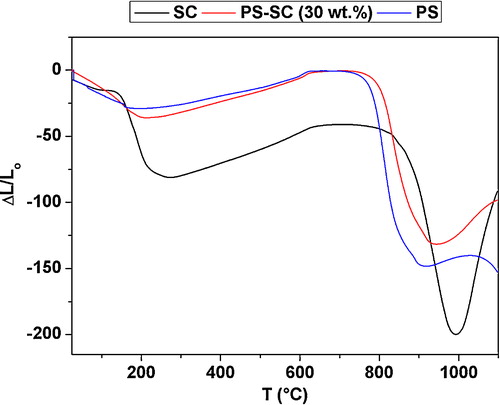
The main stage of the sintering process was accompanied by an abrupt shrinkage (). In this respect, it may be noted that samples were the subject of expansion between 200 and 600 °C, and T > 1000 °C. Because of its insignificant intensity and less energetic aspect, the former expansion was mainly due to the dehydroxylation of the clay mineral (montmorillonite) and the escape of structural water [Citation23]. The second expansion could be related to the neoformation process, which is investigated hereafter. As far as the dilatometric analysis was concerned, the shrinkage observed at T < 250 °C was essentially attributed to dehydration and the loss of the interlayer water of the swelling clay mineral [Citation23].
3.2 Microstructural investigation
Taking into consideration the mineralogical compositions of the basic materials () and the results of the X-ray diffraction analysis of the heated blends (), montmorillonite and carbonates decomposed at T < 900 °C, whereas quartz and fluorapatite persisted. In addition, gehlenite formed at low temperature. As shown in , the content of gehlenite increased with the increase of temperature, while those of quartz and fluorapatite diminished. Gehlenite (ideal chemical formula: Ca2Al2SiO7) formed from the products of carbonates and clay mineral [Citation24,Citation25], and developed at the expense of quartz and fluorapatite. SEM examinations revealed, in addition to the above-mentioned phases (a and b), the presence of a vitreous phase (c) and magnesia, which derived from decomposed dolomite.
Fig. 5 X-ray diffraction patterns. (A) Phosphate sludge samples heated at (a) 900 °C, (b) 1000 °C and (c) 1100 °C. (B) Blend of phosphate sludge-clay (30 wt.%) fired at (a) 900 °C, (b) 1000 °C and (c) 1100 °C. F: fluorapatite (PDF # 71-0880); G: gehlenite (PDF # 77-1146); Q: quartz (PDF # 5-0490).
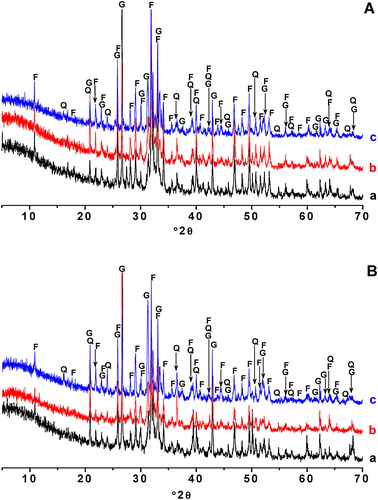
Fig. 6 Evolution of relative X-ray intensities of the main reflexions of quartz, fluorapatite and gehlenite versus heating temperature.
Fig. 7 SEM micrographs showing particles of gehlenite (G), quartz (Q), fluorapatite (F) and vitreous phase (A).

In conformity with the above comment, the observed melt (vitreous phase) played a key role in the sintering process, and improved markedly the electrical conductivity in the range of 750–1000 °C. The abundance of gehlenite at T > 1000 °C was responsible for the above increase of the energy of activation. The development of the calc-aluminosilicate phase was accompanied by a reduction of the melt [Citation26]. In such condition, sintering would mainly proceed by solid-state diffusion. The relative high value of Ea determined at 600 < T < 750 °C was chiefly due to the presence of the amorphous products of the clay mineral.
3.3 Effects of the experimental factors on physical properties of the heated blends
The ANOVA results showed that the Fisher variance ratio (F-ratio) ≫1 and the signification exceeded 99.9% (). Moreover, the values of the correlation coefficient (R2) approached unity, and those of the standard deviation were low (). These data pointed out that the adopted polynomial model described well the variation of the ceramic physical properties against the experimental parameters.
Table 3 Analysis of variance (ANOVA) and values of the fitting coefficient (R2) and the standard deviation (σ) related to the adopted models.
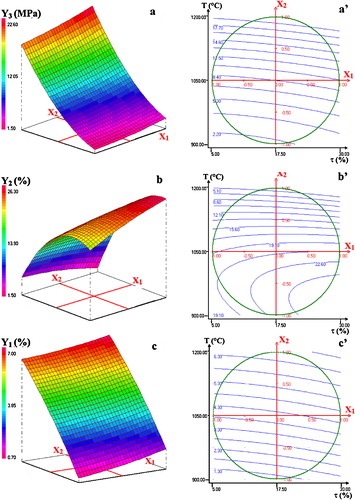
Hereafter are reported the obtained equations for shrinkage (Y1), water absorption (Y2) and compressive strength (Y3):The analysis of these equations showed that temperature was the most influencing factor, and its increase affected positively all properties. Indeed, the increase of T favored the formation of melt and activated the diffusion process; thereby the consolidation of the matrix was improved. The second important affecting factor for water absorption and mechanical strength was the clay content. By contrast with the effect of the increase of T, clay additions had an adverse impact.
As a calc-aluminosilicate mineral, gehlenite formed from decomposed clay mineral and dolomite; thereby its amount increased with clay additions. In such condition, porosity increased and the melt content diminished, and subsequently water absorption increased and compressive strength regressed.
The weight of the effect of the soaking time on the change of the above properties was less significant. Based on the algebraic values of the linear coefficient a3, the increase of the soaking time should contribute to the increase of water absorption and to the decrease of strength and shrinkage. These facts may have a relation with the positive effect of the soaking time on the development of gehlenite.
The effects of the interactions between the factors on the properties studied were evaluated based on the algebraic values of aij [Citation12,Citation13]. In this respect, it may be noted that the simultaneous increase of the clay quantity and temperature had substantial negative effect on the compressive strength, presumably because of the abundance of gehlenite. The antagonistic effects of these factors on the mechanical resistance are well visualized by the response surface of a. For water absorption and shrinkage, τ and T manifested synergistic effects, i.e., the simultaneous increase of the factors contributed to the increase of water absorption and shrinkage. The latter effects can be deduced from the plots of b and c.
4 Conclusion
The results of the impedance spectroscopy and the dilatometry analysis showed that the main stage of the sintering process of the clay-sludge blends started beyond 750 °C and extended up to about 1000 °C. During this phase, the ionic conductivity was easier and the energy of activation for ionic conduction was reduced by a factor of three. The microstructure examinations indicated that the sintering process occurred mainly by melt flow, and gehlenite formed at low temperature (T < 900 °C) from the decomposition products of the clay and carbonates. The increase of the sintering temperature resulted in the reduction of the amounts of quartz and fluorapatite because of their involvement in the neoformation process of gehlenite. The development of gehlenite, which happened at high temperature, limited the formation of the melt.
The ceramic physical properties were well correlated to the firing cycle parameters and clay additions using polynomial models. In this connection, it may be concluded that the increase of temperature had a positive and strong effect of strength and water absorption, because of its favorable influence on the melt formation. Clay additions had an adverse effect, since it favored the formation of gehlenite. The change of the soaking time had a minor impact. Regarding the effects of the mutual interactions between the factors, it may be concluded that the weight of the effect of the simultaneous increase of a couple of factors on the studied properties could not be neglected, especially in the case of the mechanical strength.
Notes
Peer review under responsibility of The Ceramic Society of Japan and the Korean Ceramic Society.
References
- M.LoutouM.HajjajiM.MansoriC.FavottoR.HakkouJ. Environ. Manage.1302013354360
- M.N.RahamanSintering of Ceramics2008CRC Press, Taylor & Francis Group
- M.J.RibeiroD.U.TulyaganovJ.M.F.FerreiraJ.A.LabrinchaJ. Mater. Process. Technol.14812004139146
- H.H.MurrayClay Miner.341199939
- M.C.SteilJ.FouletierM.KleitzP.LabruneJ. Eur. Ceram. Soc.1961999815818
- R.MuccilloJ.A.CerriE.R.LeiteE.LongoJ.A.VarelaMater. Lett.3011997125130
- C.M.S.RodriguesJ.A.LabrinchaF.M.B.MarquesJ. Eur. Ceram. Soc.182199895104
- L.DessemondR.MuccilloM.HenaultM.KleitzAppl. Phys. A: Mater.57119935760
- M.KleitzL.DessemondR.JimenezF.PetitbonR.HerbinE.MarchandProc. 2nd Int. Forum on European Solid Oxide Fuel CellOslo, Norway1996317324
- L.DessemondJ.GuindetA.HammouM.KleitzProc 2nd Int. Symp. on Solid Oxide Fuel Cells1991409416
- M.KleitzC.PescherL.DessemondProc. Int. Conf. on Science and Technology of Zirconia, VMelbourne16211992
- D.NjoyaM.HajjajiD.NjopwouoAppl. Clay Sci.652012106113
- A.KhalfaouiM.HajjajiS.KacimA.BaçaouiJ. Am. Ceram. Soc.895200615631567
- D.MathieuR.P.T.LuuSoftware Nemrod1980Université d’Aix-Marseille IIIFrance
- D.MathieuR.Phan-Tan-LuuJ.J.DroesbekeJ.FineG.SaportaApproche méthodologique des surfaces de réponse in Plans d’expériences1997TechnipParis211277
- X.WangP.XiaoJ. Eur. Ceram. Soc.2242002471478
- X.WangNon-destructive characterisation of structural ceramics using impedance spectroscopy (PhD Theses)2001Department of Mechanical Engineering, Brunel UniversityLondon, UK
- E.N.S.MuccilloM.KleitzJ. Eur. Ceram. Soc.1641996453465
- M.J.RibeiroJ.C.C.AbrantesJ.M.FerreiraJ.A.LabrinchaJ. Eur. Ceram. Soc.2415200438413848
- J.DuB.JonesM.LanaganMater. Lett.5922200528212826
- M.HajjajiS.KacimM.BoulmaneAppl. Clay Sci.2132002203212
- I.FukumotoY.KandaJ. Sol. Mech. Mater. Eng.352009739747
- D.N.TodorThermal Analysis of Minerals1976Abacus Press
- T.PetersR.IbergAm. Ceram. Soc. Bull.5751978503509
- M.HajjajiS.KacimBr. Ceram. Trans.103120042932
- M.HajjajiA.KhalfaouiConstr. Build. Mater.2322009959966