Abstract
Metal (II) complexes of Cu, Ni, and Co with Schiff base derived from potassium 2-N (4-N,N-dimethylaminobenzyliden)- 4-trithiocarbonate 1,3,4-thiadiazole (L) were synthesized and characterized by standard physico-chemical procedures i.e. (metal analysis A.A, elemental chemical analysis C.H.N.S, FTIR, UV–vis, thermal analysis TGA, magnetic susceptibility and conductometric measurements). On the basis of these studies, a six coordinated octahedral geometry for all these complexes has been proposed. The Schiff base ligand and its complexes were also tested for their antibacterial activity to assess their inhibiting potential against Pseudomonas aeruginosa (as gram negative bacteria) and Staphylococcus aureus (as gram positive bacteria) using two different concentrations (5 and 10 mM). The results showed the Ni(II) complex have the higher rate in antibacterial activity than other complexes and ligand when compared them with ampicillin as standard drug.
1 Introduction
Schiff bases and their bio-active complexes have been studied extensively over the past decade. Schiff bases provide potential sites for bio-chemically active compounds. Various transition and inner-transition metal complexes with bi, tri and tetradentate Schiff bases containing nitrogen and oxygen or sulfur donor atoms play an important role in biological systems (Malik et al., Citation2011; Raman et al., Citation2007"). The thiadiazole ring and its derivatives possess good coordination behavior, since they have sulfur atom and two nitrogen atoms in addition to the substituent having a donating group in the structure (March and Smith, Citation2007) therefore the study of its metallic complexes is of structural importance in addition to many important applications such as fungicidal and leshmanicides (Łukaszuk et al., Citation2007; Foroumadi et al., Citation2005"). Trithiocarbonate complexes have received attention because of the dual nature of metal-CS3 moiety as electrophilic and nucleophilic reagents, which make them versatile intermediates for the synthesis of other oil thio species (Vicente et al., Citation1995). Although the main application is the treatment of a variety of rheumatic diseases, some of these compounds have shown to have antileishmanial activity in vitro inhibitory effect on HIV or activity of tumor cell (Vicente et al., Citation1995; Dehmel et al., Citation2007"). Organotrithiocarbonates have found many applications in various fields such as analysis, organic synthesis, medicine, industry and agriculture, some of these applications are as flotation agents, vulcanization accelerators, pesticides, plant defoliants, rust inhibitor, lubricating oil additives, and some have recently reported to possess activity as anti-radiation drugs (Srivastava et al., Citation1990; Ali and Abbas, Citation2006"). In this paper, we focused on a synthesized new derivative of trithiocarbonate in an attempt to introduce the azomethine moiety in the structure of the thiadiazole ring with the 4-trithiocarbonate group in the same structure and investigate the coordination behavior as well as study the antibacterial activity.
2 Experimental
All the chemicals used in this work were of analytical reagent grade. Metal salts used in this study are copper chloride dihydrate CuCl2.2H2O from Fluka, nickel chloride hexahydrate NiCl2.6H2O from BDH and cobalt chloride hexahydrate CoCl2.6H2O from Fluka. The metal analysis was performed by a Perkin Elmer 5000 Atomic Absorption Spectrophotometer. Elemental Analysis (C.H.N.S) of compounds was carried out with EM-034.mth. The electronic spectra were recorded on a Shimadzu (UV–vis 1600 A) Ultra Violate Spectrophotometer using quartz cell at a wave length range of 200–1100 nm. FTIR spectra were recorded on a Shimadzu 8400 Fourier Transform Spectrophotometer by using CsI disk in the wave number range of 4000–200 cm−1. Thermal analysis TGA was performed with 4000 Perkin–Elmer. Magnetic susceptibility measurements were determined using Magnetic Susceptibility Balance of Johnson matting catalytic system division, England at room temperature. The molar conductance was measured in DMSO as a solvent at room temperature using Coring Conductivity Meter 220. Melting point apparatus of Gallencamp M.F.B-600.01 was used.
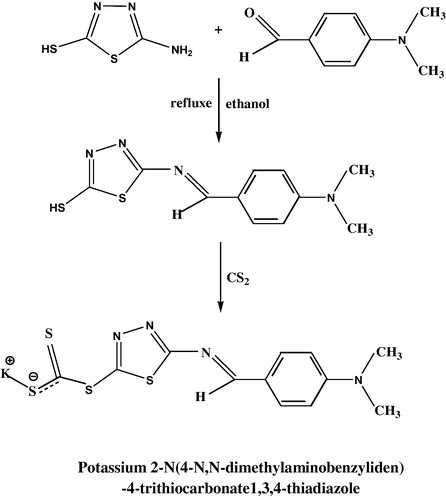
2.1 Synthesis of Schiff base potassium 2-N(4-N,N-dimethylaminobenzyliden)-4-trithiocarbonate 1,3,4-thiadiazole (L)
A mixture of 2 g, 0.015 m mol of 2-amino-5-mercapto1,3,4-thiadiazole and 3.07 g, 0.015 m mol of 4-N,N-dimethylaminobenzyliden in 10 ml of absolute ethanol and one drop of glacial acetic acid was continuously stirred and heated in a water bath at 60–70 °C then refluxed for 1 h, left to cool in ice-water bath until orange precipitate was obtained. 2 g, 0.0075 mol of the previous mixture i.e. 2-N(4,4-dimethylamin benzyliden)-5-mercapto1,3,4-thiadiazole in 20 ml of absolute ethanol was added to 1.5 ml of carbon disulfide and 0.42 g, 0.0075 mol potassium hydroxide as alkali media. The mixture was refluxed for 3 h then the solvent was distilled off and the precipitate crystals were filtered and recrystallized from ethanol and distilled water to obtain orange precipitate (). The physical properties are shown in .
Table 1 Physical data of new ligand (L) and its metal complexes.
2.2 Synthesis of complexes
A general method has been used for the preparation of the new complexes by the reaction of the hydrate metal salts of copper, cobalt and nickel which dissolved in 5 ml of absolute ethanol and 0.378 g, 1 m mol of the Schiff base ligand at a metal to ligand molar ratio of 1:1, while 0.756 g, 2 m mol at a metal to ligand molar ratio of 1:2 which dissolved in 10 ml of absolute ethanol. The metal salts were added gradually drop by drop to a solution of ligand. The reaction mixture was allowed to stir magnetically for 1 h at room temperature. The reaction mixture was filtered, washed with ethanol and dried at 50 °C by using an oven for 1 h .
2.3 Evaluation of antibacterial activity
The in vitro biological screening effects of the investigated compounds were tested against the bacteria: Pseudomonas aeruginosa and Staphylococcus aureus this was carried out by the disk diffusion technique, using agar nutrient as the medium (Arora et al., Citation2011). The stock solution (5 and 10 mM) of new compounds was prepared by dissolving the compounds in DMSO. In a typical procedure, a well was made on the agar medium inoculated with microorganisms. The well was filled with the test solution using a micropipette and the plate was incubated for 24 h at 37 °C. During this period, the test solution diffused and the growth of the inoculated microorganisms was affected. The results were recorded by measuring the growth inhibition surrounding the disk.
3 Results and discussions
3.1 General
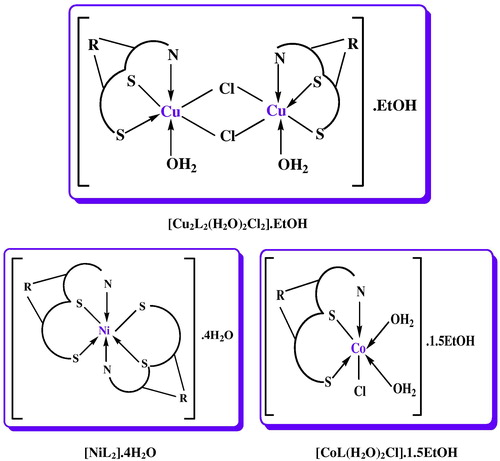
The obtained complexes were colored powders, stable for a long time in the open atmosphere. The analytical data for the ligand and its complexes together with some physical properties are summarized in . All the complexes were sparingly soluble in general organic solvents, the melting points show that all of the complexes decomposed before melting. Analytical data suggest a ratio of 1:1 (M:L) for Cu(II) and Co(II) complexes and 1:2 for Ni(II) complex. The presence of lattice water or uncoordination EtOH was confirmed by TGA analysis. The molar conductivity corresponding to the Cu(II), Ni(II) and Co(II) complexes presents low values and in this way a structural formula of non-electrolyte for these complexes can be assigned. The magnetic susceptibilities of the complexes at room temperature were consistent with octahedral geometry around the central metal ion.
3.2 Infrared spectra
The FTIR spectra provide valuable information regarding the nature of the functional group attached to the metal atom. The infrared spectra of the ligand showed a band at 1527 cm−1 which was attributed to νCN band of ring which remained unchanged in all complexes, νCN band at 1591 cm−1 was observed due to the azomethine group which shows a shift to lower frequencies in all complexes almost between 10 and 30 cm−1 which may be indicating the involvement of –CN nitrogen in coordination to the metal ion (Raman et al., Citation2007; Mitu et al., Citation2012"). The FTIR spectra of the new compounds showed a band in the region (1440–1445) cm−1 referring to νNCS band. The frequencies that appeared at 1031, 777 and 943 cm−1 regions are assigned to νCS, νC–S and νCS bands respectively, which undergo a shift toward higher frequencies in all complexes for νC–S and νCS, while in νCS band exhibit a shift toward lower frequencies in all complexes (Vicente et al., Citation1995, Citation1997") , another band exhibits at 1371 cm−1 referring to Ar–N mode which remained constant in all complexes. Accordingly, the ligand acts as a tridentate chelating agent, bonded to the metal ion via the two sulfur atoms as well as N atom of the azomethine group. Other new bands appeared which were supported by the appearance of frequencies of ν(M–S), ν(M–N), ν(M–Cl) and ν(M–O) bands. A band observed around 3350–3371 cm−1 in the spectra of metal complexes, assigned to the νOH refers to the presence of out of sphere EtOH or H2O groups (Nakomato, Citation2009; Soleimani, Citation2010").
Table 2 Characteristic FTIR bands in (cm−1) of the new ligand and its metal complexes.
3.3 Electronic spectra and magnetic moment
The electronic spectral data of the metal complexes in DMSO solution are given in . The nature of the ligand field around the metal ion has been deduced from the electronic spectra.
Table 3 Electronic spectra, conductance in DMSO solvent and magnetic moment (B.M.) for the prepared ligand and its metal complexes.
Cu(II) complex: Electronic spectrum of brown complex displayed bands in the range of 23.955–37.037 cm−1 which can be assigned to charge transfer as well as the spectrum showed d–d electronic transition at 14.814 cm−1 which was assigned to 2Eg → 2T2g transition, the broadness of the band is due to the ligand field and the Jahn–Teller effect, these absorptions prefer the distorted octahedral geometry for the Cu(II) ion (Solomon and Lever Citation2006; Alhadi et al., Citation2012"). Moreover, the magnetic moment for the Cu(II) complex is 1.2 B.M. suggesting a dimeric structure leading to spin–spin coupling (Greenwood and Ernshaw, Citation1998). Furthermore, the complex is non-electrolyte as the molar conductance was found to be 28 μscm−1 in 10−3 M in DMSO as solvent .
Ni(II) complex: In the present work, light brown color of the new complex is postulated to be distorted octahedral with its respective values. The 10Dq (10.267 cm−1) is equal to the first transition v1. The band at 13.947 cm−1 is assigned to the spin-forbidden transition 3A2g → 1Eg (Pastorek et al., Citation2001) . More evidence for the suggested octahedral geometry of present complex is supported by the absence of band in the range of 20000 cm−1 which is characteristic of square planar Ni(II) complexes (Greenwood and Ernshaw, Citation1998; Siddiqi et al., Citation2006"). Magnetic moment 3.2 B.M. and the conductivity measurement show that the complex is non-ionic.
Co(II) complexes: In the spectrum of Co(II) complex, the bands at 10.040 cm−1, 15.070 cm−1 and 19.980 cm−1 are associated to the transitions 4T1g → 4T2g (ν1), 4T1g → 4A2g (ν2) and 4T1g → 4T1g(P) (ν3) respectively. These transitions are specified to the Co(II) ion in the field of octahedral symmetry and the magnetic moment of 4.9 B.M. shows the complex is paramagnetic and has three unpaired electrons corresponding to a high spin of this geometry (Rathore et al., Citation2010; Al-Najjar, Citation2009"). The ligand field splitting energy (10 Dq), interelectronic repulsion parameter (B/) and nephelauxetic ratio (β) for the Co(II) complex were calculated, and these correspond to the 10 Dq = 11.896 cm−1, B/ = 661 cm−1, β = 0.68 values . The value of the β parameter indicates a covalent character for the metal–ligand bonds. The conductivity measurement recorded 29 μscm−1 and this refers that the complex is non-electrolyte.
3.4 Thermal analysis
The results of the solid complexes are listed in . The results show good agreement with the structures suggested from the analytical data . A general decomposition pattern was concluded, whereby the complexes decomposed in three stages for ligand this decomposition began from almost between 180 and 900 °C. Besides these three decomposition stages, those complexes which have coordinated water and/or ethanol exhibited additional decomposition steps, the structural formulas assigned to the complexes are presented in .
Table 4 Thermal analysis data for metal complexes.
3.5 Suggested structure of complexes
.
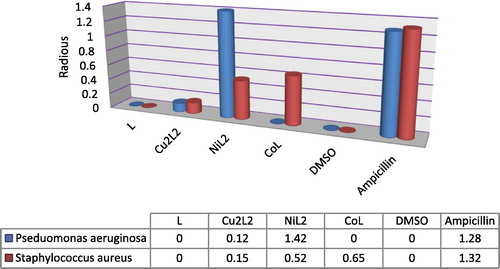
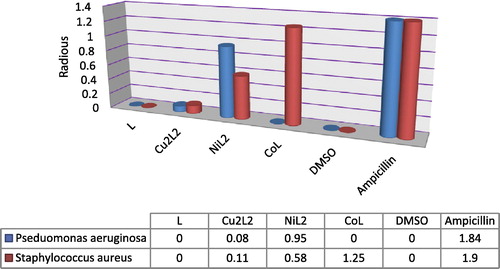
3.6 Antibacterial activities
The results obtained for biological activity for the prepared ligand and its metal complexes are given in . Diameter of inhibition zone (mm) including the disk diameter was measured for each treatment. The ligand showed no antimicrobial activity against both kinds of bacteria i.e. P. aeruginosa and S. aureus. The copper(II) complex shows a slight effect compared with ligand in both concentrations and organisms used, while nickel(II) complex exhibited the maximum antibacterial activity against all organisms used in this study and recorded high inhibition activity against P. aeruginosa comparable with standard drug. Moreover, cobalt complex showed moderate activity against S. aureus microorganism comparable with ligand and standard drug ampicillin, while against P. aeruginosa the results do not record any biological activity of this complex in both concentrations ( and ). The higher inhibition zone of metal complexes than those of the ligand can be explained on the basis of Overtone’s concept and Chelation theory. On chelation, the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelating ring and enhances the penetration of the complexes into lipid membranes and blocking of the metal binding sites in the enzymes of microorganisms. There are other factors which also increase the activity are solubility, conductivity and bond length between the metal and ligand (Chohan et al., Citation2001; Iqbal et al., Citation2006; Singh et al., Citation2008").
Table 5 Antibacterial screening data for the ligands and their complexes.
4 Conclusion
In the present research study, we synthesized new ligand of trithiocarbonate from 2-amino-5-mercapto1,3,4-thiadiazole. This new compound was used to prepare some new complexes of Cu(II), Ni(II) and Co(II), these complexes are characterized by various physicochemical and spectral analyses. The results exhibit that the synthesized ligand binds with metal ions in tridentate through S,S donor sites of trithiocarbonate as well as N atom of the azomethine group. Thermogravimetric studies of the complexes also helped to characterize the complexes. The antibacterial data show that the metal complexes have biological activity compared to that of parent ligand.
Notes
Peer review under responsibility of University of Bahrain.
References
- A.A.AlhadiS.A.ShakerW.A.YehyeH.M.AliM.A.AbdullahSynthesis, magnetic and spectroscopic studies of Ni(II), Cu(II), Zn(II) and Cd(II) complexes of a newly Schiff base derived from 5-bromo-2-hydroxxybezylidene)-3,4,5-trihydroxybenzohydrazide)Bull. Chem. Soc. Ethiop.261201295101
- M.F.AliS.AbbasA review of methods for the demetallization of residual fuel oilsFuel Process. Technol.8772006573584
- N.Al-NajjarPreparation and identification of some PVA–metal complexesIraqi J. Sci.5032009271278
- S.AroraS.VijayD.KumarPhytochemical and antimicrobial studies on the leaves of Spilanthes acmellaJ. Chem. Pharm. Res.352011145150
- Z.ChohanA.MunawarC.SupuranTransition metal ion complexes of Schiff-base. Synthesis, characterization and antibacterial propertiesMet. Based Drugs82001137143
- F.DehmelT.CiossekT.MaierS.WeinbrennerB.SchmidtM.ZocheT.BeckersTrithiocarbonates: exploration of a new head group for HDAC inhibitorsBioorg. Med. Chem. Lett.1717200747464752
- A.ForoumadiS.PournourmohammadiF.SoltaniM.Asgharian-RezaeeS.DabiriA.KharazmiA.ShafieeSynthesis and in vitro leishmanicidal activity of 2-(5-nitro-2-furyl) and 2-(5-nitro-2-thienyl)-5-substituted-1,3,4-thiadiazolesBioorg. Med. Chem. Lett.158200519831985
- N.N.GreenwoodA.ErnshawChemistry of Elements2nd ed.1998Pergamum Press
- J.IqbalS.A.TirmiziF.H.WattooM.ImranBiological properties of chloro-salicylidene aniline and its complexes with Co(II) and Cu(II)Turk. J. Biol.30200614
- C.ŁukaszukE.Krajewska-KułakA.NiewiadomyJ.StachowiczU.GłaszczE.OksiejczukIn vitro antifungal activity of 2,5 disubstituted amino-oksometyloso-arylo-thiadiazole derivativesJ. Adv. Med. Sci.52120072629
- S.MalikS.GhoshL.MituComplexes of some 3d-metals with a Schiff base derived from 5-acetamido-1,3,4-thiadiazole-2-sulphonamide and their biological activityJ. Serb. Chem. Soc.7610201113871394
- J.MarchM.B.SmithAdvanced Organic Chemistry>6th Ed2007John Wiley & Sons, HobokenNew Jersey
- L.MituM.IlisN.RamanM.ImranS.RavichandranTransition metal complexes of isonicotinoyl-hydrazone-4-diphenylaminobenzaldehyde: synthesis, characterization and antimicrobial studiesE. J. Chem.912012365372
- N.NakomatoInfrared and Raman Spectra of Inorganic and Coordination Compounds6th Ed2009SpringerBerlin
- R.PastorekJ.KamenicekJ.HusarekZ.SindelarNickel (II) dithiocarbamates with 1,4,8,11-tetraazacyclotetradecanein the coordination sphereJ. Chem.4020015763
- N.RamanS.RajaJ.JosephJ.RajaSynthesis, spectral characterization and DNA cleavage study of heterocyclic Schiff base metal complexesJ. Chil. Chem. Soc.522200711381141
- K.RathoreK.R.RajivH.B.SinghStructural, spectroscopic and biological aspects of O, N-donor Schiff base ligand and its Cr(III), Co(II), Ni(II) and Cu(II) complexes synthesized through green chemical approachEur. J. Chem.2010S566S572 7(S1)
- K.S.SiddiqiS.A.NamiLutfullahaY.ChebudeTemplate synthesis of symmetrical transition metals dithiocarbamatesJ. Braz. Chem. Soc.1712006107112
- V.P.SinghA.KatiyarS.SinghSynthesis, characterization of some transition metal(II) complexes of acetone p-amino acetophenone salicyloyl hydrazone and their anti microbial activityBiometals2142008491501
- E.SoleimaniSynthesis and characterization of a novel benziloxime ligand and its iron(III) and nickel(II) complexesJ. Chin. Chem. Soc.573A2010332337
- E.I.SolomonA.B.P.LeverInorganic Electronic Structure and Spectroscopy: Applications and Case Studiesvol. II2006John Wiley and SonsInc, New York, Chester, Singapore, Toronto
- A.SrivastavaS.K.SinghA.GuptaDetermination of organotrithiocarbonates with O-diacetoxyiodobenzoate and N-chlorosuccinimide in aqueous and non-aqueous mediaAnalyst11541990421423
- J.VicenteM.T.ChicoteP.González-HerreroP.G.JonesSynthesis of the first trithiocarbonatogold complex: [N(PPh3)2]2[Au2(μ2-η2-CS3)2]. First crystal structure of a μ2-η2-bridging trithiocarbonato complexJ. Chem. Soc Chem. Commun.71995745746
- J.VicenteM.T.ChicoteP.González--HerreroP.G.JonesComplexes with S-donor ligands. Synthesis of the first family of (trithiocarbonato) gold complexes. Crystal structure of [(PPh3)2N][AuCl2(CS3)]J. Inorg. Chem.3625199757355739