Abstract
A Monte-Carlo simulation of the growth of hydrogenated amorphous silicon (a-Si:H) thin films deposited by plasma enhanced chemical vapour deposition technique is presented in this work which is based on four fundamental processes that determine the layer growth: (i) random deposition of SiH3 radicals, (ii) desorption, (iii) “H” abstraction and (iv) sticking on a dangling bond. The essential goal of the model is to predict the bulk and the surface properties of films (hydrogen content, dangling bonds, surface roughness...) having different thicknesses and deposited at different substrate temperatures. The effects on the film properties of the incident radical flux of SiH3, directed towards the surface isotropically, on the surface are examined. A rate of content of hydrogen (8–12%) in the bulk is found. We find that surface roughness increases with increasing film thickness, though thin films (<20 mono-layers) have large hydrogen fractions on surface layer with a thickness approximately equal to the surface roughness. We also find a correlation between the average thickness and the incident radical flux of SiH3.
1 Introduction
Hydrogenated amorphous silicon (a-Si:H) is an important large area electronic material, used in solar cells and in film transistors for active matrix liquid crystal displays and sensors (Matsuda et al., Citation1990; Robertson, Citation2000a,Citationb; Street, Citation1992). There is now a trend to use microcrystalline Si (μc-Si) for these applications, because of its improved mobility and electrical stability. Both materials are grown by plasma enhanced chemical vapour deposition (PECVD) and the material quality is helped by understanding its growth process. Experimentally, it is generally accepted that SiH3 radicals dominate a-Si:H and μc-Si films growth from SiH4 plasmas in PECVD if the dissociation of the SiH4 source gas is kept below 15%, the reactions of SiH3 radical at the a-Si:H surface are still not fully understood (Dewarrat and Robertson, Citation2003). Film growth consists of three stages: the creation of reactive species in plasma, their reaction with the film surface and the conversion of the surface layers into the bulk. It is thought that growth occurs by the adsorption of SiH3 onto the hydrogen-terminated surface, it diffuses over it and abstracts a hydrogen from a surface Si–H bond to create a dangling bond (Si–). A second SiH3 then also adsorbs, diffuses around and adds to this dangling bond to give growth. Some molecular dynamics simulations (Tatsuya et al., Citation1995) of hydrogenated amorphous silicon thin film growth have been carried out. The molecular dynamics techniques need the incorporation of an interatomic potential-energy (Brenner, Citation1990; Tersoff, Citation1988) expression which can accurately describe chemical bonding. On the other hand, the Monte-Carlo method is an important tool for understanding random phenomena. Using random numbers, this technique provides solutions to problems expressed mathematically. In this paper, we present a Monte-Carlo simulation for the deposition of a-Si:H from SiH4 plasmas in PECVD technique taking into account all the surface reactions of SiH3 radicals. The model presented here emphasizes the bulk and surface properties of the film as function of average thickness and deposition substrate temperatures.
2 Surface adsorption model
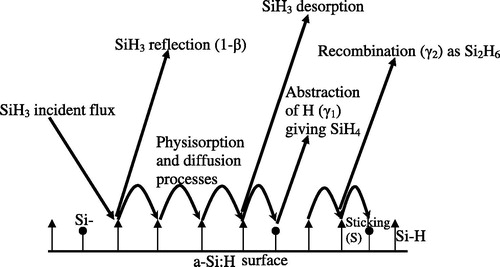
We consider surface growth processes when the aim growth species is SiH3. The a-Si:H is almost fully finished by hydrogen atoms with the existing of some dangling bonds at lower deposition temperature (less than 400 °C). It is believed that SiH3 can either be reflected (with probability 1-β) or physisorbed on the hydrogen saturated a-Si:H surface. It undergoes several processes (), it hops randomly to the neighbouring Si–H site at a rate υh, it abstracts an hydrogen atom from a-Si:H site to leave a surface dangling bond at a rate υa, or it can add to an existing surface D.B (giving growth) at a rate S0 = 1, in addition to this the SiH3 can be seen a desorption from a-Si:H surface at a rate υd as it can see a recombination with an other SiH3 giving Si2H6 (Kessels et al., Citation1998a,Citationb, Citation1999; Robertson, Citation2000a,Citationb).
3 Description of Monte-Carlo simulation
Our model uses Monte-Carlo method (MCM) to simulate the growth, random phenomenon, of a-Si:H films. In this case, a three dimensional model is proposed. All possible interactions of the SiH3 radicals with the a-Si:H surface are taken into account. We consider the main growth species is only SiH3 radicals (Kessels et al., Citation2000a,Citationb; Citation2004). These later can either react or reflect from the surface. The reaction probability at the a-Si:H surface is considered to be independent of the angle of incidence of the particles. For a typical growth rate of 1 Ǻ/s corresponds 1013–1014 particles that arrive by second on a surface containing from 1014 to 1015 sites, when 90% SiH3 radicals are reflected. The quotient of the particles by surface sites is taken in this model.
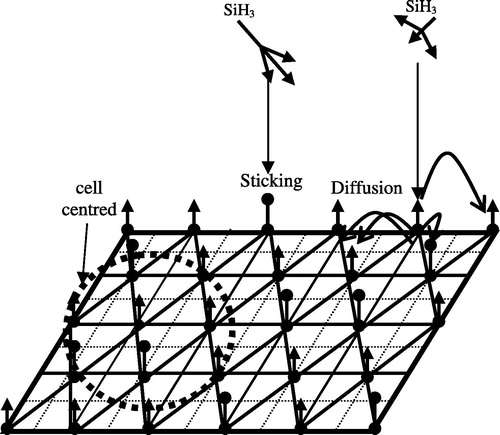
It is observed experimentally that the initial surface contains two types of bonds randomly distributed: dangling bonds (D.B) and Si–H bonds. We suppose that these bonds are located at the tops of equilateral triangles (cells), where the neighbouring of six (06) cells takes the shape of a honeycomb centred (). This choice is justified by the geometrical nature of the mesh of silicon.
Taking into account all the interactions of radicals and ionic species to simulate the growth of a-Si:H on truly atomic scale is a very complex task. However a realistic simplification can allow a Monte-Carlo simulation to be performed for reasonable computational effort. The simulation described here, written using the mathematical programme PASCAL, is based upon a matrix of (x,y) points as shown in . The dangling bonds (i–) and (SiH) are randomly distributed on the first surface that corresponds to (z = 1)(x,y) at percentages of around 30% and 70% respectively.
The simulation considers SiH3 radicals which land at random points on a-Si:H surface (in the beginning of growth the initial surface corresponds to z = 1) containing dangling and hydrogen bonds one at a time. The internal PASCAL random number generator is used to select x and y coordinates of these points on the initial surface (z = 1) in the beginning of growth and then on the a-Si:H surface (during growth). Depending on the chosen point the radical SiH3 is allowed to diffuse by one point at a time over the surface in a random fashion until it attaches onto the surface, abstracts H, and escapes into the gas phase of plasma The direction of each step is again determined using the internal PASCAL random number generator. Edge effects during diffusion, due to the finite size of the grid, are eliminated by using boundary conditions. All the interactions of SiH3 with the surface described in the following section (3.1) are taken into account in our model. The procedures used in this model are described in detail in the Sections 3.2 and 3.3.
3.1 Surface reactions of SiH3
Before interacting with the surface, the SiH3 radicals follow different trajectories; they come with a well defined flux and arrive homogeneously on the surface. The simulation shows that the average time of two successive arriving of SiH3 on the surface is very large than the average time of all possible reactions of SiH3. Furthermore we suppose in our model of simulation that the SiH3 comes one after one with the same probability for all the surface sites. So the SiH3 falls randomly on one site of the surface from which we will follow its way. The growth of the film is only assured by the sticking of SiH3 on a dangling bond.
When the SiH3 reaches the surface on a site representing Si–H bond, it will be adsorbed on this site with probability S1 (Robertson, Citation2000b) represented by the following chemical reaction.(R1)
Then it can diffuse randomly towards one of the neighbouring sites over the surface at a rate (Robertson, Citation2000b):where υh0 = 1013 Hz, Eh = 0.2 eV, T: temperature, KB: Boltzmann constant.
This phenomenon is represented by the following reaction:(R2)
SiH3 can eventually abstract a hydrogen from the surface and create a Si-dangling bond, with a frequency given by the formula:with υa0 = 3.1011 Hz, the abstraction energy Ea = 0.4 eV and as it is shown by the corresponding reaction:
(R3)
This reaction is important, because it gives a new dangling bond on the surface where the SiH3 can be stuck and assure the growth.
The SiH3 radical can also leave the surface at a rate:and υd0 = 1013 Hz, the desorption energy Ed = 0.7 eV (Robertson, Citation2000b).
The desorption from the surface is given by the chemical reaction:(R4)
We note that when the SiH3 diffuses towards a dangling bond or arrives directly on this last, it will be stuck (sticking) on the surface with a probability S0 = 1. This sticking or chimesorption is followed by an elimination of two hydrogen atoms from the three H atoms of the SiH3 stuck, as it is shown by the chemical reaction Equation(R5)(R5) :
(R5)
and we obtain two new dangling bonds.
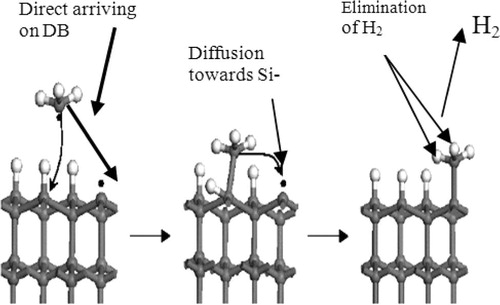
The elimination of H2 took place immediately after the sticking (), and if it did not happen after the sticking it will be incorporated into the film. Furthermore, we propose the following simulation steps:
3.2 The sticking of SiH3
By sticking on Si-dangling bond, this last is eliminated from the surface, and we obtain three hydrogen atoms in the following mono-layer surface centred on cells (triangles) as shown in .
Figure 4 (a) Sticking up of SiH3 radical, (b) elimination of H2 randomly from SiH3 fixed up on the surface (sticking up means Si–Si strong bond is pointed down and Si–H are oriented up and centred the cells).
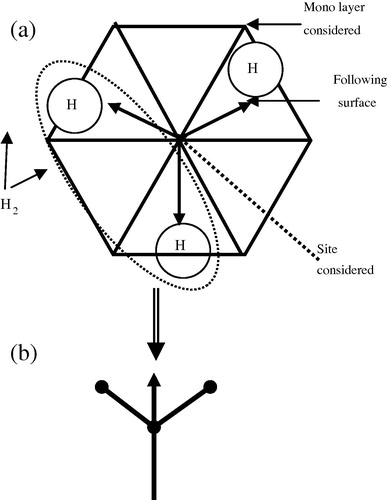
Taking into account the reaction Equation(R5)(R5) , we eliminate randomly, using the internal PASCAL random number generator, two hydrogen (H2) from the three atoms and we have two new dangling bonds and a hydrogen bond (Si–H) on the following surface. This situation is operated if the sticking of SiH3 is done.
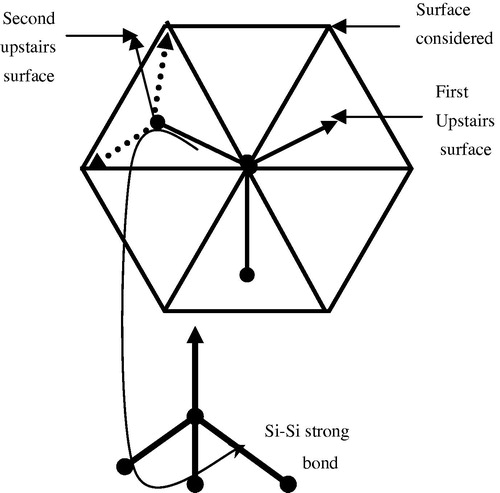
Now if the sticking is done as schematized in , this case is produced on a D.B located in the middle of cells (triangles), one Si–H bond of the SiH3 stuck points towards the following surface and the two Si–H bonds point into the nearest tops of cells in the downstairs surface. And as it is mentioned, we have an elimination of H2 randomly from the SiH3 stuck and for this situation we obtain three cases: an even dangling bonds D°–D°, even H–D° or Si–Si strong bond if the nearest top is occupied by another SiH3.
3.3 The interaction of SiH3 with Si–H
When SiH3 radical arrives on a site of the surface occupied by a hydrogen, it can diffuse on one of the nearest sites, as it can have desorption or abstraction of a hydrogen atom. These reactions have different frequencies, therefore different probabilities. It is noted that the sum of these three probabilities is equal to the unit, what enables us to standardize the probabilities and consequently one can allot a certain number for each reaction. There is affected for the diffusion a number Xh, Xa for the abstraction and Xd for desorption, these three numbers are given respectively by the following relations:where M is a very large number selected arbitrarily.
The internal PASCAL random number generator will be used to select an unspecified number among the M and each number is allotted to a given reaction. For the abstraction or desorption, the operation is simple. While for the diffusion we suppose that all the directions have the same probabilities, which requires the determination with precision in all the adjacent sites, that is to say in the same surface, or in the lower or higher mono-layer.
The same procedure repeats each time that a radical SiH3 arrives on surface. Thus the growth is done.
The simulation requires values for the abstraction, diffusion, desorption… probabilities. These values are taken from the literature (Robertson and Powell, Citation1999; Robertson, Citation2000b) and summarized in .
Table 1 Parameter values of the surface interaction of SiH3 used in the simulation model (Robertson and Powell, Citation1999; Robertson, Citation2000b).
By way of comparison, similar simulations were carried out by Flewitt et al. (Citation1999) who did not take into account all the possible interactions of SiH3 with the surface and Smilauer and Vvedensky (Citation1995) where reacting species on a (0 0 1) surface of GaAs were considered to need a greater energy to diffuse up a surface step edge than down. The difference in diffusion rate acts as the driving force for island formation.
The values mentioned in the are used in our simulation to model some mono-layers of the a-Si:H growth for calculating some physical parameters as shown in the following section of results.
4 Results and discussion
In this section, we will discuss results obtained from our model for the simulation of the growth of hydrogenated amorphous silicon films from silane plasmas when the main precursor is the SiH3 radical.
4.1 Average feature height ( )
)
shows the evolution of the average thickness (), given by Eq. Equation(1)
(1) , versus the number of the incident SiH3 radicals:
(1) where: Hi: is the height of ith site on the initial surface and N is the total number of sites.
Figure 6 The layer average thickness variation versus the SiH3 number for T = 350 °C, the initial surface contains 30% dangling bonds and 70% Si–H bonds.
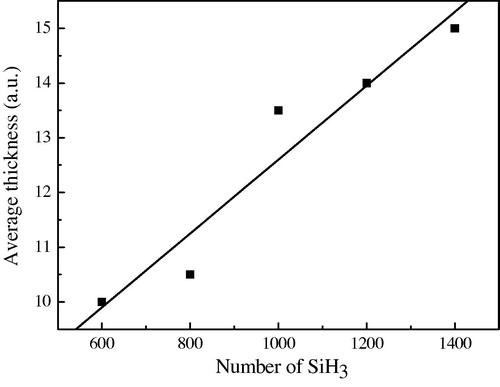
Because of the random deposition of the SiH3 arriving on the initial surface that contains N sites, the film growth will not be homogenous (voids) and the height is different from one site to other. Using Eq. Equation(1)(1) , we calculate the height (Hi) of each site (i) for all the N sites in the first surface and then we took the average.
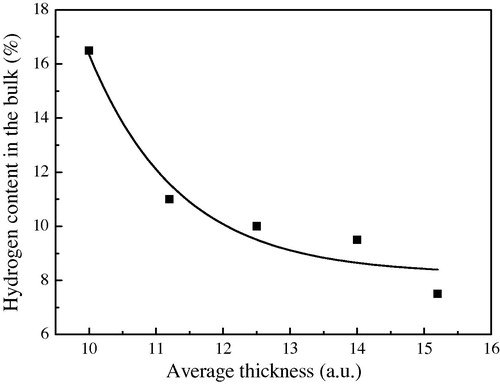
4.2 Hydrogen content in the bulk
The atomic percent hydrogen (%H) of the simulated film is calculated. shows how the %H content in the bulk of a-Si:H changes via layer average thickness and substrate temperature. We observe that the %H is from 8% to 10% such result is observed in experiments (Robertson, Citation2000a,Citationb).
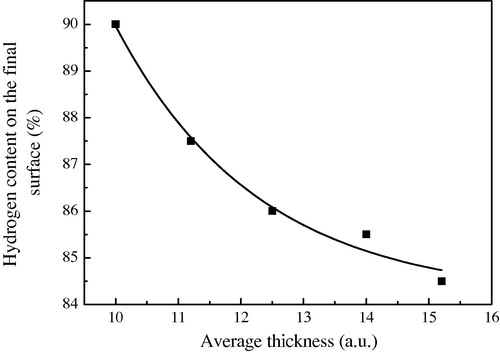
4.3 Hydrogen content on the surface
An unsolved, but fascinating, problem in a-Si:H growth is how SiH3 radicals containing 75 at %H can lead to an a-Si:H, deposited at good plasma conditions, film with a typical “H” content of 25–5% (for Ts ranging from 100 to 400 °C) (Robertson, Citation2000b). During growth, the percent of hydrogen covering the surface remains constant, and it is close to 80% as it is schematically shown in where we present the H percent versus the time (number of SiH3 arriving) for an initial surface containing 70% Si–H bond and 30% Si-D.B randomly distributed. This result is also seen (observed) while we change the number of Si–H and Si–D.B that cover the first surface and we always observe the surface during growth almost terminated by hydrogen (80%) with the existing of some dangling bonds (20%).
4.4 Surface roughness
The surface roughness of a-Si:H films is important with respect to their use in thin film devices and in multilayer structures (Gleason et al., Citation1987; Michael et al., Citation1989). The a-Si:H surface is actually extraordinarily smooth (Collins and Cavese, Citation1987; Smets et al., Citation2003), and this smoothness does not naturally follow from surface diffusion of SiH3 only, this can be simply seen from the fact that when surface dangling-bonds creation is random on the surface, film growth will be random as well. Since in the MGP model the SiH3 radical can only stick on these dangling-bonds. Therefore, SiH3 needs to have a higher probability to find a D.B and steps and valleys in the surface in order to lead to smooth film growth (Robertson, Citation2000b; Dewarrat and Robertson, Citation2003). In order to confirm this result, we calculated the layer roughness (σ), defined by Eq. Equation(2)(2) :
(2)
We have seen that roughness increases linearly with the average thickness. This can be explained by the fact that when the thickness of the sample increases, the random distribution of the SiH3 radicals on the sites becomes increasingly uniform and consequently roughness increases. It is generally believed that plasma conditions which result in radical fluxes dominated by SiH3 result in smoother films and higher quality material.
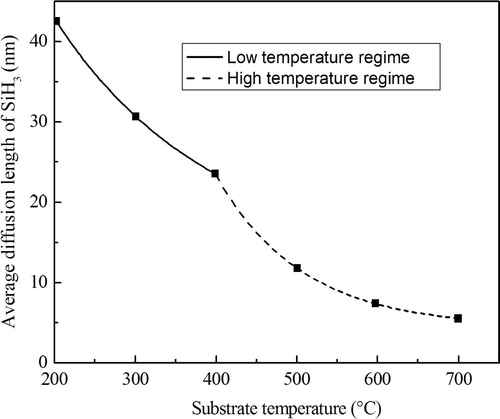
4.5 Length diffusion of SiH3
The average SiH3 diffusion length L on the final a-Si:H surface, calculated by our simulation, is shown in . L decreases continuously with substrate temperature Ts. It is clear from , that there are two regimes. At lower temperature where the thermal desorption rate of H is small. The diffusion length is determined by abstraction and addition (chimesorption) (Robertson, Citation2000a,Citationb), explained this by the fact that L increases with decreasing Ts because SiH3 diffuses to an abstraction site, and abstraction becomes increasing rate at low Ts. He supposed in this regime, that two SiH3 are needed for growth, the first to create a dangling bond and the second to add to it.
There is a second, higher temperature regime (Ts > 400 °C) of direct thermal H desorption. This regime has an even faster increase in dangling bonds D.B, determined by the competition between dangling bond creation by thermal desorption and dangling bond removal by SiH3 addition, and decrease of L with Ts.
5 Conclusion
We have examined the surface and bulk properties of a-Si:H on a three-dimensional numerical growth model of simulation; it constitutes a new general approach that allows for a better understanding of a-Si:H growth from SiH3 radicals at low temperature in plasma CVD using Monte-Carlo method. Here, only the essential growth mechanisms (random deposition of SiH3 radicals, diffusion, adsorption, abstraction and desorption) are considered. The approach described here does not critically depend on the trajectory of the incoming radical.
Our model can be used, in its simplest form, to study the evolution of the layer average thickness, roughness, defect dangling bond density and the atomic percent hydrogen in the bulk and on the surface.
References
- D.W.BrennerEmpirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond filmsPhys. Rev. B.42199094589471
- R.W.CollinsJ.M.CaveseSurface roughness evolution on glow discharge a-Si:HJ. Appl. Phys.31198716621664
- R.DewarratJ.RobertsonBinding and surface diffusion of SiH3 radicals and the roughness of hydrogenated amorphous siliconAppl. Phys. Lett.822003883885
- A.J.FlewittJ.RobertsonW.I.MilneGrowth mechanism of hydrogenated amorphous silicon studied by in situ scanning tunneling microscopyJ. Appl. Phys.8512199980328039
- K.K.GleasonK.S.WangM.K.ChenJ.A.ReimerMonte Carlo simulations of amorphous hydrogenated silicon thin film growthJ. Appl. Phys.61198728662873
- W.M.M.KesselsR.J.SeverensM.C.M.van de SandenD.C.SchramTemperature and growth-rate effects on the hydrogen incorporation in hydrogenated amorphous siliconJ. Non-Crystalline Solids1998133137
- W.M.M.KesselsM.C.M.van de SandenD.C.SchramHydrogen poor cationic silicon clusters in an expanding argon-hydrogen-silane plasmaAppl. Phys. Lett.72199823972399
- W.M.M.KesselsC.M.LeewisM.C.M.van de SandenD.C.SchramFormation of cationic silicon clusters in a remote silane plasma and their contribution to hydrogenated amorphous silicon film growthJ. Appl. Phys.86199940294039
- W.M.M.KesselsM.C.M.van de SandenR.J.SeverensD.C.SchramSurface reaction probability during fast deposition of hydrogenate amorphous silicon with a remote silane plasmaJ. Appl. Phys.87200033133320
- W.M.M.KesselsM.C.M.van de SandenD.C.SchramFilm growth precursors in a remote SiH4 plasma used for high rate deposition of hydrogenated amorphous siliconJ. Vac. Sci. Technol. A18200021532163
- W.M.M.KesselsA.H.M.SmetsM.C.M.Vand de SandenThe a-Si:H growth mechanism and the role of H abstraction from the surface by SiH3radicals via an Eley-Rideal mechanismJ. Non-Crystalline Solids20042731
- A.MatsudaK.NomotoY.TakeuchiA.SuzukiA.YuukiJ.PerrinTemperature dependence of the sticking and loss probabilities of silyl radicals on hydrogenated amorphous siliconSurf. Sci.22719905056
- J.MichaelM.CaugheyM.J.KuhnerSimulation of the bulk and surface properties of amorphous hydrogenated silicon deposited from silane plasmaJ. Appl. Phys.651989186195
- J.RobertsonGrowth mechanism of hydrogenated amorphous siliconJ. Non-Crystalline Solids20007983
- J.RobertsonDeposition mechanism of hydrogenated amorphous siliconJ. Appl. Phys.87200026082617
- J.RobertsonM.J.PowellDeposition, defect and weak bond formation processes in a-Si:HThin Solid Films33719993236
- A.H.M.SmetsW.M.M.KeelM.C.M.van de SandenTemperature dependence of the surface roughness evolution during hydrogenated amorphous silicon film growthAppl. Phys. Lett.8262003865867
- Smilauer, P., Vvedensky, D.D., 1995. Step-Edge Barriers on GaAs(001). MRS Proceedings, 399–229.
- R.A.StreetHydrogenated amorphous silicon1992Cambridge University PressCambridge
- O.TatsuyaU.OsamuA.TakeshiMolecular-dynamics simulations of SiH3 radical deposition on hydrogen-terminated silicon (1 0 0) surfacesPhys. Rev. B52199582838287
- J.TersoffNew empirical approach for the structure and energy of covalent systemsPhys. Rev. B37198869917000