Abstract
Ni-impregnated nickel over mesoporous silica with different Ni contents has been synthesized and the resulting samples were characterized by elemental analysis, N2 sorpometry at 77 K, XRD, TEM and temperature programmed reduction (TPR). The mesoporous nickel-containing materials showed both high activity and high selectivity for benzylation of benzene. More interesting is the observation that this catalyst is always active and selective for large molecules like naphthenic compounds such as methylnaphthalene and it can also be reused in the benzylation of benzene for several times.
1 Introduction
Friedel–Crafts alkylations comprise a very important class of reactions which are of common use in organic chemistry. These reactions are habitually catalyzed by Lewis acids in liquid phase (Olah, Citation1973), and the substitution of liquid acids by solid acid catalysts is a challenging task. The alkylation of benzene by benzyl chloride is interesting for the preparation of substitutes of polychlorobenzenes used as dielectrics. In homogeneous phase this reaction is catalyzed at the industrial scale by AlCl3, FeCl3, BF3, ZnCl2 and H2SO4 (Olah, Citation1973; Olah et al., Citation1985). The new environmental legislation pushes for the replacement of all liquid acids by solid acid catalysts which are environmentally more friendly catalysts and which lead to minimal pollution and waste. Indeed, several solid acid catalysts have already been proposed which are efficient catalysts such as: Fe-modified ZSM-5 and H-β zeolites; Fe2O3 or FeCl3 deposited on micro-, meso and macro-porous (Choudhary et al., Citation2002a); Fe-containing mesoporous molecular sieve materials (He et al., Citation1998; Bachari et al., Citation2004); Ga- and Mg-oxides and chlorides derived from Ga–Mg-hydrotalicite (Choudhary et al., Citation2002b); Ga-SBA-15 (El Berrichi et al., Citation2006); Ga-HMS (Bachari and Cherifi, Citation2006a); transition metal chloride supported mesoporous SBA-15 (Bachari and Cherifi, Citation2006b); supported thallium oxide catalysts (Choudhary and Jana, Citation2001); Sb supporting K10 (Deshpande et al., Citation2001); Si-MCM-41-supported Ga2O3 and In2O3 (Choudhary et al., Citation2000a); alkali metal salts and ammonium salts of keggin-type heteropolyacids (Izumi et al., Citation1995); ion-exchanged clays (Cseri et al., Citation1995); Cu-HMS (Bachari and Cherifi, Citation2006c); Pt(II) clays, Cu(II) clay, and sol–gel hybrid materials of Cu clay (Choudhary and Misra, Citation2011); nanocrystalline sulfonated titania systems (Devi et al., Citation2012); chrysotile-supported transition metal salts (Pinto et al., Citation1995); different ferrites viz. CuFe2O4, NiFe2O4, CoFe2O4, ZnFeO4, MgFe2O4 (Shinde and Sawant, Citation2003) for the benzylation of benzene and other aromatic compounds.
The discovery of the new family of mesoporous silica molecular sieves with pore diameters in the 2.0–10 nm range, designated as M41S, is of considerable interest for heterogeneous catalysis and material science (Kresge et al., Citation1992). Depending on the synthesis conditions, different phases could be obtained, like the hexagonal phase MCM-41, the cubic one MCM-48 as well as the lamellar compound MCM-50. Furthermore, another pathway was proposed by Tanev et al. (Citation1994) to prepare mesoporous silicas at room temperature by neutral templating route (S0I0). In this case, the organic surfactant is not quaternary ammonium cation but a primary amine, and the assembly involves hydrogen-bonding interactions between neutral primary amines and neutral inorganic precursors. These materials denoted HMS (hexagonal mesoporous silica), reveal excellent catalytic capacity for macro molecular reactions and suggest new opportunities for transition metal incorporation into silica frameworks. In the present work, we report the synthesis and characterization of such materials impregnated nickel and their test as catalysts for the benzylation of benzene with benzyl chloride. The kinetics of the reaction over these catalysts have been investigated and the reaction has been extended to other substrates like toluεne, p-xylene, anisole, naphthalene and methylnaphthalene.
2 Experimental
2.1 Materials
Samples were synthesized with hexadecylamine (Aldrich), tetraethyl orthosilicate (TEOS, Aldrich), nickel-nitrate (Ni(NO3)2·6H2O, Merck) and ethanol (Rh𢦬ne – Poulenc).
2.2 Catalysts preparation
The HMS material was prepared following the pathway reported by Tanev et al. (Citation1994). In a representative preparation, hexadecylamine (HDA) (0.3 mol) was added to a solution containing water (36 mol) and ethanol (EtOH) (7 mol) and the mixture was stirred until homogeneous. Then 1 mol of tetraethyl orthosilicate (TEOS) was added under vigorous stirring. This solution was then stirred at room temperature for 24 h to obtain the products. The solids were recovered by filtration, washed with distilled water, and air-dried at 393 K. Organic molecules occluded in the mesopores were removed by solvent extraction. The dried precursor was dispersed in ethanol (5 g/100 ml) and the mixture was refluxed under vigorous stirring for 2 h. The solid was then filtered and washed with cold ethanol. The extraction procedure was repeated twice before drying the samples at 393 K in an oven. Finally the samples were calcined at 823 K in air for 6 h. Impregnated mesoporous materials Ni (%)/HMS with Ni (%) = 2 and 8 are prepared as follows: the amount of nickel nitrate is added to 5 g of pure silica mesoporous material (HMS) and 50 g of methanol. The mixture is agitated at ambient temperature during 2 h, the solvent is then rapidly evaporated under vacuum and the solid is calcined under air at 723 K overnight at a heating rate of 1 K/min.
2.3 Characterization of the samples
The chemical compositions of the samples were determined by a combination of wet chemical methods and atomic absorption spectrometry (HITACHI Z 800). Powder X-ray diffraction patterns were recorded on SIEMENS D500 diffractometer with Cu-Kα radiation. They were recorded with 0.02° (2θ) steps and 1 s counting time per step over two angular domains from 1° to 10° (2θ) and from 10° to 80° (2θ). Adsorption/desorption experiments using N2 were carried out at 77 K on a NOVA 2000 porosimeter (Quantachrome) instrument. The N2 isotherms were used to determine the specific surface areas and pore volumes and diameters of the samples using the BET equation and BJH theory (Garbowski and Praliaud, Citation1988). Transmission electron microscopy (TEM) measurements were performed with a Philips CM10 electron microscope operated at 100 kV. Temperature programmed reduction (TPR) experiments were performed with a TPDRO1100 apparatus from Thermo Quest CE Instruments. For the TPR measurements the calcined catalyst samples were pelletised, ground and sieved. Sieve fractions of 425–850 μm were placed in a fixed bed reactor and used for analysis. A flow containing 5% hydrogen and 95% argon (Hoek Loos) was passed downward through the catalyst bed at a rate of 20 ml min−1. After water removal from the outcoming flow (mol sieves) hydrogen consumption was measured using a tungsten thermal conductivity detector.
2.4 Catalytic testing
The benzylation reactions over a series of impregnated nickel over mesoporous silicas catalysts were carried out in a magnetically stirred glass reactor (25 cm3) fitted with a reflux condenser, having a low dead volume, mercury thermometer and arrangement for continuously bubbling moisture free nitrogen N2 (flow rate = 30 cm3 min−1) through the liquid reaction mixture, at the following reaction conditions: reaction mixture = 15 ml of moisture-free liquid aromatic compound (or 2.5 ml of moisture-free aromatic compound mixes with 12.5 ml of moisture-free solvent) + 1.0 ml of benzyl chloride, amount of catalyst = 0.1 g and reaction temperature = 353 K. The reaction was started by injecting benzyl chloride in the reaction mixture, containing catalyst and aromatic compound with or without solvent. Measuring quantitatively the HCl evolved in the reaction by acid–base titration (by absorbing the HCl carried out by N2 in a 0.1 M NaOH solution containing phenolphthalein indicator) followed the course of the reaction. The polybenzyl chloride (which is formed by the condensation of benzyl chloride) was isolated from the reaction mixture by the procedure given by Choudhary et al. (Citation2000b). In all the cases, the major product formed was mainly mono-benzylated compound along with polybenzyl chloride as side product depending upon the condition used. Samples were analyzed periodically on a gas chromatograph (HP-6890) equipped with a FID detector and a capillary column RTX-1 (30 m × 0.32 nm i.d.). The products were also identified by GC–MS (HP-5973) analysis.
3 Results and discussion
3.1 Characterization
The results of the chemical composition and characteristics of the catalysts are given in . The nickel compositions of the solids corresponded relatively well to those fixed for the synthesis. The specific surface areas of the solids were very larger, which were typical mesoporous materials (Tanev et al., Citation1994). When the nickel content increased, they decreased slightly. In the other hand, a very narrow pore size distribution, centered on 3 nm, was calculated from nitrogen physisorption data. Furthermore, a very high pore volume (0.96 ml g−1) was measured. This high value reflects the very high surface area of the support.
Table 1 Chemical composition and characteristics of the catalysts.
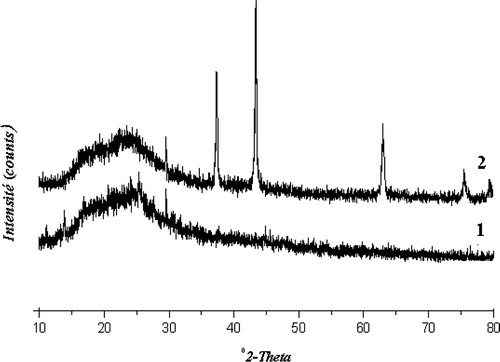
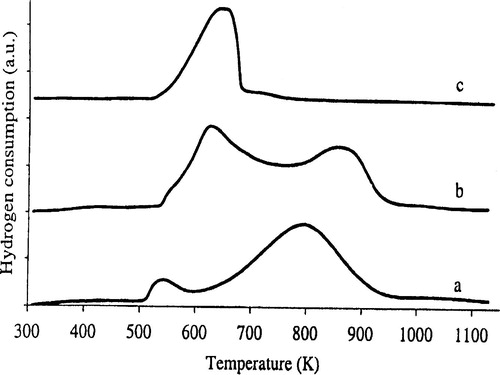
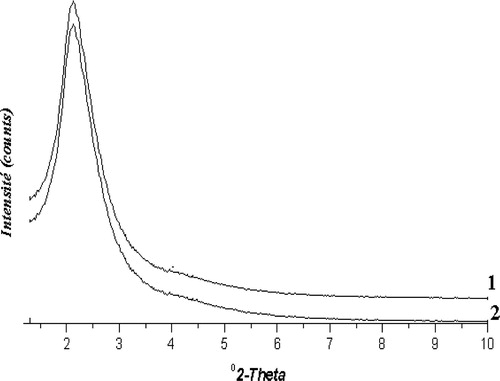
The X-ray powder diffraction patterns of the solids showed a broad peak at (2θ) = 2.2° () characterizing an amorphous materials not well-crystallized. The intensity of the peak decreased slightly when the nickel content increased showing that the addition of nickel has not a negative effect on the crystallinity. At the same time, X-ray diffractograms of the solids in the 10–80° (2θ) range displaying the diffraction pattern characteristic of nickel oxide are presented in . In fact, only, the Ni (8%)/HMS displays intense and well resolved NiO peaks, indicating that relatively large nickel oxide particles are present. TEM analyses of the catalysts indicated that large nickel oxide particles had formed with the catalyst Ni (8%)/HMS (11.3 nm), whereas no large nickel oxide particles could be visualized with the Ni (2%)/HMS catalyst. Reduction plots of the catalysts are given in . To enable comparison a reduction plot of bulk NiO, obtained from Ni(NO3)2 by calcination, is also included. Indeed, TPR analysis was used to attain information about the reducibility of the deposited nickel oxide of the catalysts. In the case of the catalyst Ni (8%)/HMS two large reduction stages are observed. The large low-temperature reduction stage coincides with the reduction of bulk NiO, indicating the presence of nickel oxide with a bulk character. As a consequence, the nickel oxide particles associated with this reduction stage have to be rather large. Thus, the second reduction stage is associated with the presence of very small nickel oxide particles, stabilized by the HMS framework. The catalyst Ni (2%)/HMS mainly contains small nickel oxide particles, which are demonstrated by the high temperature of reduction. Nevertheless, the very small low-temperature reduction peak points to the presence of some slightly enlarged particles too, which are not observed with the other characterization techniques.
Figure 1 DRX patterns of the Ni (%)/HMS catalysts in the domain of 1–10° (2θ). (1) Ni (2%)/HMS, (2) Ni (8%)/HMS.
3.2 Catalytic performances of Ni(%)/HMS materials in the benzylation of benzene
A comparison of the catalytic properties of the solids tested is presented in . The pure silicic compound (HMS) was totally inactive. The other compounds showed an activity increasing with their nickel content. However, the selectivity to diphenylmethane remains constant.
Table 2 Catalytic properties of the catalysts in the benzylation of benzene with benzyl chloride at 348 K, Bz/BzCl ratio = 15 and mcat = 0.1 g.
3.3 Reaction kinetics
The kinetic data for the benzene benzylation reaction in excess of benzene (stoichiometric ratio Bz/BzCl = 15) over the Ni (8%)/ HMS catalyst could be fitted well to a pseudo-first-order rate law: Log [1/1 − x] = (ka/2.303)(t − t0) where ka is the apparent first-order rate constant, x the fractional conversion of benzyl chloride, t the reaction time and t0 the induction period corresponding to the time required for reaching equilibrium temperature. A plot of Log [1/1 − x] as a function of the time gives a linear plot over a large range of benzyl chloride conversions.The effect of temperature on the rate was studied by conducting the reaction at 343, 348, and 353 K under the standard reaction conditions (stoichiometric ratio Bz/BzCl = 15 and 0.1 g catalyst). The results showed that the catalytic performances of the catalyst strongly increased with the reaction temperature ( and ). By contrast, the selectivity to diphenylmethane remains approximately constant. The activation energy estimated thus obtained was 96.7 kJ mol−1. In fact, this value can probably suggest that no interference of diffusional limitations is existed. Two Bz/BzCl ratios have been investigated. The results obtained are reported in . It appears that the stoichiometric ratio between benzene and benzyl chloride has a strong influence on the selectivity to diphenyl methane. With a low ratio, the secondary reactions to dibenzyl benzenes and tribenzyl benzene were favored. Results showing the influence of different substituent groups attached to aromatic benzene nucleus on the conversion of benzyl chloride in the benzylation of corresponding substituted benzenes at 353 K over the Ni (8%)/HMS catalyst are presented in . According to the classical mechanism of the Friedel–Crafts type acid catalyzed benzylation reaction, the benzylation of an aromatic compound is easier if one or more electron donating groups are present in the aromatic ring (Olah, Citation1973). Hence, the order for the rate of benzylation for the aromatic compound is expected as follows: anisole > p-xylene > toluene >> benzene. But, what is observed in the present case is totally opposite to that expected according to the classical mechanism. The first-order rate constant for the benzylation of benzene and substituted benzenes is in the following order: benzene > toluene > p-xylene > anisole. This indicates that, for this catalyst, the reaction mechanism is different from that for the classical acid catalyzed benzylation reactions. In fact, the probable redox mechanism for the activation of both benzyl chloride and benzene by these catalysts leading to the benzylation of benzene reaction is proposed:where M = Ni; n = 2 and m = 1.
Figure 4 The conversion vs reaction time plots for benzylation of benzene at: (▾) 343 K, (𥭟) 348 K, (▪) 353 K.
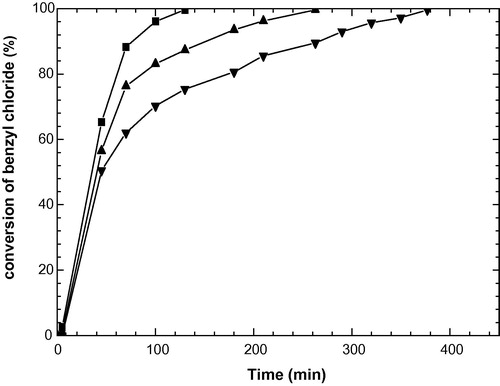
Table 3 Catalytic activities of Ni (8%)/HMS at different temperatures: 343, 348 and 353 K, Bz /BzCl ratio = 15 and mcat = 0.1 g.
Table 4 Influence of the stoichiometric ratio between benzene and benzyl chloride for the benzylation of benzene at 348 K over Ni (8%)/HMS catalyst.
Table 5 Catalytic properties of substituted benzenes and substituted naphthalene at 353 K over Ni (8%)/HMS catalyst, Bz/BzCl ratio = 15 and mcat = 0.1 g.
The redox mechanism is similar to that proposed earlier for the benzene benzylation and acylation reactions (Choudhary and Jana, Citation2001; Cseri et al., Citation1995; Brio et al., Citation2000). Furthermore, in order to rule out the influence of a sterric effect on the rate of reaction, we have applied the Taft relation (March, Citation1985). According to this relation when a sterric effect influences the reaction, there is a linear relation between the rate and the parameter Es values considered to be representative of the size of the substituting group of the studied aromatic compounds. Using the Es parameter tabulated by Charton (March, Citation1985) we have shown that such a relation did not exist. Furthermore, the catalyst Ni (8%)/HMS is always active and selective for larger molecules like naphthenic compounds such as methylnaphthalene (). The large pores of the mesoporous support permit the conversion of these molecules that could not be done on other supports. In conclusion, the kinetic data for the benzene benzylation reaction have been found over Ni (8%)/HMS catalyst. This catalyst possesses larger amount of bulk NiO (confirmed by XRD, TEM and TPR) and provides a better catalytic activity with greater independence to the effect of substituents. This result shows that the bulk NiO can be admitted as the active site in this reaction. Thus, the proposed redox mechanism for the reaction of benzylation used in our contribution could be thoroughly considered. Moreover, this mechanism has been supported by Hammett relationship.
3.4 Effect of solvent
To understand the role of solvent in benzylation of benzene by nickel-containing samples with benzyl chloride, the reaction was carried out with different solvents, such as dichloroethane and and n-heptane. The reaction conditions and the results of benzene benzylation with Ni (8%)/HMS catalyst are presented in . The conversion of the catalyst decreased in the presence of aprotic solvent due to the interaction of negative charge or the electron lone pair of solvent with acidic sites of the catalyst. The reaction rate is highest in the absence of any solvent. It is decreased when the solvent (via dichloroethane and n-heptane) is used, the decrease is quite large when n-heptane is used as a solvent but it is small for dichloroethane as a solvent. In conclusion, the results show that between the two solvents, dichloroethane is a better solvent for the benzylation reaction.
Table 6 Effect of solvent on the conversion of benzyl chloride at 353 K in the benzylation of benzene over Ni (8%)/HMS, Bz/BzCl ratio = 15 and mcat = 0.1 g.
3.5 Recycling of the catalysts
The stability of the catalysts has been studied by running the reaction successively with the same catalyst (Ni (8%)/HMS) under the same conditions without any regeneration between two runs. The reaction was first run under the standard conditions (benzene to benzyl chloride ratio of 15, 353 K) to the complete conversion of benzyl chloride. Then after a period of 10 min another quantity of benzyl chloride was introduced in the reaction mixture leading to the same benzene to benzyl chloride ratio. After the achievement of the second run, the same protocol was repeated a second time. The results, presented in , showed that the catalyst could be used several times in the benzene benzylation process without a significant change of its catalytic activity.
Table 7 Effect of recycling of the catalysts in the benzylation of benzene with benzyl chloride at 353 K over Ni (8%)/HMS catalyst, Bz/BzCl ratio = 15 and mcat = 0.1 g.
4 Conclusion
In conclusion, Ni(%)/HMS catalysts show remarkable activities for the benzylation of aromatics. The mechanism involves a redox step at the reaction initiation. This gives a greater independence to the effect of substituents, and these catalysts can therefore be used with substrates of low reactivity. Furthermore, the large pores of the mesoporous catalyst do not limit the size of the molecules that could be reacted.
References
- K.BachariO.CherifiGallium-containing mesoporous silicas as a catalyst for alkylation of benzene and other aromatics by benzyl chlorideJ. Mol. Catal. A: Chem.2532006187191
- K.BachariO.CherifiStudy of the benzylation of benzene and other aromatics by benzyl chloride over transition metal chloride supported mesoporous SBA-15 catalystsJ. Mol. Catal. A: Chem.26020061923
- K.BachariO.CherifiBenzylation of benzene and other aromatics by benzyl chloride over copper-mesoporous molecular sieves materialsCatal. Commun.72006928930
- K.BachariJ.M.M.MilletB.BenaichoubaO.CherifiF.FiguerasBenzylation of benzene by benzyl chloride over iron mesoporous molecular sieves materialsJ. Catal.22120045561
- K.BrioS.BekassyB.AgaiF.FiguerasHeterogeneous catalysis for the acetylation of benzo crown ethersJ. Mol. Catal. A: Chem.1512000179184
- T.ChoudharyN.M.MisraRole of clay as catalyst in Friedel–Craft alkylationBull. Mater. Sci34201112731279
- V.R.ChoudharyS.K.JanaB.P.KiranHighly active Si-MCM-41-supported Ga2O3 and In2O3 catalysts for Friedel–Crafts-type benzylation and acylation reactions in the presence or absence of moistureJ. Catal.1922000257261
- V.R.ChoudharyS.K.JanaB.P.KiranHighly active and moisture-insensitive solid catalysts – GaCl3 and InCl3 supported on montmorillonite-K10 and Si-MCM-41 for benzylation of benzeneCatal. Lett.642000223226
- V.R.ChoudharyS.K.JanaHighly active and low moisture sensitive supported thallium oxide catalysts for Friedel–Crafts-type benzylation and acylation reactions: strong thallium oxide–support interactionsJ. Catal.2012001225235
- V.R.ChoudharyS.K.JanaA.S.MammanBenzylation of benzene by benzyl chloride over Fe-modified ZSM-5 and H-β zeolites and Fe2O3 or FeCl3 deposited on micro-, meso- and macro-porous supportsMicropor. Mesopor. Mater.5620026571
- V.R.ChoudharyS.K.JanaV.S.NarkhedeBenzylation and benzoylation of substituted benzenes over solid catalysts containing Ga- and Mg-oxides and/or chlorides derived from Ga–Mg-hydrotalcite by its HCl pre-treatment or calcinationAppl. Catal. A: Gen.2352002207215
- T.CseriS.BekassyS.RiznerF.FiguerasBenzylation of aromatics on ion-exchanged claysJ. Mol. Catal. A: Chem.981995101107
- A.B.DeshpandeA.R.BajpaiS.D.SamantThe enhanced activity of Sb after supporting on K10 in the benzylation of benzene using benzyl chloride and benzyl alcoholAppl. Catal. A: Gen.2092001229235
- K.R.P.S.DeviP.B.SreejaS.SugunanEnvironmentally benign Freidel–Crafts benzylation over nano TiO2/SO42Int. Nano Lett.220123039
- Z.El BerrichiL.CherifO.OrsenJ.FraissardJ.P.TessonnierE.VanhaeckeB.LouisM.J.LedouxC.Pham-HuuGa doped SBA-15 as an active and stable catalyst for Friedel–Crafts liquid-phase acylationAppl. Catal. A2982006194202
- Garbowski, E., Praliaud, H., 1988. Les techniques physiques d’étude des catalyseurs. In: Imelik, b., Védrine, J.C. (Eds.) Editions Techniq-Paris, p. 115.
- N.HeS.BaoQ.XuFe-containing mesoporous molecular sieves materials: Very active Friedel–Crafts alkylation catalystsAppl. Catal. A: Gen.16919982936
- Y.IzumiM.OgawaK.UrabeAlkali metal salts and ammonium salts of Keggin-type heteropolyacids as solid acid catalysts for liquid-phase Friedel–Crafts reactionsAppl. Catal. A: Gen.1321995127140
- C.T.KresgeM.E.LeonowiczW.J.RothJ.C.BeckOrdered mesoporous molecular sieves synthesized by a liquid-crystal template mechanismNature3591992710712
- J.MarchAvanced Organic Chemistrythird ed.1985Wiley/InterscienceNew York
- G.A.OlahFriedel–Crafts Chemistry1973Wiley/InterscienceNew York
- G.A.OlahG.K.S.J.PrakashJ.SommerSuperacids1985Wiley/InterscienceNew York
- R.O.PintoJ.A.R.RodriguesP.J.S.MoranI.JoekesChrysotile-supported transition metal salts as Freidel–Crafts catalystsJ. Braz. Chem. Soc.61995373376
- M.M.ShindeM.R.SawantLiquid phase Freidel–Crafts alkylation over mixed metal oxide catalyst(J. Chinese Chem. Soc.50200312211226
- P.T.TanevM.ChibweT.J.PinnavaiaTitanium-containing mesoporous molecular sieves for catalytic oxidation of aromatic compoundsNature3681994317321