Abstract
Molecular sieves or zeolites are important widespread industrial materials of unique chemical structure, which are used in most industrial plants as an adsorbent for gases or even as catalysts.
1 Introduction
Zeolites are crystalline, hydrated alumino-silicates of group I and II elements, in particular, sodium, potassium, magnesium, calcium, barium and strontium. Over 150 synthetic and 40 naturally occurring zeolites are known (Marcus and Cormier, Citation1999). Initially only natural zeolites are used but more recently synthetic forms have been made on a industrial scale giving rise to tailor made zeolites that are highly replicable. Structurally, zeolites are framework alumino-silicates, which are based on infinitely extending three dimensional AlO4 and SiO4 tetrahedrals linked to each other by sharing all the oxygen (Berck, Citation1974; Richardson, Citation1989"). They can be represented by the empirical formula:In this oxide formula, x is generally equal to or greater than 2 since tetrahedral AlO4 are joined only to tetrahedral SiO4 and n is the valency of the cation.
They have three properties that make them unique and deserving of a separate category:
| (1) | They are highly crystalline with a well defined structure that encloses the aluminum silicate framework cavities occupied by large ions and water molecules, the cavities opening ranging from 0.8 to 1 nm in diameter, which are of the order of molecular dimensions. The size and shape of these pores determine which molecules enter the cavities and which are excluded, so they are called molecular sieves. | ||||
| (2) | Cations within the cavities are easily replaced with a large number of different valency cations which exert a large number of electrostatic or polarizing forces across the small dimensions of the cavity. | ||||
| (3) | The introduced cation into the cavities by ion exchange will have separate activities of their own; this facilitates the opportunity of dual function catalysts involving acidity with other activities. Zeolite A is synthesized in the sodium form and has the general chemical formula: | ||||
According to the Database of Zeolite Structure (Citation2002), Zeolites type A are classified into three different grades, 3A, 4A and 5A, all of which posses the same general formula but have different cation types. When 75% of its sodium is replaced by potassium, it is referred to as zeolite (3A). Alternatively replacing of sodium by calcium gives rise to type (5A) one.
Synthetic zeolites are widely used in the industry as an adsorbent for various gases and vapors (Chandrasekhar and Pramada, Citation1999) and as catalysts for many petroleum industries of great importance in industrial application as described by Speight (Citation1999). They are used for drying of gases and liquids from low humidity where it shows a higher adsorption capacity than other adsorbents. They have a high tendency to adsorb water and other polar compounds, for examples, NH3, H2S, SO2 and CO2 and a good capacity at very low temperatures if compared with other adsorbents.
Traditionally, zeolites are commercially produced from hydro gels of sodium aluminate and silicate and was the work of Copperwaite et al. (Citation1986). However, production of zeolites from clay, as a source of alumina and silica are being continuously investigated with positive results. Of the work done by Atta et al. (Citation2007) for the synthesis of Faujasite zeolites from Kankara kaolin clay and Chandrasekhar and Pramada (Citation1999) for zeolite synthesis using Kerala kaolin.
The aim of this manuscript is to prepare zeolite type 4A from Iraqi kaolin and characterize and test its activity.
2 Materials and methods
2.1 Materials
2.1.1 Kaolinite clay
The kaolinite clay used in the present investigation was supplied from Doukhla site in the west part of Iraq with the chemical composition of Al2Si2O5(OH)4.
2.1.2 Sodium hydroxide
8 M sodium hydroxide solution was used in the preparation of 4A zeolite for the ion exchange procedure.
2.2 Preparation of zeolite type 4A
The preparation was done using the following steps:
2.2.1 Calcination of the raw kaolinite
A process in which the kaolinite was exposed to a high temperature of about 550 °C for about 1.5 h where its structure is destroyed and any undesired volatile matter was removed and converted to a metakaolinite, a material, which readily accepts and exchanges sodium as a guest in its structure and is having the chemical composition given in .
Table 1 The chemical composition of Doukhla kaolinite used in the preparation of type 4A zeolite.
The chemical reaction in this step is:
2.2.2 Ion exchange
The procedure of Rollmann et al. (Citation1982) was employed, the above produced metakaolinite was treated with sodium hydroxide solution with a ratio of 1:5 and reflux heated to 90 °C with continuous vigorous stirring for 4 h in order to insert the sodium ion in the metakaolinite structure where the following reaction occurs:Concentration of sodium hydroxide higher than the usual one was experimented in order to accelerate the ion exchange process because we used raw materials and not pure aluminate and silicate.
2.3 Washing, filtration and drying
The treated kaolin slurry was left to settle for several hours and was washed three times with distilled water to remove the excess unreacted sodium hydroxide. Then it was filtered by any suitable mean and dried in an oven at 110 °C for 3 h.
2.4 Formulation and calcination
This is important because most fixed bed reactors necessitate that zeolites have to be formulated in to spheres, extrudates or tablets. Zeolite powder obtained above was crushed, milled and a suitable grain size (Bashir, Citation1997; Flank, Citation1989") was mixed with 15% raw metakaolinite and 30% water to make a paste that can be easily dealt with and then passed through a laboratory extruder to obtain extrudates of about 1.6 × 3 mm dimensions; this was then dried at 80 °C for 3 h and finally calcined for another 4 h at 500 °C.
2.5 Characterization
The elemental composition analysis of raw kaolin and prepared zeolite was obtained using X-ray fluorescence (Siemens D500) and metal content analysis being determined by standard atomic absorption method. Pore volume was determined experimentally using liquid impregnation method as described by Satter and Charles (Citation1980), Richardson (Citation1989), Karim (Citation2010)". Hardness was determined with the ERWEKA.TBH28 hardness meter (Le Pag and Miquel, Citation1976) while crushing value was determined by placing 25 g of the sample under pressure of 18 kN for 20 min and measuring the amount of powder that passed through 53 μm mesh as a percent relative to the total weight. Loss on ignition (L.O.I) was calculated from the weight being lost after roasting the sample at 900 °C for 3 h.
For powder, X-ray diffraction patterns were recorded using a Phillips X-ray diffract meter and the peaks were compared and assigned by comparison with the published data from the international zeolite association (Database of Zeolite Structure, Citation2002) and those of typical compounds given in ASTM (Citation1986 powder diffraction files).
The bulk density was determined by placing a weight catalyst in a graduated cylinder then shaking it by a vibrator and reading the volume of it and then calculating the density, i.e., it is the packing or load density of the reactor.
2.6 Testing of the efficiency
The adsorption capacity of any adsorbent as a dimensionless quantity was defined as the weight of adsorbate being adsorbed by 100 g of adsorbent; it can be represented as a percent ratio or as milliequivalent adsorbate. It was tested for the prepared samples as explained by Aderemi (Citation2004), ASTM (Citation1986), Armor (Citation1995)" in a simple laboratory designed apparatus where air saturated with water vapor is passed over a previously weighted sample of 4A zeolite and the increase in weight that resulted from the adsorption of water on zeolite is then monitored and measured after a suitable time.
3 Results and discussion
The chemical analysis of the prepared type 4A zeolite, shown in , indicates that the major components of zeolite present in the structure, in addition to trace amounts of impurities associated the raw kaolin used in the preparation, cannot be removed through out the preparation procedure used and this has no effect on the activity as will be seen later.
Table 2 Chemical analysis of prepared zeolite, type 4A.
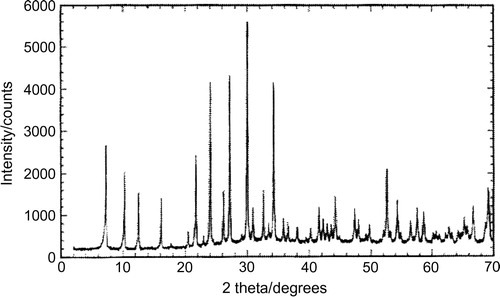
A powder X-ray diffraction pattern from prepared type 4A zeolite is shown in . This pattern is just a plot of the X-ray intensity scattered from the sample versus the scattering angle (Bragg angle, 2θ). The positions and intensities of the peaks in the diffraction pattern are a fingerprint of the crystalline components that are presents in the sample. In , we list the peak positions and intensities (area under the peaks) that can be expected for 4A zeolite pure sample. Additional peaks in the powder diffraction pattern would indicate the presence of a crystalline impurity. The nature of the impurity could be established and it is not essential that the identity of the sample be confirmed exactly by powder diffraction as the synthesis is reliable (see ).
Table 3 Powder diffraction peaks positions and intensities for prepared zeolite 4A.
The physical properties of the prepared samples are demonstrated in , which shows that a relatively good adsorption capacity of 23 was obtained, a value that corresponds to 92% efficiency based on maximum adsorption capacity of 25 in most zeolites type 4A. In addition to that other properties such as crushing strength, attrition, porosity etc., which are not very far or even consistent with those mentioned in the literatures by Atta et al. (Citation2007) and documented in the Database of Zeolite Structure (Citation2002).
Table 4 The major properties and characteristics of the prepared zeolites 4A.
The main object in the preparation is to select clay that satisfies the stochiometric ratio of silicate and aluminate to build the AlO4 and SiO4 in the zeolite structure.
4 Conclusions
We may state that it is possible to use Iraqi kaolinite clay to prepare type 4A zeolite by converting it to metakaolinite via calcinations at 550 °C and then using sodium hydroxide for ion exchange to insert the sodium ion into its structure. The structure was characterized by XRD spectroscopy and water adsorption capacity of 92% efficiency was obtained.
References
- B.O.AderemiPreliminary studies on synthesis of zeolites from locally clayNiger. J. Sci. Res.422004712
- J.N.ArmorMolecular sieve for air separation, material chemistryAdv. Chem. Ser.131995245
- ASTM, 1986. Annual Book of ASTM Standards, Catalysis, D 4058-81 and D 4179.
- A.Y.AttaO.A.AjayiS.S.AdefilaSynthesis of Faujasite zeolites from Kankara kaolin clayJ. Appl. Sci. Res.310200710171021
- Bashir, B.N., 1997. Preparation of Zeolite Binder Agglomerates from Locally Available Raw Material as Cylindrical Pellets, M.Sc. Thesis, University of Baghdad, Iraq.
- D.W.BerckZeolite Molecular Sieves1974John Wiley & Sons, Inc.USA
- S.ChandrasekharP.N.PramadaInvestigation on the synthesis of zeolites NaX from Kerala kaolinJ. Porous. Mater.641999283297
- R.G.CopperwaiteG.T.HutchingsM.Van Der RietPreparation and evaluation of a synthetic zeolite catalyst: an undergraduate chemistry laboratory experimentsJ. Chem. Educ.71986632634
- Database of Zeolite Structures, 2002. <www.zeolitesethz.ch/zeolites/StdAtlas.htm/>.
- Flank, W.H., 1989. Process for Preparing Molecular Sieve Bodies, US Patent, 4818 508.
- H.H.KarimRegeneration and activity test of spent zinc oxide hydrogen sulfide removal catalystEur. J. Sci. Res.3922010289295
- J.F.Le PagJ.MiquelB.DelmonP.A.JacobsG.Poncelt>Preparation of Catalysts1976ElsevierAmsterdam3943
- B.K.MarcusW.E.CormierGoing green with zeolitesChem. Eng. Prog.95619994753
- J.T.RichardsonPrinciples of Catalysts Developments1989Plenum PressNew York and London
- L.D.RollmannE.ValyocsikR.ShannonZeolite molecular sievesInorg. Synth.2219826168
- S.SatterN.CharlesHeterogeneous Catalysis in Practice1980McGraw-Hill, Inc.New York, USA
- J.G.SpeightThe Chemistry and Technology of Petroleum1999Marcel Dekker, Inc.New York