Abstract
This work involves the influence of high pressure on some thermodynamical properties of solid aluminum (Al) using a 3rd and 4th order Birch–Murnaghan equation of state based on the finite Eulerian strain theory.
1 Introduction
The equation of state (EOS) of a system basically describes the relationships among thermodynamic variables such as pressure, temperature and volume. The study of equation of state for solids has been extremely useful in the field of geophysics and condensed matter physics (Stacey et al., Citation1981; Anderson, Citation1995).
The isothermal EOS expresses the relation between pressure and volume at constant temperature while the relation between the volume and temperature at constant pressure is known as the isobaric EOS. However, the isochoric EOS relates temperature and pressure at constant volume (Tripathi et al., Citation2006).
It should be mentioned that the EOS expression for pressure (P), bulk modulus (KT) and its derivatives () and (
) are not easy to obtain (Sushil et al., Citation2004).
The EOS has been derived by many authors based on different physical assumptions. Among these assumptions, the equation of state depends on finite-strain theory. This theory has played a very significant role in the development of equation of state (EOS) for solid (Stacey et al., Citation1981).
Stacey recently developed a method for obtaining higher order finite-strain equations which can be used for deriving and obtaining EOS. Other researches have obtained the EOS based on the finite Eulerian strain or the finite Lagrangian strain theories (Stacey, Citation2001). The equation of state based on the Eulerian strain yields good agreement with the EOS developed by Kushwah et al. (Citation2007) and also with the generalized Rydberg EOS (Shanker et al., Citation2009). It is well known that the formulation based on the Eulerian strain is more appropriate than that based on the Lagrangian strain, since the Taylor series expansion for free energy in powers of the strain parameter is more convergent in the case of the Eulerian strain (Birch, Citation1952, Citation1986). The Lagrangian strain is the strain relative to the unstrained state, whereas the Eulerian strain represents the strain relative to the strained state (Sushil et al., Citation2004). The general form of strain (f) in terms of volume compression (V/V0) is given by (Holzapfel, Citation1996)(1) where x = (V/V0), V0 is the volume at P0, V is the volume at P. n is the power constant that depends upon the type of strain. For example, the value of n for the Eulerian strain is equal to 2 (Stacey, Citation2001).
The aim of this work is to study some thermodynamical properties for solid aluminum (Al) under high pressure using the 3rd and 4th order Birch–Murnaghan EOS based on the finite Eulerian strain theory. This study includes the variation of relative compression volume, isothermal bulk modulus (KT) and the first derivative of bulk modulus () with pressure. Moreover, the effect of high pressure on the melting temperature of solid (Al) was investigated using the Mie–Gruneison–Debye equation based upon the 3rd and 4th order Birch–Murnaghan EOS, Lindemann and Kumar’s equations.
2 Theory
2.1 The isothermal Birch–Murnaghan EOS based on Eulerin strain
The Birch–Murnaghan EOS (B–M EOS) based on the Eulerian strain theory has been widely used for understanding high pressure behavior of solids (Gaurav et al., Citation2002). The Eulerian strain (fe) can be obtained from Eq. Equation(1)(1) by substituting n = 2. Therefore
(2) The B–M EOS is obtained by expanding a series of powers of the Eulerian strain (Birch, Citation1952).
The 2nd order isothermal EOS is a function of two measurable parameters, x and isothermal bulk modulus (K0) at P = 0, so that P = f(x, K0).
Similarly, the 3rd and 4th order isothermal B–M EOS, have the forms and
, respectively.
The coefficient of the term of degree 3 in the expansion can be neglected in many cases (corresponding to ) so that the equation of state at the 2nd order is good enough. However Stacey et al. (Citation1981) and Anderson (Citation1995) remarked that there is a truncation problem, since the coefficient of the term of degree 4 of the expansion is larger than that of the term of degree 3.
The 2nd, 3rd and 4th B–M EOS are written as below:(3)
(4)
(5) where
(6)
(7)
(8)
(9) where K0 is the isothermal bulk modulus at P0,
is the first pressure derivative of bulk modulus and
is the second pressure derivative of bulk modulus.
2.2 Isothermal bulk modulus (KT) of 3rd and 4th order Birch–Murnghan EOS
Results obtained from the P–V relationship in a pressure study give information regarding higher order thermodynamic parameters. This isothermal P–V relationship yields bulk modulus and is defined as (Sushil et al., Citation2004)(10) the bulk modulus for 3rd and 4th B–M EOS is written as below:
(11) and
(12)
2.3 The pressure derivative of bulk modulus ( ) at constant temperature
) at constant temperature
The pressure derivative of KT for the 3rd and 4th order B–M EOS is given:(13)
(14)
3 Result and discussion
The pressure has been calculated at different relative compression volumes ranging from 1 to 0.65 for solid aluminum (Al) using the 3rd and 4th order B–M EOS equations Equation(4)(4) and Equation(5)
(5) . The input parameters V0 = 10 cm3/mole, isothermal bulk modulus K0 = 72.7 GPa at P0 and its first pressure derivative
are taken from Hänström and Lazor (Citation2000). The value of the second pressure derivative of K0 has been calculated from the following equation (Stacey, Citation2000, Citation2001):
(15) where the value of
is obtained from Hama and Suito (Citation1996).
shows a comparison between the calculated pressure (P) as a function of relative compression (V/V0) for the 3rd and 4th order B–M EOS with experimental data obtained from Hänström and Lazor (Citation2000) and Boehler and Ross (Citation1997).
Figure 1 Comparison between the calculated isothermal compression curves for Al at room temperature using B–M EOS with experimental data.
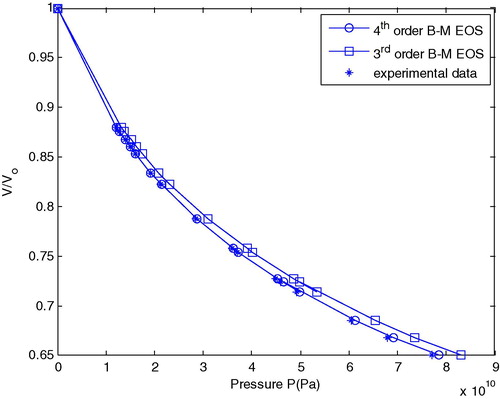
From this figure one can see that the relative compression volume decreases continually with the increase in pressure. It also indicates that up to 20 GPa, the compression curve coincides with experimental data. Beyond this and up to 100 GPa, the 3rd order B–M EOS diverges less than the 4th order B–M EOS in which it satisfies Eq. Equation(5)(5) alongside experimental data.
The isothermal bulk modulus (KT) and its first pressure derivative () have been calculated at different relative compression volumes from 1 to 0.65 for solid Al using the 3rd and 4th order B–M EOS according to Eqs. (11)–(14). The same input parameter values were used as above.
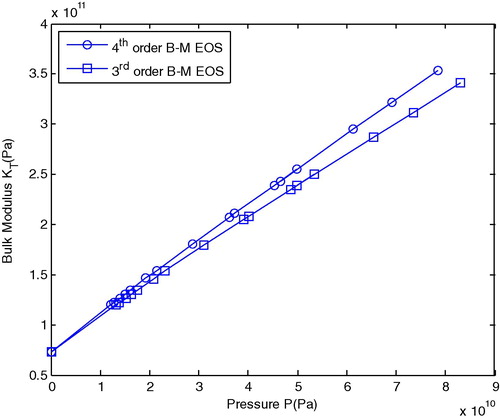
shows the isothermal bulk modulus (KT) versus pressure for the 3rd and 4th order B–M EOS at room temperature.
shows that the isothermal bulk modulus (KT) increases continuously with an increase in pressure and which satisfies the equation of compressibility Equation(10)(10) . The increase in pressure at constant temperature will reduce the volume for which dV will be negative. Thus KT is increased above 20 GPa, the 3rd order B–M EOS slightly differs from the 4th order B–M EOS. This concept can be explained as due to the pressure of the term
originally at the 4th order B–M EOS, while the 3rd order B–M EOS does not contain that term.
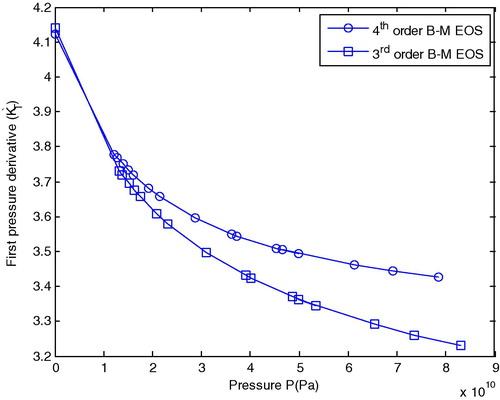
shows the first pressure derivative () and isothermal bulk modulus (KT) as a function of pressure for the 3rd and 4th order B–M EOS at room temperature.
This figure shows that the first pressure derivative of isothermal bulk modulus () decreases as pressure increases for both Eqs. Equation(13)
(13) and Equation(14)
(14) and the results coincide with each other. It has been seen that above 20 GPa, the results of these equations do not coincide, but diverge away from each other. We can interpret this as the original 3rd order B–M EOS does not contain the term
. The most important reason for this is that
provides a close control of the curvature of the plot of
versus P for the 4th order B–M EOS.
4 The effect of high pressure on melting temperature of solid
4.1 Preamble
The effect of pressure on melting temperature studied by Clapeyron was deduced a latent heat equation from Maxwell’s thermodynamical relation to obtain the following equation (Cohen and Weitz, Citation1996):(16) Determination of the pressure dependence of the melting temperature of solids is so important in condensed matter. A number of different theoretical expressions for the pressure dependence of melting temperature of solids have been discussed in the literature (Schlosser et al., Citation1989).
4.2 EOS of the melting curve of solid
Our calculation follows the recommendation of Gilvary (Citation1956), who suggested that Tm(P) could be found by first calculating P(Vm) coupled with Tm(Vm), where Tm(Vm) is found by the Lindemann law. Gilvary used the Murnghan EOS and the Lindemann formula in its simplest form, i.e. where γ (first Grunisen parameter) is independent of Vm while in this work we do not assume that γ is independent of Vm.
The melting equation of state appropriate to temperature above Tm0 (Tm0 is the temperature of melting at ambient pressure, i.e. P = 0 GPa) is(17) which is the EOS of melting curve and called Mie–Grunisen–Debye EOS where
(18) where ED is the harmonic internal energy according to Debye theory and here
(19) is given by Anderson (Citation1995), where R is the gas constant and Tm is the melting temperature along melting curve at any P
(20) where γm0 is the first Gruneisen parameter at zero pressure and melting temperature (Tm0), q is the second Gruneisen parameter = 1, the equation of state P(V, T300) (Hofmeister, Citation1993; Hama and Suito, Citation1996) and equal isothermal EOS Equation(4)
(4) and Equation(5)
(5) .
Vm is melting molar volume at Tm and Pm (GPa) and is given by Kumer (Citation1995, Citation1996)" and is equal to(21) where Vm0 is melting molar volume at Tm0 and P = 0 GPa, B0 is bulk modulus at P = 0 GPa, A = δT + 1 and δT is Anderson Gruneisen parameter.
4.3 Lindemann’s equation
One of the oldest and most widely used attempts to predict the melting curves of solids is the Lindemann (Citation1910) equation. Lindemann assumed that a solid melts when the mean-square amplitude of vibrations of atoms about their equilibrium position become larger than a certain fraction of the lattice spacing. Lindemann then straightforwardly arrived at a relationship between the melting temperature (Tm) and melting volume (Vm).
The famous Lindemann’s equation is written as below:(22) The melting gradient (dTm/dPm) can be obtained from Eq. Equation(22)
(22) as
(23) where KT is the bulk modules of solid and is a function of compression and temperature (Kumer, Citation1995, Citation1996).
5 Result and discussion
The melting pressure (Pm) and melting temperature (Tm) have been calculated for solid Al using the 3rd and 4th order B–M EOS.
To calculate the melting temperature (Tm), Eq. Equation(22)(22) has been used. The melting pressure (Pm) has been worked out using Eq. Equation(17)
(17) . The first term of Eq. Equation(17)
(17) represented the value of P obtained from the 3rd and 4th order B–M EOS which is illustrated in . The second term of Eq. Equation(17)
(17) Pth(Vth, Tm) has been calculated using Eqs. Equation(19)
(19) and Equation(20)
(20) . To calculate the melting volume (Vm), Eq. Equation(21)
(21) has been used, while the melting gradient has been calculated by using Eq. Equation(23)
(23) . The basic physical specific values of Al are Vm0 = 10.65 cm3/mol, Tm0 = 933 K, γm0 = 2.42, q = 1, Km0 = 58.71 GPa obtained from Kumer (Citation1995, Citation1996)" and R = 8.31 J/mole.
shows a comparison between the calculated melting temperature (Tm) versus (Vm/Vm0) using the 3rd and 4th order B–M EOS with experimental data obtained from Hänström and Lazor (Citation2000) and Boehler and Ross (Citation1997).
Figure 4 Comparison between calculated melting temperature versus Vm/Vm0 for Al with experimental data.
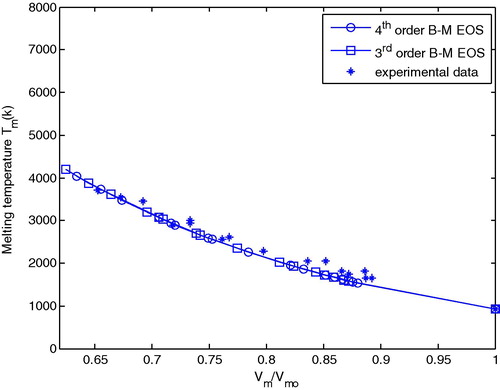
One can see from this figure that Vm/Vm0 decreases gradually with increasing melting temperature.
shows a comparison between the calculated melting temperature (Tm) versus melting pressure (Pm) for Al using the Lindemann equation which involves the 3rd and 4th order B–M EOS with experimental data obtained from Hänström and Lazor (Citation2000) and Boehler and Ross (Citation1997).
Figure 5 Comparison between calculated melting temperature versus melting pressure for Al with experimental melting data.
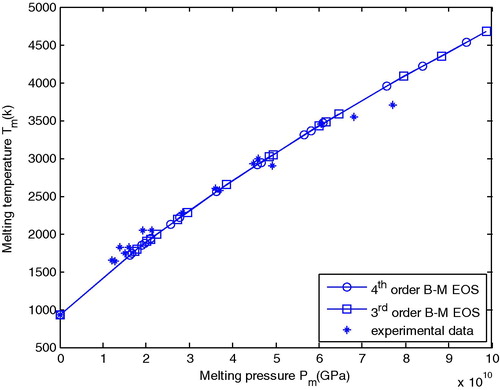
exhibited that the results obtained from the 3rd and 4th order B–M EOS are quite matchable and they fit with experimental data.
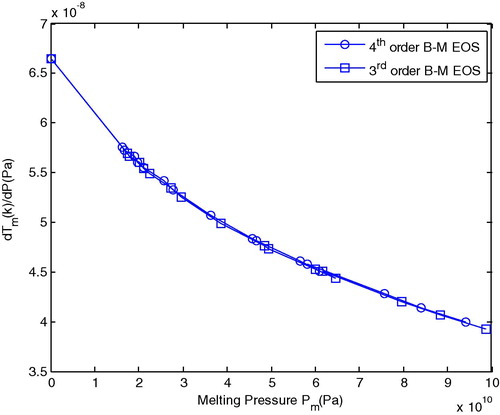
shows the melting gradient with melting pressure (Pm) for Al using the 3rd and 4th order B–M EOS. In this figure we show that the rate of variation in melting temperature with melting pressure decrease gradually with increases melting pressure and at a high melting pressure the rate of melting gradient decreases.
6 Conclusions
The results for (V/Vo) versus (P) obtained for 4th order B-M EOS are more agreement with experimental data than 3rd order B-M EOS. The isothermal bulk modulus (KT) and its first pressure derivative () for the 3rd and 4th order B–M EOS give different results at high pressure, this difference is due to the 4th order B–M EOS containing the term (
).
It has been found that the 3rd and 4th order B–M EOS gave same results with melting experiment data. The experimental melting compression curve was found in good agreement with the 3rd and 4th order B–M EOS. The melting temperature value obtained from Eq. Equation(22)(22) as well as the experimental data at 0–100 GPa are in good matching to each other.
The Kumar equation gives the melting volume (Vm/Vm0) at any high pressure and temperature, which is far better than the other relation. At any high pressure the rate of variation of melting temperature with pressure decreases.
References
- O.L.AndersonEquations of State of Solids for Geophysics and Ceramic Science1995Oxford University PressNew York
- F.J.BirchElasticity and constitution of the earth interiorGeophys. Res.571952227286
- F.J.BirchEquation of state and thermodynamic parameters of NaCl to 300 kbar in the high temperature domainJ. Geophys. Res.91198649494954
- R.BoehlerM.RossMelting curve of aluminum in a diamond cell to 0.8 Mbar: implications for ironEarth Planet. Sci. Lett.1531997223227
- R.E.CohenJ.S.WeitzThe Melting Curve and Premelting of MgO1996Geophysical Laboratory and Center for High Pressure Research, Carnergie Institution of WashingtonWashington, DC
- S.GauravB.S.SharmaS.B.SharmaS.C.UpadhayaAnalysis of equations of state for solids under compressionPhysica B3222002328339
- I.J.GilvaryThe Lindemann and Gruneisen lawPhys. Rev.1021956308316
- J.HamaK.SuitoThe search for a universal equation of state correct up to very high pressuresJ. Phys.: Condens. Matter819966781
- A.HänströmP.LazorHigh pressure melting and equation of state of aluminiumJ. Alloys Compd.3052000209215
- A.HofmeisterInteratomic potentials calculated from equations of state: limitation of finite strain to moderate K′Geophys. Res. Lett.2071993 http://dx.doi.org/10.1029/93GL00388, Issn: 0094-8276
- W.B.HolzapfelPhysics of solids under strong compressionRep. Prog. Phys.5919962990
- M.KumerHigh pressure equation of state for solidsPhysica B2121995391394
- M.KumerApplication of high pressure equation of state for different classes of solidPhys. B: Condens. Matter2171996143148
- S.S.KushwahH.C.ShrivastavaK.S.SinghStudy of pressure–volume relationships and higher derivatives of bulk modulus based on generalized equations of statePhysica B38820072025
- F.A.LindemannÜber die Berechnung Moleclarar EigenfrequenzenPhysica Z111910609612
- H.SchlosserP.VinetJ.FerrantePressure dependence of the melting temperature of metalsJ. Phys. Rev. B40198959295935
- J.ShankerB.P.SinghK.JitendraExtreme compression behaviour of higher derivative properties of solids based on the generalized Rydberg equation of stateCondens. Matter Phys.122009205213
- F.D.StaceyThe K-primed approach to high-pressure equations of stateGeophys. J. Int.1432000621628
- F.D.StaceyFinite strain, thermodynamics and the earth’s corePhys. Earth Planet. Inter.1282001179193
- F.D.StaceyB.J.BrennanR.D.IrvineFinite strain theories and comparison with seismological dataGeophys. Surveys431981189232
- K.SushilK.AruneshP.K.SinghB.S.SharmaAnalysis of finite-strain equations of state for solids under high pressuresPhysica B3522004134146
- P.TripathiG.MisraS.C.GoyalEquation of state for group IV–IV semiconductorsSolid State Commun.1392006132137