Abstract
The urgent need in search of new biological entities to fight back with recent drug-resistant microbial flora, has led us report a library of s-triazine derivatives. The intermediate 4-((4-chloro-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile 3 was substituted with various thiophenol, phenol, aniline and piperazine/piperidine/morpholine moieties to furnish the final 35 target compounds i.e. (4a–j), (5a–j), (6a–g) and (7a–h), respectively. These compounds were screened for in vitro antibacterial evaluation against bacteria (Staphylococcus aureus MTCC 96, Bacillus cereus MTCC 619, Escherichia coli MTCC 739, and Pseudomonas aeruginosa MTCC 741) and antifungal activity against fungi (Candida albicans MTCC 183, Aspergillus niger MTCC 282, and Aspergillus clavatus MTCC 1323). The title compounds were further subjected for antituberculosis activity against Mycobacterium tuberculosis H37Rv strain using the BACTEC MGIT method. In this biological evaluation, thiophenol derivatives were found to be more active than the rest (i.e. -Thiophenol > -piperazine > -Aniline > -phenol). The final compounds were characterized by FT-IR, 1H NMR, 13C NMR, mass spectroscopy and elemental analysis.
1 Introduction
Multidrug resistant (MDR) strains, a rapid development of pathogens causing a severe resistance toward currently available standard drugs, pose a frightening threat by increasing severe opportunistic microbial infections in past decades (Gootz, Citation2010; Niccolai and Tarsi, Citation1997; Overbye and Barrett, Citation2005"). Such resistant organisms were due for a dramatic and alarming increase in microbial infections which results in pressing problem worldwide. On the other hand, MDR-Tuberculosis (TB) and extensively drug-resistant XDR-TB, caused by some mycobacteria of the Mycobacterium tuberculosis complex which most commonly affect the lungs, emerged as one of the most infectious diseases in the recent era (Ducati et al., Citation2006; Gandhi et al., Citation2010; Udwadia et al., Citation2012"). The latest statistics of World health Organization (WHO) reported that about one third of the human population were infected with TB which showed the urgent need to combat such dilemma (Citation2012).
Surprisingly, 8.7 million new cases of TB were reported in 2011 from which 13% co-infected with HIV (Human Immuno Deficiency); 1.4 million people died from TB, including almost one million deaths among HIV-negative individuals and 0.4 million people allied to HIV-positive which scores around 25% death due to TB (Citation2013). In view of the above, consequences of these problems highlight the urgent need to develop new agents with specific activity with increased potency to sustain a pool of new bioactive entities. Therefore, design and synthesis of new compounds likely to be unaffected by existing resistance mechanisms are an area of immense significance for medicinal chemists.
Owing to a wide range of biological applications, s-triazine nucleus has received an immense attention among chemists through fertile source of pharmacological activities such as antibacterial (Bhushan Singh et al., Citation2012; Gahtori et al., Citation2012b; Kumar Ghosh et al., Citation2012"), antimalarial (CitationGahtori et al., 2012a), antiprotozoal (CitationBaliani et al., 2005), antifungal (CitationSingh et al., 2012), anticancer (CitationMenicagli et al., 2004), antimycobacterial (CitationPatel et al., 2012), and antiviral (CitationChen et al., 2012). In addition to this several s-triazine derivatives bearing p-amino benzonitrile moiety have been found to possess an enhanced antimicrobial profile and improved antitubercular (CitationPatel et al., 2011b) and profound anticancer activity (CitationPatel et al., 2011a) as well. Consequences of such potential effects of triazine and an imperative need in search of new chemical entities lead us to synthesize some biologically efficient molecules.
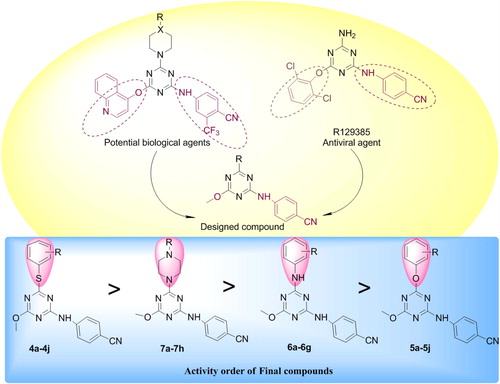
Recently our research group has reported 2,4,6-trisubstituted triazine derivatives endowing promising biological activity (CitationModh et al., 2012a,Citationb,Citationc, Citation2013a,Citationb; CitationPatel et al., 2012, Citation2011a,Citationb); hence it is worthy to synthesize novel compounds which elicit a series of antimicrobial and anti tuberculosis agents. Recent studies have confirmed that several s-triazine derivatives bearing morpholine, piperidine and some piperazine moieties are effective against M. tuberculosis H37Rv strain (CitationSunduru et al., 2010). Prompted by such facts it is worthy to envisage that combination of such bioactive moieties in a compact system may arise with new biologically active agents. We introduced synthetic strategy to acquire triazine nucleus with biolabile derivatives viz. phenol, thiophenol, aniline and piperazine/piperidine/morpholine. Target compounds were rationalized and designed using the hits obtained from the (piperazinyl/piperidinyl)-s-triazines derivatives (CitationPatel et al., 2011a), which were previously reported for their antimicrobial, antimycobacterial and anticancer activities besides this, compound R129385 (CitationDas et al., 2004) with s-triazine nucleus was reported as an effective antiviral agent (). Adopting all such criteria, herewith, a library of 35 s-triazine based compounds were synthesized and evaluated for their biological potential which may lead to future prospects in drug design and discovery.
2 Experimental section
2.1 Materials and methods
All chemicals as well as solvents were procured from sigma Aldrich, Merck and Fluka. Solvents taken were of analytical grade and used without further purification. All reactions were routinely checked by TLC. TLC was performed on aluminum-backed silica gel plates (Silica gel 60 F254 grade, Merck DC) with spots visualized by UV light. Column chromatography was performed on silica gel LC 60A (70–200 μ). Melting points were determined in open capillaries on a Veego electronic apparatus VMP-D (Veego Instrument Corporation, Mumbai, India) and are uncorrected. FT-IR spectra were recorded on a perkin–Elmer 257 spectrometer using KBr disks. 1H NMR and 13C NMR spectra were recorded on a Bruker 400 MHz model spectrometer using DMSO-d6 as a solvent and TMS as an internal standard. The chemical shifts were reported as parts per million (ppm) downfield from TMS (Me4Si) with 1H resonant frequency of 400 MHz and 13C resonant frequency of 100 MHz. Purity of all tested compounds was ensured on the basis of their elemental analyses (C, H, N) and were performed using a Heraeus Carlo Erba 1180 CHN analyzer (Hanau, Germany). The electron spray mass spectra were recorded on a triple quadrupole mass spectrometer with the ionization potential of 70 eV .
2.2 Chemistry: general methods
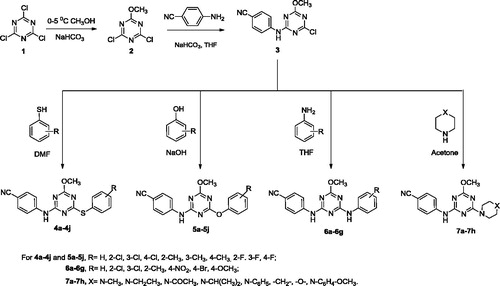
2.2.1 Synthesis of 2,4-dichloro-6-methoxy-1,3,5-triazine (2)
A mixture of 2,4,6-trichloro-1,3,5-triazine 1 (5.0 g, mol) and sodium bicarbonate (2.5 g, 0.02982 mol) in methanol (10 mL) was stirred at 0–5 °C for 4 h. The progress of the reaction was monitored by TLC using hexane:ethyl acetate (4:1) solvent system as an eluent. After the completion of the reaction, the reaction mass was poured into crushed ice. The solid was separated, washed with cold water, dried and recrystallized from ethanol to give compound 2 (CitationDudley et al., 1951). Yield: 79%; m.p. 88–90 °C; IR (KBr cm−1): 2815 (–OCH3), 826 (C3N3, s-triazine); 1H NMR (400 MHz, DMSO-d6): δ 3.77 (s, 3H–Ar–OCH3); 13C NMR 179.8, 167.5, 58.3; ESI-MS (M+1): 180.99.
2.2.2 Synthesis of 4-((4-chloro-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (3)
To a stirred solution of compound 2 (5.0 g, 0.02778 mol) and sodium bicarbonate (2.56 g, 0.03056 mol) in THF (20 mL), a solution of 4-amino benzonitrile (3.28 g, 0.02778 mol) was added and stirred for 6 h at room temperature. The progress of the reaction was monitored by TLC using toluene:acetone (4:1) solvent system as an eluent. After the completion of the reaction, resultant mixture was poured into crushed ice. The solid product obtained was filtered, washed with distilled water, dried and purified by column chromatography using toluene:acetone solvent system as an eluent. Yield: 85%; m.p.167 °C; IR (KBr cm−1): 3372 (N–H), 2210 (C=N), 845 (C3N3, s-triazine); 1H NMR (400 MHz, DMSO-d6): DMSO-d6: δ 2.97 (s, 3H–Ar–OCH3), 9.8 (s,1H, –NH); 13C NMR 177.1, 169.2, 168.9, 144.6, 135.9, 119.7, 118.5, 105.1, 54.8; ESI-MS (m/z): 262.67.
2.2.3 General procedure for the preparation of ((4-methoxy-6-(substituted phenylthio)-1,3,5-triazin-2-yl)amino)benzonitrile (4a–j)
A stirred mixture of appropriate thiophenol (0.0191 mol), 4-((4-chloro-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile 3 (5.0 g, 0.0191 mol) and anhydrous K2CO3 (2.92 g, 0.0211 mol) in DMF (20 mL) was refluxed for 20 h. The progress of the reaction was monitored by TLC using toluene:acetone (7:3) solvent system as an eluent. After the completion of the reaction, the reaction mass was poured into ice. The product was extracted with 25 mL ethyl acetate and then the organic layer was washed with first brine and then with water. The organic layer was separated, dried over Na2SO4 and concentrated to dryness to give a yellow solid, which was re-crystallized from n-hexane to give light yellow powder.
2.2.3.1 4-((4-Methoxy-6-(phenylthio)-1,3,5-triazin-2-yl)amino)benzonitrile (4a)
Yield: 83%; m.p. 166 °C; IR (KBr cm−1): 3271 (N–H), 2909 (C–H), 2240 (C≡N), 1305 (C–N in 2° aromatic amine), 1210 (C–O), 1100 (C–S), 830 (C3N3–s-triazine); 1H NMR (400 MHz, DMSO-d6): δ ppm 7.54 (d, J = 7.5 Hz, 2H), 7.25–6.89 (m, 7H), 6.33 (s, 1H), 3.82 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 191.4, 177.9, 166.3, 143.6, 134.4, 132.1, 129.9, 128.1, 126.6, 119.2, 118.1, 103.3, 54.7; Anal. Calcd. for C17H13N5OS: C, 60.88; H, 3.91; N, 20.88; O, 4.77; S, 9.56, Found: C, 60.74; H, 3.79; N, 20.78; O, 4.67; S, 9.45; ESI-MS (M+1): 336.08.
2.2.3.2 4-((4-((2-Chlorophenyl)thio)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (4b)
Yield: 79%; m.p. 183 °C; IR (KBr cm−1): 3260 (N–H), 2890 (C–H), 2231 (C≡N), 1309 (C–N in 2° aromatic amine), 1221 (C–O), 1121 (C–S), 841 (C3N3–s-triazine), 564 (C–Cl); 1H NMR (400 MHz DMSO-d6): δ 7.57 (d, J = 7.5 Hz, 2H), 7.34–7.12 (m, 4H), 7.08–7.01 (m, 2H), 6.09 (s, 1H), 3.84 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 189.4, 179.9, 168.3, 148.6, 137.8, 134.8, 132.4, 130.1, 127.6, 129.9, 126.8, 119.2, 118.2, 103.3, 54.1; Anal. Calcd. for C17H12ClN5OS: C, 55.21; H, 3.27; Cl, 9.59; N, 18.94; O, 4.33; S, 8.67, Found: C, 55.11; H, 3.17; Cl, 9.49; N, 18.83; O, 4.21; S, 8.55; ESI-MS (M+1): 370.83.
2.2.3.3 4-((4-((3-Chlorophenyl)thio)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (4c)
Yield: 70%; m.p. 174 °C; IR (KBr cm−1): 3283 (N–H), 2921 (C–H), 2228 (C≡N), 1312 (C–N in 2° aromatic amine), 1215 (C–O), 1110 (C–S) 822 (C3N3–s-triazine), 594 (C–Cl); 1H NMR (400 MHz DMSO-d6): δ 7.51 (d, J = 7.5 Hz, 2H), 7.46 (t, J = 1.3 Hz, 1H), 7.24–7.06 (m, 5H), 6.12 (s, 1H), 3.83 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 193.4, 174.3, 164.8, 149.7, 135.2, 133.7, 131.2, 130.4, 129.6, 127.2, 126.2, 119.2, 118.6, 103.9, 54.1; Anal. Calcd. for C17H12ClN5OS; C, 55.21; H, 3.27; Cl, 9.59; N, 18.94; O, 4.33; S, 8.67, Found: C, 55.10; H, 3.17; Cl, 9.49; N, 18.84; O, 4.23; S, 8.57; ESI-MS (M+1): 370.83.
2.2.3.4 4-((4-((4-Chlorophenyl)thio)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (4d)
Yield: 82%; m.p. 180 °C; IR (KBr cm−1): 3273 (N–H), 2890 (C–H), 2240 (C≡N), 1309 (C–N in 2° aromatic amine), 878 (C3N3–s-triazine), 1247 (C–O), 580 (C–Cl), 1197 (C–S); 1H NMR (400 MHz DMSO-d6): δ 7.41 (d, J = 7.5 Hz, 2H), 7.28 (d, J = 7.5 Hz, 2H), 7.20 (dd, J = 7.5, 4.4 Hz, 4H), 6.29 (s, 1H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 181.1, 172.9, 161.3, 140.6, 135.7, 131.4, 129.4, 127.4, 126.3, 119.8, 118.2, 103.7, 56.9; Anal. Calcd. for C17H12ClN5OS; C, 55.21; H, 3.27; Cl, 9.59; N, 18.94; O, 4.33; S, 8.67, Found: C, 55.21; H, 3.27; Cl, 9.59; N, 18.94; O, 4.33; S, 8.67; ESI-MS (M+1): 370.83.
2.2.3.5 4-((4-Methoxy-6-(o-tolylthio)-1,3,5-triazin-2-yl)amino)benzonitrile (4e)
Yield: 77%; m.p. 179 °C; IR (KBr cm−1): 3212 (N–H), 2798 (C–H), 2264 (C≡N), 1397 (C–N in 2° aromatic amine), 812 (C3N3–s-triazine), 1264 (C–O), 978 (C–S); 1H NMR (400 MHz, DMSO-d6): δ 7.53 (d, J = 7.5 Hz, 2H), 7.50–7.16 (m, 3H), 7.14–6.89 (m, 3H), 6.06 (s, 1H), 3.83 (s, 3H), 2.41 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 195.1, 171.8, 167.3, 156.7, 144.6, 136.9, 133.4, 130.8, 125.6, 123.2, 119.4, 118.6, 115.2, 103.7, 53.9, 20.1; Anal. Calcd. for C17H12FN5OS: C, 57.78; H, 3.42; F, 5.38; N, 19.82; O, 4.53; S, 9.07, Found: C, 57.65; H, 3.31; F, 5.25; N, 19.78; O, 4.41; S, 9.10; ESI-MS (M+1): 350.41.
2.2.3.6 4-((4-Methoxy-6-(m-tolylthio)-1,3,5-triazin-2-yl)amino)benzonitrile (4f)
Yield: 62%; m.p. 154 °C; IR (KBr cm−1): 3376 (N–H), 2815 (C–H), 2120 (C≡N), 1264 (C–N in 2° aromatic amine), 892 (C3N3–s-triazine), 1254 (C–O), 1163 (C–S); 1H NMR (400 MHz, DMSO-d6): δ 7.62–7.47 (m, 2H), 7.30 (s, 1H), 7.22–7.18 (m, 3H), 7.13 (s, 1H), 6.95 (s, 1H), 6.30 (s, 1H), 3.86–3.82 (m, 3H), 2.36–2.32 (m, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.7, 176.1, 163.2, 154.2, 143.1, 137.9, 134.6, 130.9, 128.6, 124.2, 122.6, 119.7, 118.9, 103.1, 59.4, 21.1; Anal. Calcd. for C17H12FN5OS: C, 57.78; H, 3.42; F, 5.38; N, 19.82; O, 4.53; S, 9.07, Found: C, 57.69; H, 3.32; F, 5.28; N, 19.72; O, 4.44; S, 9.05; ESI-MS (M+1): 350.41.
2.2.3.7 4-((4-Methoxy-6-(p-tolylthio)-1,3,5-triazin-2-yl)amino)benzonitrile (4g)
Yield: 76%; m.p. 137 °C; IR (KBr cm−1): 3346 (N–H), 2912 (C–H), 2194 (C≡N), 1245 (C–N in 2° aromatic amine), 866 (C3N3–s-triazine), 1226 (C–O), 1167 (C–S): 1H NMR (400 MHz, DMSO-d6): δ 7.56 (d, J = 7.5 Hz, 2H), 7.27 (d, J = 7.5 Hz, 2H), 7.21 (d, J = 7.5 Hz, 2H), 7.05 (d, J = 7.5 Hz, 2H), 6.30 (s, 1H), 3.78 (s, 3H), 2.32 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 191.6, 175.3, 166.8, 154.9, 143.6, 142.9, 136.1, 133.4, 129.7, 119.7, 118.2, 103.7, 54.2, 23.9; Anal. Calcd. for C17H12FN5OS: C, 57.78; H, 3.42; F, 5.38; N, 19.82; O, 4.53; S, 9.07, Found: C, 57.66; H, 3.30; F, 5.26; N, 19.70; O, 4.50; S, 9.10; ESI-MS (M+1): 350.41.
2.2.3.8 4-((4-((2-Fluorophenyl)thio)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (4h)
Yield: 68%; m.p. 146 °C; IR (KBr cm−1): 3243 (N–H), 2887 (C–H), 2251 (C≡N), 1309 (C–N in 2° aromatic amine), 814 (C3N3–s-triazine), 1210 (C–O), 1320 (C–F), 900 (C–S) 1H NMR (400 MHz, DMSO-d6): δ 7.61–7.46 (m, 2H), 7.31–7.14 (m, 3H), 7.11 (s, 1H), 6.93 (d, J = 10.7 Hz, 2H), 6.29 (s, 1H), 3.78–3.74 (m, 3H); 13C NMR (100 MHz, DMSO-d6): δ 188.4, 176.9, 168.9, 158.2, 143.5, 134.6, 128.7, 127.4, 126.1, 124.7, 119.2, 118.7, 115.4, 103.7, 54.8; Anal. Calcd. for C18H15N5OS; C, 61.87; H, 4.33; N, 20.04; O, 4.58; S, 9.18, Found: C, 61.75; H, 4.21; N, 20.10; O, 4.44; S, 9.07; ESI-MS (M+1): 354.37.
2.2.3.9 4-((4-((3-Fluorophenyl)thio)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (4i)
Yield: 82%; m.p. 164 °C; IR (KBr cm−1): 3273 (N–H), 2840 (C–H), 2240 (C≡N), 1344 (C–N in 2° aromatic amine), 897 (C3N3–s-triazine), 1364 (C–F), 1296 (C–O), 964 (C–S): 1H NMR (400 MHz, DMSO-d6): δ 7.53 (d, J = 7.5 Hz, 2H), 8.59–6.81 (m, 8H), 6.15 (s, 1H), 3.83 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 196.3, 177.5, 167.8, 161.9, 145.8, 138.1, 135.2, 128.7, 124.1, 119.2, 118.7, 114.7, 112.3, 101.3, 52.1; Anal. Calcd. For C18H15N5OS C, 61.87; H, 4.33; N, 20.04; O, 4.58; S, 9.18, Found: C, 61.74; H, 4.23; N, 20.11; O, 4.43; S, 9.05; ESI-MS (M+1): 354.37.
2.2.3.10 4-((4-((4-Fluorophenyl)thio)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (4j)
Yield: 65%; m.p. 183 °C; IR (KBr cm−1): 3245 (N–H), 2863 (C–H), 2221 (C≡N), 1364 (C–N in 2° aromatic amine), 897 (C3N3–s-triazine), 1252 (C–O), 1347 (C–F), 946 (C–S): 1H NMR (400 MHz, DMSO-d6): δ 7.49 (d, J = 7.5 Hz, 2H), 7.37–7.28 (m, 2H), 7.21 (d, J = 7.5 Hz, 2H), 6.91 (t, J = 7.7 Hz, 2H), 6.30 (s, 1H), 3.85 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.6, 178.3, 168.3, 154.1, 141.6, 131.4, 129.2, 127.4, 120.4, 118.4, 114.9, 105.7, 56.5; Anal. Calcd. for C17H12N5OS: C, 61.06; H, 3.62; N, 20.94; O, 4.78; S, 9.59, Found: C, 61.10; H, 3.52; N, 20.83; O, 4.66; S, 9.48; ESI-MS (M+1): 354.37.
2.2.4 General procedure for the preparation of 4-((4-(substituted phenoxy)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5a–j)
A mixture of 4-((4-chloro-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile 3 (5.0 g, 0.0191 mol), appropriate phenol (0.0191 mol) and sodium hydroxide (0.93 g, 0.0232 mol) in THF (20 mL) was stirred and refluxed for 5 h. The progress of the reaction was monitored by TLC using ethyl acetate:hexane (4:1) solvent system as an eluent. After the completion of the reaction, resultant mixture was poured into crushed ice. The solid product obtained was filtered, washed with distilled water, dried and purified by column chromatography using ethyl acetate:hexane solvent as an eluent.
2.2.4.1 4-((4-Methoxy-6-phenoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5a)
Yield: 82%; m.p. 140 °C; IR (KBr cm−1): 3397 (N–H), 2788 (C–H), 2164 (C≡N), 1268 (C–N in 2° aromatic amine), 866 (C3N3–s-triazine), 1234 (C–O); 1H NMR (400 MHz, DMSO-d6): δ 7.55 (d, J = 7.5 Hz, 2H), 7.26–7.12 (m, 4H), 6.98–6.85 (m, 3H), 6.27 (s, 1H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 195.4, 173.9, 167.8, 152.8, 144.1, 136.4, 128.9, 123.6, 122.8, 119.3, 118.7, 104.1, 56.8; Anal. Calcd. for C17H13N5O2: C, 63.94; H, 4.10; N, 21.93; O, 10.02, Found: C, 63.80; H, 4.00; N, 21.82; O, 10.12; ESI-MS (M+1): 320.11.
2.2.4.2 4-((4-Methoxy-6-(o-tolyloxy)-1,3,5-triazin-2-yl)amino)benzonitrile (5b)
Yield: 73%; m.p. 168 °C; IR (KBr cm−1): 3245 (N–H), 2858 (C–H), 2232 (C≡N), 1301 (C–N in 2° aromatic amine), 864 (C3N3–s-triazine), 1214 (C–O); 1H NMR (400 MHz, DMSO-d6): δ 7.55 (d, J = 7.5 Hz, 2H), 7.22 (d, J = 7.5 Hz, 2H), 7.06 (ddd, J = 14.3, 7.4, 1.5 Hz, 2H), 6.92–6.82 (m, 2H), 6.27 (s, 1H), 3.81 (s, 3H), 2.35 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 188.3, 175.9, 164.3, 157.2, 154.9, 146.1, 134.3, 129.2, 127.6, 124.8, 119.1, 118.8, 114.2, 103.8, 58.1, 18.5; Anal. Calcd. for C18H15N5O2: C, 64.86; H, 4.54; N, 21.01; O, 9.60, Found: C, 64.75; H, 4.44; N, 21.11; O, 9.51; ESI-MS (M+1): 334.12.
2.2.4.3 4-((4-Methoxy-6-(m-tolyloxy)-1,3,5-triazin-2-yl)amino)benzonitrile (5c)
Yield: 74%, m.p. 139 °C; IR (KBr cm−1): 3369 (N–H), 2887 (C–H), 2186 (C≡N), 1362 (C–N in 2° aromatic amine), 826 (C3N3–s-triazine), 1171 (C–O); 1H NMR (400 MHz, DMSO-d6): δ 7.47 (d, J = 7.5 Hz, 2H), 7.22 (d, J = 7.5 Hz, 2H), 7.12 (t, J = 7.4 Hz, 1H), 6.85–6.74 (m, 3H), 6.27 (s, 1H), 3.81 (s, 3H), 2.34 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 186.9, 179.2, 162.8, 158.5, 151.3, 145.7, 133.4, 127.6, 124.9, 121.1, 120.6, 116.8, 112.3, 106.1, 57.4, 22.6; Anal. Calcd. for C18H15N5O2; C, 64.86; H, 4.54; N, 21.01; O, 9.60, Found: C, 64.73; H, 4.44; N, 21.00; O, 9.58; ESI-MS (M+1): 334.12.
2.2.4.4 4-((4-Methoxy-6-(p-tolyloxy)-1,3,5-triazin-2-yl)amino)benzonitrile (5d)
Yield: 69%; m.p. 161 °C; IR (KBr cm−1): 3361 (N–H), 2859 (C–H), 2114 (C≡N), 1285 (C–N in 2° aromatic amine), 897 (C3N3–s-triazine), 1216 (C–O); 1H NMR (400 MHz, DMSO-d6): δ 7.22 (d, J = 7.5 Hz, 2H), 7.04 (d, J = 7.5 Hz, 2H), 6.84 (d, J = 7.5 Hz, 2H), 6.27 (s, 1H), 8.35 to −2.37 (m, 15H), 7.50 to −2.37 (m, 13H), 3.81 (s, 3H), 2.33 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 191.3, 173.9, 162.9, 156.5, 152.3, 147.4, 132.1, 129.4, 121.6, 119.2, 114.1, 104.3, 58.4, 20.4; Anal. Calcd. for C18H15N5O2: C, 64.86; H, 4.54; N, 21.01; O, 9.60, Found: C, 64.76; H, 4.42; N, 20.95; O, 9.50; ESI-MS (M+1): 334.12.
2.2.4.5 4-((4-(2-Fluorophenoxy)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5e)
Yield: 68%; m.p. 166 °C; IR (KBr cm−1): 3297 (N–H), 2879 (C–H), 2144 (C≡N), 1321 (C–N in 2° aromatic amine), 844 (C3N3–s-triazine), 1164 (C–O), 1341 (C–F); 1H NMR (400 MHz, DMSO-d6): δ 7.56 (d, J = 7.5 Hz, 2H), 7.22 (d, J = 7.5 Hz, 2H), 7.07–6.64 (m, 4H), 6.26 (s, 1H), 3.79 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 191.9, 178.3, 164.3, 154.8, 152.3, 141.6, 138.2, 127.2, 125.1, 121.4, 119.4, 117.2, 113.7, 104.3, 56.7; Anal. Calcd. for C17H12FN5O2 C, 60.53; H, 3.59; F, 5.63; N, 20.76; O, 9.49, Found: C, 60.42; H, 3.47; F, 5.53; N, 20.65; O, 9.33; ESI-MS (M+1): 338.10.
2.2.4.6 4-((4-(3-Fluorophenoxy)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5f)
Yield: 81%; m.p. 175 °C; IR (KBr cm−1): 3364 (N–H), 2851 (C–H), 2261 (C≡N), 1327 (C–N in 2° aromatic amine), 832 (C3N3–s-triazine), 1234 (C–O), 1399 (C–F); 1H NMR (400 MHz, DMSO-d6): δ 7.51 (d, J = 7.5 Hz, 2H), 7.26–7.12 (m, 3H), 6.68 (ddd, J = 14.2, 5.7, 1.3 Hz, 3H), 6.27 (s, 1H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 194.7, 173.9, 161.3, 154.6, 153.2, 148.6, 134.3, 127.7, 125.1, 123.6, 119.8, 118.4, 115.4, 108.1, 57.2; Anal. Calcd. for C17H12FN5O2 C, 60.53; H, 3.59; F, 5.63; N, 20.76; O, 9.49, Found: C, 60.41; H, 3.48; F, 5.54; N, 20.64; O, 9.32; ESI-MS (M+1): 338.10.
2.2.4.7 4-((4-(4-Fluorophenoxy)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5g)
Yield: 73%; m.p. 175 °C; IR (KBr cm−1): 3313 (N–H), 2812 (C–H), 2245 (C≡N), 1297 (C–N in 2° aromatic amine), 889 (C3N3–s-triazine), 1145 (C–O), 1362 (C–F); 1H NMR (400 MHz, DMSO-d6): δ 7.57 (d, J = 7.5 Hz, 2H), 7.26 (d, J = 7.5 Hz, 2H), 6.96–6.83 (m, 4H), 6.27 (s, 1H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.9, 174.3, 163.3, 157.1, 151.5, 144.2, 133.7, 125.4, 120.1, 117.8, 114.6, 107.4, 56.9; Anal. Calcd. for C17H12FN5O2 C, 60.53; H, 3.59; F, 5.63; N, 20.76; O, 9.49, Found: C, 60.45; H, 3.50; F, 5.57; N, 20.66; O, 9.34; ESI-MS (M+1): 338.10.
2.2.4.8 4-((4-(2-Chlorophenoxy)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5h)
Yield: 64%; m.p. 136 °C; IR (KBr cm−1): 3293 (N–H), 2866 (C–H), 2164 (C≡N), 1284 (C–N in 2° aromatic amine), 837 (C3N3–s-triazine), 1164 (C–O), 596 (C–Cl); 1H NMR (400 MHz, DMSO-d6): δ 7.50 (d, J = 7.5 Hz, 2H), 7.29–7.11 (m, 3H), 7.08 (td, J = 7.5, 1.4 Hz, 1H), 6.91–6.78 (m, 2H), 6.29 (s, 1H), 3.80 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 195.7, 176.3, 164.8, 155.1, 146.8, 133.4, 131.7, 129.2, 127.4, 124.6, 122.9, 120.1, 117.8, 106.3, 57.9; Anal. Calcd. For C17H12ClN5O2: C, 57.72; H, 3.42; Cl, 10.02; N, 19.80; O, 9.05, Found: C, 57.61; H, 3.30; Cl, 10.15; N, 19.69; O, 8.96; ESI-MS (M+1): 354.07.
2.2.4.9 4-((4-(3-Chlorophenoxy)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5i)
Yield: 68%; m.p. 167 °C; IR (KBr cm−1): 3212 (N–H), 2832 (C–H), 2156 (C≡N), 1244 (C–N in 2° aromatic amine), 846(C3N3–s-triazine), 1161 (C–O), 569 (C–Cl); 1H NMR (400 MHz, DMSO-d6): δ 7.58 (d, J = 7.5 Hz, 2H), 7.22 (d, J = 7.5 Hz, 2H), 7.12 (t, J = 7.5 Hz, 1H), 7.01–6.92 (m, 2H), 6.79 (dt, J = 7.3, 1.4 Hz, 1H), 6.27 (s, 1H), 3.80 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 194.1, 176.9, 164.2, 153.2, 147.2, 139.5, 134.4, 131.9, 126.1, 122.6, 120.2, 119.5, 118.3, 103.2, 56.3; Anal. Calcd. for C17H12ClN5O2: C, 57.72; H, 3.42; Cl, 10.02; N, 19.80; O, 9.05, Found: C, 57.60; H, 3.31; Cl, 10.14; N, 19.71; O, 8.97; ESI-MS (M+1): 354.07.
2.2.4.10 4-((4-(4-Chlorophenoxy)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (5j)
Yield: 79%; m.p. 164 °C; IR (KBr cm−1): 3361 (N–H), 2843 (C–H), 2120 (C≡N), 1320 (C–N in 2° aromatic amine), 829 (C3N3–s-triazine), 1156 (C–O), 561 (C–Cl); 1H NMR (400 MHz, DMSO-d6): δ 7.49 (d, J = 7.5 Hz, 2H), 7.21 (dd, J = 13.6, 7.5 Hz, 4H), 6.83 (d, J = 7.5 Hz, 2H), 6.27 (s, 1H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 189.2, 177.3, 161.8, 150.9, 145.2, 136.4, 132.9, 130.2, 120.1, 118.6, 114.2, 107.2, 57.4; Anal. Calcd. for C17H12ClN5O2: C, 57.72; H, 3.42; Cl, 10.02; N, 19.80; O, 9.05, Found: C, 57.62; H, 3.32; Cl, 10.12; N, 19.70; O, 8.95; ESI-MS (M+1): 354.07.
2.2.5 General procedure for the synthesis of 4-(4-methoxy-6-phenyl amino-[1,3,5 trazine-2-ylamino)-benzonitrile (6a–g)
A stirred solution of 4-((4-chloro-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile 3 (5.0 g, 0.0191 mol), appropriate aniline (0.0191 mol) and sodium bicarbonate (1.77 g, 0.0210 mol) in THF (20 mL) was refluxed for 5 h. The progress of reaction was monitored by TLC using hexane:ethyl acetate (4:1) as an eluent. After the completion of reaction, the refluxed content was poured into crushed ice. The solid product obtained was filtered and dried. The crude product was purified by crystallization from acetone to get the title compound.
2.2.5.1 4-((4-Methoxy-6-(phenylamino)-1,3,5-triazin-2-yl)amino)benzonitrile (6a)
Yield: 78%; m.p. 113 °C; IR (KBr cm−1): 3278 (N–H), 1248 (C–O–C), 1310 (CN), 3085 (Aromatic CH str), 836 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.60 (d, J = 7.5 Hz, 2H), 7.30–7.16 (m, 4H), 7.03–6.92 (m, 3H), 4.79 (s, 1H), 4.51 (s, 1H), 3.87 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 189.7, 172.3, 166.3, 148.8, 136.2, 132.7, 128.7, 122.1, 121.6, 118.4, 113.9, 103.1, 56.8; Anal. Calcd. for C17H14N6O: C, 64.14; H, 4.43; N, 26.40; O, 5.03, Found: C, 62.14; H, 4.33; N, 26.40; O, 5.03; ESI-MS (M+1): 319.12.
2.2.5.2 4-((4-((2-Chlorophenyl)amino)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (6b)
Yield: 81%; m.p. 144 °C; IR (KBr cm−1): 3276 (NH), 1245 (C–O–C), 1314 (CN), 3064 (Aromatic CH str), 849 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.60 (d, J = 7.5 Hz, 2H), 9.47–7.22 (m, 3H), 9.47–7.08 (m, 6H), 9.47–7.01 (m, 7H), 9.47–6.94 (m, 7H), 9.47–5.22 (m, 9H), 4.77 (s, 1H), 3.92 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 189.8, 170.9, 167.7, 145.8, 140.5, 137.6, 135.1, 130.8, 127.6, 122.1, 120.6, 118.5, 112.4, 102.1, 52.7; Anal. Calcd. for C17H13ClN6O: C, 57.88; H, 3.71; Cl, 10.05; N, 23.82; O, 4.54, Found: C, 56.84; H, 3.69; Cl, 10.05; N, 22.82; O, 4.52; ESI-MS (M+1): 353.08.
2.2.5.3 4-((4-((3-Chlorophenyl)amino)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (6c)
Yield: 70%; m.p. 138 °C; IR (KBr cm−1): 3259 (NH), 1259 (C–O–C), 1325 (CN), 3059 (Aromatic CH str), 831 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.55 (d, J = 7.5 Hz, 2H), 7.24–7.16 (m, 3H), 7.02–6.92 (m, 3H), 4.70 (s, 1H), 4.60 (s, 1H), 3.88 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 193.3, 174.3, 162.8, 146.3, 143.5, 135.8, 133.1, 128.6, 126.6, 122.1, 118.5, 116.6, 112.3, 106.1, 58.3; Anal. Calcd. for C17H13ClN6O: C, 57.88; H, 3.71; Cl, 10.05; N, 23.82; O, 4.54, Found: C, 57.70; H, 3.55; Cl, 10.05; N, 23.83; O, 4.55; ESI-MS (M+1): 353.08.
2.2.5.4 4-((4-Methoxy-6-(o-tolylamino)-1,3,5-triazin-2-yl)amino)benzonitrile (6d)
Yield: 72%; m.p. 164 °C; IR (KBr cm−1): 3255 (NH), 1256 (C–O–C), 1338 (CN), 3052 (Aromatic CH str), 831 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.84 (t, J = 177.8 Hz, 2H), 9.35–7.07 (m, 6H), 9.35–6.95 (m, 7H), 9.35–4.93 (m, 8H), 8.33–11.38 (m, 16H), 7.65–11.38 (m, 16H), 4.65 (s, 1H), 4.30 (s, 1H), 3.87 (s, 3H), 2.31 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 194.3, 176.2, 164.4, 146.1, 142.3, 139.8, 135.1, 130.0, 128.2, 126.6, 121.6, 119.3, 118.2, 105.8, 57.6, 18.9; Anal. Calcd. for C18H16N6O: C, 65.05; H, 4.85; N, 25.29; O, 4.81, Found: C, 63.05; H, 3.85; N, 23.29; O, 4.85; ESI-MS (M+1): 333.14.
2.2.5.5 4-((4-Methoxy-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)amino)benzonitrile (6e)
Yield: 68%; m.p. 171 °C; IR (KBr cm−1): 3253 (NH), 1239 (C–O–C), 1349 (CN), 3055 (Aromatic CH str), 855 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 8.16 (d, J = 7.5 Hz, 2H), 7.60 (d, J = 7.5 Hz, 2H), 7.27 (d, J = 7.5 Hz, 2H), 7.18 (d, J = 7.5 Hz, 2H), 4.79 (s, 1H), 4.74 (s, 1H), 3.89 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.0, 177.9, 162.8, 151.9, 144.8, 134.1, 132.5, 126.7, 119.2, 116.8, 113.1, 105.3, 58.2; Anal. Calcd. for C17H13N7O3: C, 56.20; H, 3.61; N, 26.99; O, 13.21, Found: C, 56.20; H, 3.61; N, 26.99; O, 13.21; ESI-MS (M+1): 364.11.
2.2.5.6 4-((4-((4-Bromophenyl)amino)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (6f)
Yield: 73%; m.p. 143 °C; IR (KBr cm−1): 3254 (NH), 1230 (C–O–C), 1339 (CN), 3054 (Aromatic CH str), 850 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.69 (d, J = 7.5 Hz, 2H), 7.42 (d, J = 7.5 Hz, 2H), 7.11 (d, J = 7.5 Hz, 2H), 6.87 (d, J = 7.5 Hz, 2H), 5.65 (s, 1H), 4.46 (s, 1H), 3.81 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 191.4, 180.7, 169.2, 156.5, 141.9, 136.6, 130.7, 125.1, 120.2, 117.6, 114.9, 107.1, 56.1; Anal. Calcd. for C17H13BrN6O: C, 51.40; H, 3.30; Br, 20.12; N, 21.16; O, 4.03, Found: C, 51.40; H, 3.35; Br, 20.05; N, 21.12; O, 4.04; ESI-MS (M+1): 397.03.
2.2.5.7 4-((4-Methoxy-6-((4-methoxyphenyl)amino)-1,3,5-triazin-2-yl)amino)benzonitrile (6g)
Yield: 82%; m.p. 166 °C; IR (KBr cm−1): 3255 (NH), 1239 (C–O–C), 1347 (CN), 3055 (Aromatic CH str), 856 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.59 (d, J = 7.5 Hz, 2H), 7.17 (d, J = 7.5 Hz, 2H), 6.96 (d, J = 7.5 Hz, 2H), 6.85 (d, J = 7.5 Hz, 2H), 4.58 (s, 1H), 4.31 (s, 1H), 3.86 (s, 3H), 3.82 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 195.1, 171.6, 164.4, 152.9, 144.8, 137.2, 1326, 125.2, 120.8, 118.1, 114.9, 105.5, 59.7, 54.2; Anal. Calcd. for C18H16N6O2: C, 62.06; H, 4.63; N, 24.12; O, 9.19, Found: C, 61.06; H, 4.60; N, 24.11; O, 9.18; ESI-MS (M+1): 349.13.
2.2.6 General procedure of triazine based morpholine piperidine and piperazine derivatives (7a–h)
To a stirred solution of 4-((4-chloro-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile 3 (5.0 g, 0.0191 mol) and sodium bicarbonate (1.77 g, .0210 mol) in acetone (10.0 mL), a solution of appropriate piperazines or morpholine or piperidine (0.0191 mol) in 5 mL acetone was added dropwise and refluxed for 4–5 h. The progress of reaction was monitored by TLC using toluene:ethyl acetate (6:4) as an eluent. After the completion of the reaction, the refluxed content was poured into crushed ice. The solid product obtained was filtered and dried. The crude product was purified by crystallization from ethyl acetate to get the title compound.
2.2.6.1 4-((4-Methoxy-6-(4-methylpiperazin-1-yl)-1,3,5-triazin-2-yl)amino)benzonitrile (7a)
Yield: 76%; m.p. 139 °C; IR (KBr cm−1): 3233 (NH), 1274 (C–O–C), 1325 (CN), 3048 (Aromatic CH str), 863 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.81 (d, J = 7.5 Hz, 2H), 7.18 (d, J = 7.5 Hz, 2H), 4.48 (s, 1H), 3.85 (s, 3H), 3.69 (t, J = 5.2 Hz, 2H), 3.55 (t, J = 5.2 Hz, 2H), 2.77 (t, J = 5.1 Hz, 2H), 2.59 (t, J = 5.2 Hz, 2H), 2.34 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 189.5, 172.4, 168.3, 142.7, 134.1, 120.9, 118.4, 105.6, 58.3, 50.8, 49.2, 45.7; Anal. Calcd. for C16H19N7O: C, 59.06; H, 5.89; N, 30.13; O, 4.92, Found: C, 58.06; H, 5.88; N, 30.12; O, 4.92; ESI-MS (M+1): 326.17.
2.2.6.2 4-((4-(4-Ethylpiperazin-1-yl)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (7b)
Yield: 69%; m.p. 156 °C; IR (KBr cm−1): 3254 (NH), 1265 (C–O–C), 1339 (CN), 3024 (Aromatic CH str), 844 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.49 (d, J = 7.5 Hz, 2H), 7.14 (d, J = 7.5 Hz, 2H), 4.41 (s, 1H), 3.89 (s, 3H), 3.67 (t, J = 5.1 Hz, 2H), 3.52 (t, J = 5.2 Hz, 2H), 2.81 (t, J = 5.2 Hz, 2H), 2.59–2.49 (m, 4H), 1.08 (t, J = 6.3 Hz, 3H); 13C NMR (100 MHz, DMSO-d6): δ 194.6, 176.1, 167.7, 145.6, 135.4, 120.2, 114.9, 105.3, 59.3, 55.6, 50.0, 45.1, 13.8; Anal. Calcd. for C17H21N7O: C, 60.16; H, 6.24; N, 28.89; O, 4.71, Found: C, 65.16; H, 6.15; N, 28.89; O, 4.66; ESI-MS (M+1): 340.18.
2.2.6.3 4-((4-(4-Acetylpiperazin-1-yl)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (7c)
Yield: 71%; m.p. 136 °C; IR (KBr cm−1): 3240 (NH), 1270 (C–O–C), 1358 (CN), 3076 (Aromatic CH str), 821 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.67 (d, J = 7.5 Hz, 2H), 7.12 (d, J = 7.5 Hz, 2H), 4.58 (s, 1H), 4.36–3.76 (m, 5H), 4.36–2.26 (m, 11H), 2.08 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 191.1, 177.9, 167.6, 162.9, 145.5, 134.9, 120.3, 115.1, 106.8, 58.4, 52.5, 46.9, 22.7; Anal. Calcd. for C17H19N7O2: C, 57.78; H, 5.42; N, 27.75; O, 9.06, Found: C, 58.78; H, 5.44; N, 26.75; O, 9.06; ESI-MS (M+1): 354.16.
2.2.6.4 4-((4-(4-Isopropylpiperazin-1-yl)-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (7d)
Yield: 84%; m.p. 157 °C; IR (KBr cm−1): 3245 (NH), 1271 (C–O–C), 1355 (CN), 3076 (Aromatic CH str), 850 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.65–7.51 (m, 2H), 7.25–7.10 (m, 2H), 4.48 (s, 1H), 3.87–3.83 (m, 3H), 3.71–3.67 (m, 2H), 3.58–3.54 (m, 2H), 3.16 (s, 1H), 2.79–2.75 (m, 2H), 2.63–2.59 (m, 2H), 1.18 – 1.05 (m, 6H); 13C NMR (100 MHz, DMSO-d6): δ 189.1, 172.9, 161.3, 149.4, 135.5, 121.8, 118.1, 104.9, 69.2, 58.4, 54.9, 50.7, 16.5; Anal. Calcd. for C18H23N7O: C, 61.17; H, 6.56; N, 27.74; O, 4.53, Found: C, 62.15; H, 6.56; N, 25.74; O, 4.53; ESI-MS (M+1): 354.20.
2.2.6.5 4-((4-Methoxy-6-(4-phenylpiperazin-1-yl)-1,3,5-triazin-2-yl)amino)benzonitrile (7e)
Yield: 67%; m.p. 164 °C; IR (KBr cm−1): 3241 (NH), 1270 (C–O–C), 1358 (CN), 3076 (Aromatic CH str), 844 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.66 (d, J = 7.5 Hz, 2H), 7.17 (d, J = 7.5 Hz, 2H), 7.11 (dd, J = 10.7, 4.4 Hz, 2H), 6.71–6.62 (m, 3H), 4.55 (s, 1H), 3.86 (s, 3H), 4.36–3.37 (m, 11H); 13C NMR (100 MHz, DMSO-d6): δ 195.8, 176.3, 165.2, 144.9, 141.6, 134.1, 130.9, 125.3, 119.7, 116.6, 112.1, 104.8, 59.5, 47.8, 46.1; Anal. Calcd. for C21H21N7O: C, 65.10; H, 5.46; N, 25.31; O, 4.13, Found: C, 62.10; H, 5.45; N, 24.31; O, 4.10; ESI-MS (M+1): 388.18.
2.2.6.6 4-((4-Methoxy-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)amino)benzonitrile (7f)
Yield: 73%; m.p. 148 °C; IR (KBr cm−1): 3240 (NH), 1272 (C–O–C), 1355 (CN), 3176 (Aromatic CH str), 823 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.58 (d, J = 7.5 Hz, 2H), 7.21 (d, J = 7.5 Hz, 2H), 4.61 (s, 1H), 3.86 (s, 3H), 3.84–3.39 (m, 2H), 3.31–2.74 (m, 2H), 2.17–1.53 (m, 6H); 13C NMR (100 MHz, DMSO-d6): δ 192.1, 177.2, 161.3, 148.9, 135.7, 120.8, 114.6, 104.2, 58.7, 55.1, 28.6, 23.2; Anal. Calcd. for C16H18N6O: C, 61.92; H, 5.85; N, 27.08; O, 5.16, Found: C, 59.92; H, 5.83; N, 27.05; O, 5.14; ESI-MS (M+1): 311.15.
2.2.6.7 4-((4-Methoxy-6-morpholino-1,3,5-triazin-2-yl)amino)benzonitrile (7g)
Yield: 64%; m.p. 152 °C; IR (KBr cm−1): 3249 (NH), 1270 (C–O–C), 1358 (CN), 3077 (Aromatic CH str), 821 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.66 (d, J = 7.5 Hz, 2H), 7.16 (d, J = 7.5 Hz, 2H), 4.51 (s, 1H), 3.86 (s, 3H), 3.78 (t, J = 4.8 Hz, 4H), 3.54 (t, J = 4.9 Hz, 2H), 3.43 (t, J = 4.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ 187.1, 169.4, 162.3, 148.4, 139.6, 121.5, 118.3, 107.6, 67.5, 55.3, 48.2; Anal. Calcd. for C15H16N6O2: C, 61.92; H, 5.85; N, 27.08; O, 5.16, Found: C, 58.68; H, 5.12; N, 24.91; O, 10.25; ESI-MS (M+1): 313.13.
2.2.6.8 4-((4-Methoxy-6-(4-(4-methoxyphenyl)piperazin-1-yl)-1,3,5-triazin-2-yl)amino)benzonitrile (7h)
Yield: 78%; m.p. 133 °C; IR (KBr cm−1): 3249 (NH), 1270 (C–O–C), 1358 (CN), 3074 (Aromatic CH str), 825 (s-triazine C–N str.); 1H NMR (400 MHz, DMSO-d6): δ 7.58 (d, J = 7.5 Hz, 2H), 7.19 (d, J = 7.3 Hz, 2H), 6.72 (d, J = 7.5 Hz, 2H), 6.65 (d, J = 7.5 Hz, 2H), 4.54 (s, 1H), 3.86 (s, 3H), 4.36 – 3.52 (m, 14H); 13C NMR (100 MHz, DMSO-d6): δ 189.9, 173.8, 169.2, 156.4, 146.2, 142.3, 134.5, 127.7, 119.6, 114.9, 112.6, 108.3, 59.1, 56.8, 49.2, 44.5; Anal. Calcd. for C22H23N7O2: C, 63.30; H, 5.55; N, 23.49; O, 7.67, Found: C, 60.30; H, 5.55; N, 22.49; O, 7.65; ESI-MS (M+1): 418.19.
2.3 Biological assays
2.3.1 In vitro antimicrobial assays
A stock solution of the final synthesized compounds (200 μg/ml) was prepared in dimethyl sulfoxide and test compounds were taken in a specified quantity of molten sterile agar, i.e., nutrient agar and dextrose agar for antibacterial and for antifungal screening, respectively. Such medium enclosing the test compound was poured into a Petri dish at a depth of 4–5 mm and allowed to solidify under aseptic conditions. A suspension of the respective microorganism of 105 CFU/ml was prepared and added to plates with serially diluted compounds with concentrations in the range of 3.12–200 μg/ml in dimethyl sulfoxide and incubated at (37 ± 1) °C temperature for 24 h (bacteria) or 48 h (fungi). Minimum concentration of the substance that prevents the development of visible growth is considered to be the MIC value.
2.3.2 In vitro antituberculosis assays
The Mycobacteria Growth Indicator Tubes (MGIT) containing 4 ml of modified Middle brook 7H9 Broth Base were numbered as per the final compounds to be tested for antituberculosis activity by means of various concentrations prepared. The solution was allowed to sit for 20 min, and the tubes were centrifuged at 3000 rpm for 15–20 min. After that about 104–107 CFU/ml of H37RV M. tuberculosis strain suspension was added into the medium to be incubated. The MGIT tubes were then closed tightly, stirred well and incubated in a BACTEC MGIT instrument at 37 °C until positivity is observed. The readings were measured from the second day of incubation onwards. Positive cultures were generally detected within 10 days. To observe actual results, the MGIT tubes were removed from incubator and placed under the UV light. Bright fluorescence perceived by the corresponding MGIT tube was noticed in the form of bright orange color in the bottom of the tube showing an orange reflection on the meniscus. The primary screening was carried out at concentration of 12.5 μg/ml against M. tuberculosis H37RV in BACTEC MGIT system. Compounds possessing 99% inhibition in the primary screen were described as most active compounds.
3 Results and discussion
3.1 Chemistry
The designed library of target compounds and respective intermediates were synthesized as outlined in Scheme. The first step comprises the nucleophilic substitution of first chlorine atom of cyanuric chloride (1) by methanol to give 2,4-dichloro-6-methoxy-1,3,5-triazine (2) intermediate with an efficient yield. Appearance of IR absorption peak at 2820 cm−1 confirms the presence of the methoxy group in s-triazine. The intermediate 4-((4-chloro-6-methoxy-1,3,5-triazin-2-yl)amino)benzonitrile (3) was achieved by condensation of compound (2) with 4-amino benzonitrile. It displayed absorption band at 2235 cm−1 and 3294 cm−1 and showed the attachment of cyano and 2° amine group.
The target compound, third chlorine atom of cyanuric chloride was replaced by various substituted phenol, thiophenol, aniline and piperazine/piperidine/morpholine derivatives using appropriate solvents and formed final 4a–j, 5a–j, 6a–g and 7a–h compounds respectively which were further characterized by FT-IR, 1H NMR, 13C NMR, Mass and elemental analyses. Compounds 4a–j’s derivatives endowing thiophenol substituents were confirmed by peaks at 1120 cm−1; phenol substituents of 5a–j were verified by characteristic –C–O– stretching peaks at 1210 cm−1. Additional proton peak of –NH, in 1H NMR, confirmed the substitution with aniline derivatives in the formation of 6a–g compounds whereas besides of –OCH3 peak, 1H NMR spectra of 7a–h compounds appeared with distinguishable –CH2–N–CH2– peaks in the range from 3.70 to 3.51 ppm.
3.2 Biological evaluation
All the synthesized compounds (4a–j, 5a–j, 6a–g and 7a–h) were examined for their antibacterial activities (against four strains of bacteria- Staphylococcus aureus MTCC 96, Bacillus cereus MTCC 430, Escherichia coli MTCC 739, and Pseudomonas aeruginosa MTCC 741) and antifungal activities (against three strains of fungi- Candida albicans MTCC 183, Aspergillus niger MTCC 282, and Aspergillus clavatus MTCC 1323) using the broth dilution technique (CitationHawkey and Lewis, 2004) and were also checked for their antituberculosis activity (against M. tuberculosis H37Rv) using the BACTEC MGIT method as reported earlier (CitationIsenberg and Microbiology, 1992). Ciprofloxacin and ketoconazole were used as standard drugs for antibacterial and antifungal activities, respectively, whereas isoniazid, rifampicin, ethambutol and pyrazinamide are used as standard drugs for antituberculosis activity.
3.2.1 In vitro antibacterial activity
shows that all synthesized compounds exhibited well to moderate activity, among them, the chloro group containing moiety 4d was found to be highly active for the bacterial strain B. cereus MTCC 430. The electron donating group i.e. methyl group containing compound 4g was found superior to others against the bacterial species E. coli MTCC 739. Compounds 4i and 4j possessing the fluoro group exhibited excellent inhibitory profile against the bacterial strains P. aeruginosa MTCC 741 and S. aureus MTCC 96 respectively. Compound 4c having the chloro group showed very good efficacy for the strain B. cereus MTCC 430. Another moieties possessing the chloro group i.e. 4c and 4d were found to be active for the bacterial strains P. aeruginosa MTCC 741 and S. aureus MTCC 96 respectively. The fluorinated compounds 4h and 4i showed excellent inhibitory profile for the bacterial strains S. aureus MTCC 96 and B. cereus MTCC 430, respectively. Again compounds 4i and 4j also exhibited potent inhibitory profile against the bacterial strains, E. coli MTCC 739 and B. cereus MTCC 430, respectively. Antibacterial efficacy study showed that out of the compounds 5a–j, compound 5e endowed with the fluoro group was only found to be highly potent for the bacterial strain P. aeruginosa MTCC 741. Compounds 5e and 5g possessing the fluoro group displayed potent inhibitory profile for the strains S. aureus MTCC 96 and B. cereus MTCC 430, respectively. The chloro group containing scaffolds i.e. 5h, 5i and 5j also gave very good antibacterial activity against the strains, B. cereus MTCC 430, E. coli MTCC 739, and S. aureus MTCC 96, respectively. Among the synthesized scaffolds 6a–g, compounds 6c and 6f which have the halogen group were found to exhibit excellent inhibitory effect on the bacterial strains B. cereus MTCC 430 and P. aeruginosa MTCC 741, respectively. The chlorinated moiety 6b was also found to be active against the bacterial species B. cereus MTCC 430. The methyl group containing scaffold i.e. 6d showed very good antibacterial activity against the bacterium E. coli MTCC 739. The brominated compound 6f exhibited excellent inhibitory effect on the bacterial strain S. aureus MTCC 96. Compound 6g possessing the methoxy group was found to be active for both the bacterial strains, S. aureus MTCC 96 and E. coli MTCC 739. The antibacterial activity study of compounds 7a–h revealed that there are three triazine moieties which exhibited superior antibacterial efficacy against the specific strain of bacteria. Compound 7c having the N-acetyl group was found to be highly potent against the bacterial strain S. aureus MTCC 96. Compound 7e endowed with N-phenyl piperazinyl molecule was found superior to other with respect to inhibiting the growth of P. aeruginosa MTCC 741. A triazine scaffold 7h having p-methoxy phenyl piperazinyl entity gave excellent inhibitory effect on the bacterial strain E. coli MTCC 739. The N-isopropyl piperazinyl triazine moiety 7d was found as a potent antibacterial agent against both the strains B. cereus MTCC 430 and P. aeruginosa MTCC 741. A piperidinyl substituted triazine derivative 7f showed good efficiency against both the bacterial strains S. aureus MTCC 96 and E. coli MTCC 739. The morpholine substituted triazine scaffold 7g exhibited potent antibacterial activity for both the strains S. aureus MTCC 96 and P. aeruginosa MTCC 741. Compound 7h also showed potency against the strain P. aeruginosa MTCC 741.
Table 1 In vitro antimicrobial activity of compounds 4a–j, 5a–j, 6a–g and 7a–h.
3.2.2 In vitro antifungal activity
All synthesized triazine scaffolds were examined for their antifungal potency, which is outlined in shows that compounds having the halogen group exhibited highly potential antifungal efficacy against specific strain of fungi. The chlorinated moieties 4c and 4d displayed excellent inhibitory profile against the fungal strains C. albicans MTCC 183 and A. clavatus MTCC 1323, respectively. The compound containing the fluoro group i.e. 4i was found to be highly active for the strain A. niger MTCC 282. Another chlorinated moiety 4b also showed very good antifungal activity against the strain A. niger MTCC 282. Compound 4d was also found to be active against the strain C. albicans MTCC 183. The fluorinated compounds 4h, 4i and 4j exhibited good antifungal efficacy against the fungal strains A. niger MTCC 282, A. clavatus MTCC 1323, and C. albicans MTCC 183, respectively. Antifungal activity revealed that none of the compound of 5a-j exhibited highly potent activity against the fungal strains. However, some of them were found to be active for the specific fungal species into some extent. Among which, the methyl group containing entities, both 5b and 5d showed good potency against the strain A. niger MTCC 282. The fluoro group containing scaffold 5f gave inhibitory effect to the strain C. albicans MTCC 183. Compounds 5i and 5j were found to be active for the strains A. clavatus MTCC 1323 and C. albicans MTCC 183, respectively. The chlorinated compound 5i also gave inhibitory effect on the growth of the fungal strain C. albicans MTCC 183. Among the series of compounds 6a–g, the bromo group containing entity 6f was found superior to others against A. clavatus MTCC 1323. Compounds 6b and 6c having the chloro group gave good antifungal activity against the fungal strains A. clavatus MTCC 1323 and C. albicans MTCC 183, respectively. The electron donating methyl group possessing moiety 6d was found to be highly active for the strain A. niger MTCC 282. The study of compound 7a–h indicates that the triazine scaffold substituted with N-acetyl piperazine i.e. 7c exhibited excellent inhibitory profile for the fungal strain C. albicans MTCC 183. A piperidinyl derivative of triazine i.e. 7f displayed a highly potent inhibitory effect on the strain A. niger MTCC 282. Compound 7a, an N-methyl piperazine derivative of triazine was found to be active into some extent against A. clavatus MTCC 1323. Again compound 7c gave very good inhibiting effect on A. clavatus MTCC 1323. Compound 7e endowed with N-phenyl piperazinyl molecule showed very high potency to inhibit the growth of both the fungal strains C. albicans MTCC 183 and A. niger MTCC 282. The morpholine derivative of triazine i.e. 7g displayed high potency for both the strains A. niger MTCC 282 and A. clavatus MTCC 1323. The triazine scaffold incorporated with N-(4-methoxy phenyl) piperazinyl derivative i.e. 7h was found to be highly potent against the fungal strain A. clavatus MTCC 1323.
Table 2 In vitro antifungal activity of compounds 4a–j, 5a–j, 6a–g and 7a–h.
3.2.3 In vitro antituberculosis activity
Further all the synthesized triazine scaffolds were examined for their antituberculosis activities against the tubercular strain M. tuberculosis H37Rv using the BACTEC MGIT method. The results for this study show that the thiophenol substituted triazine scaffolds incorporated with the halogen group i.e. 4b and 4i were found to be highly active against the mentioned tubercular strain. The electron donating methyl group containing scaffold 4g also showed high effectiveness against the mycobacterium species. The study of antituberculosis activity () indicates that among the series of compounds 5a-j, none of the compound was active to inhibit the growth of strain. The chlorinated triazine scaffold 6b was found to give superior antibacterial efficacy. The triazine molecule endowed with N-isopropyl piperazinyl derivative i.e. 7d exhibited potent antibacterial profile. The compound containing N-(4-methoxy phenyl)piperazinyl moiety i.e. 7h was also found to be effective in inhibiting the growth of the above mentioned tubercular species.
Table 3 In vitro antituberculosis activity of compounds 4a–j, 5a–j, 6a–g and 7a–h.
3.2.4 SAR (structure–activity relationship)
3.2.4.1 Antibacterial activity
Among all synthesized compounds, possessing thiophenol moiety appeared with better antibacterial activity. Interestingly, halogen substitution at para position of thio-phenol ring provides good efficiency against Gram +ve bacteria. When the position of this substituent is replaced with meta, it showed promising activity against Gram −ve bacterial strain. Meanwhile instead of thiophenol ring, incorporation of phenol, amine or piperazine ring deviated the microbial activity but not up to the mark. The presence of two nitrogen atoms in piperazine ring with electron withdrawing acetyl group increases the biological potential than aniline and phenol substituted compounds.
3.2.4.2 Antifungal activity
Only halogen substituted thiophenol compounds provided good inhibition growth of fungal strains. Compared to other compounds, piperazine with acetyl group showed excellent antifungal activity as shown for bacterial strains as well. Phenol substituted derivatives were not as active as arylamino substituted compounds among which 4-fluoro substituent appeared with good inhibition.
3.2.4.3 Anti-tuberculosis activity
Halogen at ortho and meta position of thiophenol ring showed about 95% inhibition of H37Rv strain and methyl substituent at para position provided same activity with less MIC values. Isopropyl and anisole substituent in piperazine ring showed better anti-tuberculosis activity than the rest of the compounds. Unfortunately, none of the phenol substituted derivatives showed % inhibition of tuberculosis strain however, ortho substituted chloro group in aniline exhibited excellent inhibition. Nevertheless, rest of the compounds appeared with good to moderate activity due to variation of substituent positions. These positional isomers may affect the potency of titled compounds and hence deviation of biological activity as well.
4 Conclusion
The present work is basically focused on the development of novel s-triazinyl derivatives with wide therapeutic windows. Out of 35 compounds screened, majority of the compounds came out with promising activity against a wide range of pathogenic bacteria, fungi and mycobacteria. From the bioassay results, it was also possible to make a number of correlations regarding the relationship between the structure of the newer scaffolds and their antimicrobial activities. The thiophenol derivatives bearing chloro and fluoro were found to be the most active among the four series of final s-triazine based congeners. Marking on this order, piperazine substituted compounds displayed better activity than aniline and phenol substituted derivatives. N-acetyl (7c) with 3.12 μg/mL of MIC and N-(4-methyl phenoxy) (7h) analogous emerged as potential agents against bacteria and M. tuberculosis H37Rv strain as well. These privileged structures with enhanced bioactivities lead to provide enough scope to develop new scaffolds for further drug discovery process.
Acknowledgements
Authors are very thankful to Prof. Nisha K. Shah, Head, Department of Chemistry, School of Sciences, Gujarat University, Ahmedabad, India for her kind cooperation in providing support and research facility. The authors wish to offer their deep gratitude to the Microcare Laboratory, Surat, India for carrying out the biological screenings. We are also thankful to SAIF, Chandigarh, India for carrying out IR and 1H NMR, 13C NMR analyses. Mr. Dhruvin Shah and Mrs. Nirali Mewada would like to acknowledge UGC New Delhi, for Junior Research Fellowship.
Notes
Peer review under responsibility of University of Bahrain.
References
- A.BalianiG.J.BuenoM.L.StewartV.YardleyR.BrunM.P.BarrettI.H.GilbertDesign and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasitesJ. Med. Chem.48200555705579
- R.Bhushan SinghN.DasS.JanaA.DasSynthesis and in vitro antibacterial screening of some new 2,4,6-trisubstituted-1,3,5-triazine derivativesLett. Drug Des. Discov.92012316321
- X.ChenP.ZhanX.LiuZ.ChengC.MengS.ShaoC.PannecouqueE.De ClercqDesign, synthesis, anti-HIV evaluation and molecular modeling of piperidine-linked amino-triazine derivatives as potent non-nucleoside reverse transcriptase inhibitorsBioorg. Med. Chem.20201238563864
- K.DasA.D.ClarkP.J.LewiJ.HeeresM.R.de JongeL.M.KoymansH.M.VinkersF.DaeyaertD.W.LudoviciM.J.KuklaRoles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variantsJ. Med. Chem.47200425502560
- R.G.DucatiA.Ruffino-NettoL.A.BassoD.S.SantosThe resumption of consumption: a review on tuberculosisMem. Inst. Oswaldo Cruz1012006697714
- J.R.DudleyJ.T.ThurstonF.C.SchaeferD.Holm-HansenC.J.HullP.AdamsCyanuric chloride derivatives. III. Alkoxy-s-triazinesJ. Am. Chem. Soc.73195129862990
- P.GahtoriS.K.GhoshP.ParidaA.PrakashK.GogoiH.R.BhatU.P.SinghAntimalarial evaluation and docking studies of hybrid phenylthiazolyl-1,3,5-triazine derivatives: a novel and potential antifolate lead for Pf-DHFR-TS inhibitionExp. Parasitol.1302012292299
- P.GahtoriS.K.GhoshB.SinghU.P.SinghH.R.BhatA.UppalSynthesis, SAR and antibacterial activity of hybrid chloro, dichloro-phenylthiazolyl-s-triazinesSaudi Pharm. J.2020123543
- N.R.GandhiP.NunnK.DhedaH.S.SchaafM.ZignolD.Van SoolingenP.JensenJ.BayonaMultidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosisLancet375201018301843
- T.D.GootzThe global problem of antibiotic resistanceCrit. Rev. Immunol.3020107993
- P.HawkeyD.A.LewisMedical Bacteriology: A Practical Approach2004OUPOxford
- H.D.IsenbergA.S.F.MicrobiologyClinical Microbiology Procedures Handbook1992American Society of Microbiology
- S.Kumar GhoshA.SahaB.HazarikaU.Pratap SinghH.Raj BhatP.GahtoriDesign, facile synthesis, antibacterial activity and structure–activity relationship of novel di- and tri-substituted 1,3,5-triazinesLett. Drug Des. Discov.92012329335
- R.MenicagliS.SamaritaniG.SignoreF.VagliniL.Dalla ViaIn vitro cytotoxic activities of 2-alkyl-4,6-diheteroalkyl-1,3,5-triazines: new molecules in anticancer researchJ. Med. Chem.47200446494652
- R.P.ModhA.C.PatelK.H.ChikhaliaDesign, synthesis biological evaluation of some novel quinazolinone scaffoldsMed. Chem.82012182192
- R.P.ModhA.C.PatelD.H.MahajanC.PannecouqueE.De ClercqK.H.ChikhaliaSynthesis and evaluation of novel 4-substituted styryl quinazolines as potential antimicrobial agentsArch. Pharm. Chem. Life Sci.3452012964972
- R.P.ModhS.Prasanth KumarY.T.JasraiK.H.ChikhaliaDesign, synthesis, biological evaluation, and molecular modeling of coumarin-piperazine derivatives as acetylcholinesterase inhibitorsArch. Pharm. Chem. Life Sci.346201210.1002/ardp.201300242
- R.P.ModhE.De ClercqC.PannecouqueK.H.ChikhaliaDesign, synthesis, antimicrobial activity and anti-HIV activity evaluation of novel hybrid quinazoline–triazine derivativesJ. Enzyme Inhib. Med. Chem.201310.3109/14756366.2012.755622
- R.P.ModhA.C.PatelK.H.ChikhaliaDesign, synthesis, antibacterial, and antifungal studies of novel 3-substituted coumarinyl-triazine derivativesHeterocycl. Commun.201310.1515/hc-2013-0104
- D.NiccolaiL.TarsiR.J.ThomasThe renewed challenge of antibacterial chemotherapyChem. Commun.199723332342
- K.M.OverbyeJ.F.BarrettAntibiotics: where did we go wrong?Drug Discov. Today1020054552
- R.V.PatelP.KumariD.P.RajaniK.H.ChikhaliaSynthesis and studies of novel 2-(4-cyano-3-trifluoromethylphenyl amino)-4-(quinoline-4-yloxy)-6-(piperazinyl/piperidinyl)-s-triazines as potential antimicrobial, antimycobacterial and anticancer agentsEur. J. Med. Chem.46201143544365
- R.V.PatelP.KumariD.P.RajaniK.H.ChikhaliaSynthesis, characterization and pharmacological activities of 2-[4-cyano-(3-trifluoromethyl)phenyl amino)]-4-(4-quinoline/coumarin-4-yloxy)-6-(fluoropiperazinyl)-s-triazinesJ. Fluorine Chem.1322011617627
- A.B.PatelR.V.PatelP.KumariD.P.RajaniK.H.ChikhaliaSynthesis of potential antitubercular and antimicrobial s-triazine-based scaffolds via Suzuki cross-coupling reactionMed. Chem. Res.222012367381
- U.P.SinghH.R.BhatP.GahtoriAntifungal activity, SAR and physicochemical correlation of some thiazole-1,3,5-triazine derivativesJ. Mycol. Med.222012134141
- N.SunduruL.GuptaV.ChaturvediR.DwivediS.SinhaP.M.ChauhanDiscovery of new 1,3,5-triazine scaffolds with potent activity against Mycobacterium tuberculosis H37RvEur. J. Med. Chem.45201033353345
- Tuberculosis Fact sheet, 2013. Available from: <http://www.who.int/mediacentre/factsheets/fs104/en/> (accessed September 05, 2013).
- Z.F.UdwadiaR.A.AmaleK.K.AjbaniC.RodriguesTotally drug-resistant tuberculosis in IndiaClin. Infect. Dis.542012579581
- World Health Organization, 2012. Global tuberculosis report 2012. Available from; http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf (accessed September 05, 2013).