Abstract
As novel green pigment, various sodium iron phosphates, Na4Fe7(PO4)6, NaFe4(PO4)3, and Fe3(PO4)2, imitated with Xenophillite and Vivianite, were synthesized using a hydrothermal process. The obtained powders were estimated with X-ray diffraction (XRD), Infrared (IR) spectra, ultraviolet–visible (UV–vis) reflectance spectra, and L*a*b* color space. The hydrothermal temperature and time, volume of water, phosphorus resource were studied in this process. All samples prepared in this work were dark green powders. The weak peaks of Na4Fe7(PO4)6 and Fe3(PO4)2 were observed in XRD patterns of all samples. Hydrothermal treatment of a long duration produced high greenish powders. The large volume of water used improved the greenish and bluish colors of the phosphate powders.
1 Introduction
Recently, the use of harmful metals is restricted around world. However, because suitable substitutes have not been obtained, some materials containing harmful metals have been used in many fields [Citation1,Citation2]. For example, inorganic color pigments containing metals such as mercury, cadmium, and lead, have some merits, including high light stability, heat resistant coloring visibility, cost, etc. [Citation3 Citation2013;Citation5]. In addition, because oxide pigments have low coloring and covering, they are difficult to use for paint and plastics [Citation6]. Sulfate and nitrate pigments have lower heat resistance than oxide pigments, and require harmful and/or combustible gas to synthesize. Furthermore, it is difficult to obtain sulfide and nitrate pigments with repeatability [Citation7,Citation8]. Therefore, novel inorganic pigments are required with suitable properties and without difficult production methods.
There are some kinds of inorganic green pigments, for example, that are available for use, green earth, CuCO3Cu(OH)2, Zn–Co oxide, Cr2O3, Co–Zn–Cr–Al oxide, and so on [Citation9 Citation2013;Citation12]. However, they have some problems, there is a limited volume of green earth, which is distributed unevenly. These pigments also include harmful metals. Therefore, novel green pigment without precious and harmful metals is required. We focus on the natural ores, Xenophillite, Na4Fe7(PO4)6, and Vivianite, Fe3(PO4)2, because these ores have no precious and harmful metals [Citation13,Citation14]. Natural ores have high light stability and heat resistance. Because of their solidity, they are expected to have applications for plastics, paint, ceramics, and so on. Because iron cation is bivalent in Xenophillite and Vivianite, they have a dark green color. Because the compounds with trivalent iron cation are generally brown in color phase, the valence of iron is important to prepare the novel green pigments imitated with these ores.
In this work, novel inorganic green pigments imitated with natural ores, Na4Fe7(PO4)6 and Fe3(PO4)2, were synthesized in hydrothermal method and then estimated from the viewpoint of pigment. The middle composition, NaFe4(PO4)3, between Na4Fe7(PO4)6 and Fe3(PO4)2, was also treated using the same methods.
2 Experimental
Sample (2 g, 7Fe-sample) imitated of Xenophillite, Na4Fe7(PO4)6, was prepared as follows. Iron powder was mixed with ammonium di-hydrogen phosphate, sodium di-hydrogen phosphate in the following reaction:(1)
To improve the reaction, 5 mL of water was added to the mixture, and then let stand for 1 day. The mixture was treated with hydrothermal treatment at 120, 140, 160, and 180 °C for 1, 3, and 6 h. To clarify the suitable condition, the volume of water was changed to 10 and 20 mL. Other samples imitated of NaFe4(PO4)3 (Na2Fe8(PO4)6; 8Fe-sample) and Fe3(PO4)2 (Vivianite, Fe9(PO4)6, 9Fe-sample) were also prepared using the same method. The chemical equations are as follows:(2)
(3)
The phosphorus resource for 9Fe-sample was changed from NH4H2PO4 to H3PO4 in following reaction (9Fe′-sample).(4)
The obtained samples were dried and then analyzed. All chemicals were of commercial purity (Wako Chemical Industries Ltd., Osaka, Japan) and were used without further purification.
The chemical compositions of these materials were analyzed using X-ray diffraction (XRD) and Infrared (IR) spectra. The XRD patterns were recorded on an X-ray diffractometer (MiniFlex, Rigaku Corp.) using monochromatic CuKα radiation. IR spectra of samples were recorded on a HORIBA FT-IR 720 (Horiba Ltd.) using the KBr disk method.
The color of phosphate pigments was estimated from the ultraviolet–visible (UV–vis) reflectance spectra (UV2100; Shimadzu Corp., Kyoto, Japan) (reference compound: BaSO4). The color of the pigments was also estimated with a TES135 plus color analyzer (TES Electrical Electronic Corp, Taipei, Taiwan). The L* value means the whiteness of powder, in which 100 is white, on the opposite site, 0 is black. The a* value means the redness of materials, with positive (maximum; +60) and negative (−60) values are corresponding with red and green, respectively. The b* value indicates the yellowish, in which positive (maximum; +60) and negative (−60) values correspond with yellow and blue, respectively.
3 Results and discussion
3.1 Xenophillite composition
First, we tried to obtain the green pigment only by heating the mixtures; however, black and brown powders were obtained. When the iron did not react sufficiently, the materials became black powder. On the opposite site, when the powder iron reacted with phosphate to iron (+III) salts, the materials became brown powder. To control the oxidation of iron, a hydrothermal treatment was adopted in this experiment.
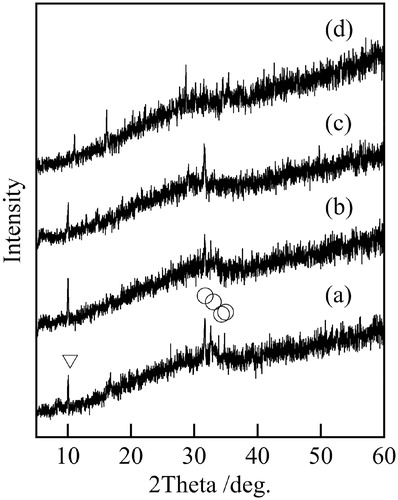
Fig. 1 XRD patterns of samples prepared at various temperatures (1 h, 5 mL), (a) 120 °C, (b) 140 °C, (c) 160 °C, (d) 180 °C, ○; Na4Fe7(PO4)6, ▽; Fe3(PO4)2.
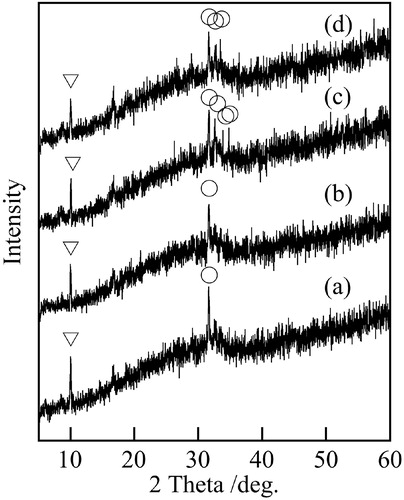
Fig. 2 IR spectra of samples prepared at various temperatures, (a) 120 °C, (b) 140 °C, (c) 160 °C, (d) 180 °C (1 h, 5 mL).
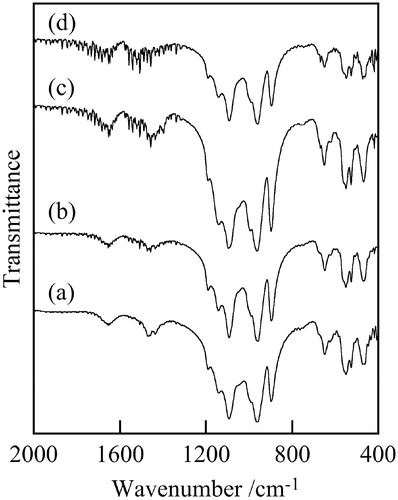
shows XRD patterns of samples prepared at various temperatures. All samples had weak peaks of Na4Fe7(PO4)6 and Fe3(PO4)2. The crystallization of materials was insufficient because of the low temperature of the hydrothermal process. The influence of hydrothermal temperature was small in XRD patterns. shows the IR spectra of samples prepared at various temperatures. The peaks at 1090, 960, 900, and 650 cm−1, due to phosphate anion were observed in IR spectra of all samples [Citation15]. Therefore, all samples were phosphate materials.
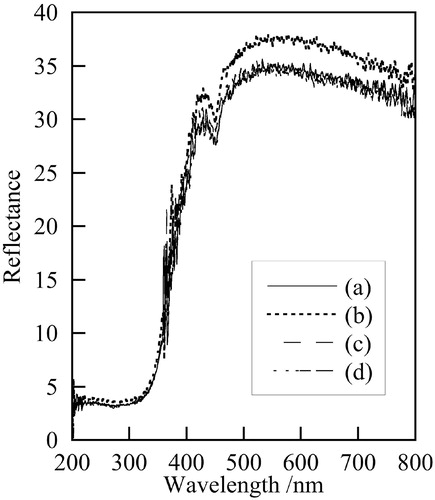
shows photographs of samples prepared at various temperatures. All samples were dark green powder. Samples prepared at 140 °C and 180 °C had a little brighter color than those at 120 °C and 160 °C. shows UV–vis reflectance spectra of samples prepared at various temperatures. Samples prepared at 140 °C indicated higher reflectance than others. Because all samples had broad reflectance at 500–600 nm, they were dark green powder. shows the color of 7Fe-sample powders prepared in various conditions. Samples prepared at 140 °C indicate high L* value, which corresponds with . All samples had from −3 to +1 in a* value and from 2 to 8 in b* value, which corresponds to dark green. The a* and b* value became small with increasing of hydrothermal time.
Table 1 Color of 7Fe-sample powders.
Fig. 3 Photographs of samples prepared at various temperatures, (a) 120 °C, (b) 140 °C, (c) 160 °C, (d) 180 °C (1 h, 5 mL).
Fig. 4 UV–vis reflectance spectra of samples prepared at various temperatures, (a) 120 °C, (b) 140 °C, (c) 160 °C, (d) 180 °C (1 h, 5 mL).
To improve the reaction, the volume of water used was varied in 7Fe-sample preparations. However, the peak intensity was little changed by the volume of water. shows the color of samples prepared with various amount of water. Samples prepared with 10 mL of water indicated higher L* value than others. When the materials included the unreacted iron, the color of pigments became dark (low L* value). Samples with high L* value means that iron reacted with phosphate easily under this condition. The a* value became smaller with the increase of water. Lowest b* value was obtained in sample prepared with 20 mL of water. The volume of water had influence on the color of phosphate pigments.
Table 2 Color of 7Fe-sample powders (hydrothermal treatment; 120 °C, 1 h).
3.2 Other compositions
To obtain the variety of color phase, the chemical composition and phosphorus resources were changed. shows XRD patterns of samples prepared at various compositions. All samples were near amorphous state in XRD patterns, because of low temperature in hydrothermal process. The peaks due to Na4Fe7(PO4)6 disappeared in XRD patterns of samples prepared at Vivianite composition (9Fe, 9Fe′). shows the color of samples prepared in various conditions. The 9Fe-samples had lower L* values than others, because the unreacted iron in materials was black. Samples prepared with H3PO4 (9Fe′) indicated enough high L* value compared with those prepared with NH4H2PO4 (9Fe), because H3PO4 easily reacts with iron powder. The 8Fe-samples indicated high b* value, dark yellowish green. The novel green pigments imitated with Vivianite were prepared using the same process with Xenophillite.
Table 3 Color of sample powders (hydrothermal treatment; 1 h).
4 Conclusions
The novel green phosphate pigments imitated with Xenophyllite and Vivianite were obtained by hydrothermal process. All samples were near amorphous state in XRD patterns, because of the low temperature used in hydrothermal process. From IR spectra, all samples were phosphate materials. Hydrothermal treatment for a long duration produced high greenish powders. A large volume of water improved the greenish and bluish color of the phosphate powders. Samples prepared with H3PO4 indicated enough high L* value compared with those prepared with NH4H2PO4.
References
- A.AmatC.MillaniS.FantacciRSC Adv.620163633636344
- Z.TaoW.ZhangY.HuangD.WeiH.J.SeoSolid State Sci.3420147884
- M.RadepontY.ConquinotK.JanssensJ.J.EzratiM.CotteJ. Anal. Atomic Spectrom.302015599612
- F.RosiC.GraziaF.GabrieliA.RomaniM.PaolantoniR.VivaniB.G.BrunettiP.ColombanC.MilianiMicrochem. J.1242016856867
- M.LiJ.WandQ.MaJ. Cultural Herit.1642015575578
- S.JiangL.PengR.GuoD.MiaoS.ShangJ.XuA.LiCeram. Int.421620161938619392
- C.MigureiJ.V.PintoM.ClarkeM.J.MeloDyes Pig.1022014210217
- M.OhashiK.KusumotoT.SugiyamaK.KatoJ. Ceram. Soc. Jpn.12492016959962
- F.OspitaliD.BersaniG.D.LonardoP.P.LotticiJ. Raman Spectrosc.39200810661073
- B.GilbertS.DenoëlG.WeberD.AllartAnalyst28200312131217
- S.LiangH.ZhangM.LuoK.LuoP.LiH.XuY.ZhangCeram. Int.40201443674373
- H.R.HedayatiA.A.S.AlvaniH.SameieR.SalimiS.MoosakhaniF.TabatabaeeA.A.ZarandiDyes Pig.1132015588595
- V.Kostov-KytinR.I.KostovP.IvanovaRev. Bulg. Geolog. Soc.741–32013111130
- D.C.ReedB.G.GustafssonC.P.SlompEarth Planet Sci.434152016241251
- D.E.C.CorbridgeE.J.LoweJ. Chem. Soc.493195445554564