Abstract
In the present study the bulk etch rate, the bulk activation energy, the track density and the degree of crystallinity percentage have been examined for gamma irradiated samples with 60Co source at doses ranging from 0 to 200 kGy. After gamma irradiation the samples were exposed to zirconium sand to collect α-particle tracks. Samples were etched at 60, 65, 70, 75 and 80 °C in 6.25 N NaOH solution for 4 h. Results indicated that, the bulk etch rate increases with the increase of gamma absorbed dose at different etching temperatures. The bulk activation energy and the track density decreased with the increase of the gamma absorbed dose. The degree of crystallinity percentage for un-etched and etched samples also has been studied for different gamma doses. The increase in bulk etch rate and the decrease in both of bulk activation energy and track density with increase in the gamma dose may be due to the degradation in CR-39 polymeric material.
1 Introduction
Solid state nuclear track detectors (SSNTDs) have some potential advantages such as low cost, light weight and the ability to discriminate against light ionizing particles (CitationMukhtar et al., 2000). CR-39 detector is one of the most frequently used of SSNTDs and is a thermoset plastic made by polymerization of the allyl diglycol carbonate monomer (Papachristodoulou et al., Citation2007; Solarz and He, Citation1995; Tse et al., Citation2007"). Deep chemical changes occur in polymers under the action of ionizing radiations regardless of their type (X-rays, gamma rays, fast and slow neutrons, fast electrons, alpha particles, protons, and other products of nuclear reactions) (CitationKuleznev and Shershnev, 1990). In the field of radiation dosimetry by using SSNTDs, it is very important to fix and enlarge the damage trail along the charged particle trajectory through the detector material. This process is known as chemical etching treatment (CE) (CitationFleischer et al., 1967). Bulk etching enlarges the track region to the point where it becomes visible under an ordinary optical microscope. In the case of CR-39, a sodium hydroxide etchant solution has been proved (CitationDurrani and Bull, 1986).
Polymer properties are strongly affected with the presence of crystalline material in the polymeric material (CitationAhluwalia and Anuradha, 2008). The degree of crystallinity can be determined with different experimental techniques; such as X-ray diffraction, density measurements, infrared spectroscopy IR and calorimeters (CitationIUPAC, 1997). The mechanical properties of polymers are strongly depending on the degree of crystallinity, where the crystal regions of polymer are much stiffer and stronger than the amorphous regions. The crystallinity makes the polymeric material strong; on the other hand it also makes the polymeric material brittle. The polymer which is completely crystalline is too brittle; while the amorphous regions give polymer toughness (polymer can bend without breaking) (CitationFried, 2005). The present study aims to examine the behavior of bulk etch rate, bulk activation energy, track density and the degree of crystallinity percentage under the effect of different gamma absorbed doses.
2 Experimental details
2.1 Material and irradiation conditions
CR-39 samples of average thickness 1 mm obtained from TASTRAK were cut into small pieces of 1 × 1 cm2. CR-39 detector samples were irradiated with different gamma doses (0, 10, 50, 100, 150, and 200 kGy) using 60Co, gamma-ray source with 3.09 kGy/h dose rate from Gamma Irradiation Unit, Nuclear Research Center at the Atomic Energy Authority of Egypt (EAEA). The gamma irradiated detector samples were exposed to zirconium sand (zirconium sand used as natural source of α-particles) to collect α-particle tracks at room temperature (nearly about 30 °C) for three months. The irradiated samples attached to the plastic cover at the top of plastic cups at an altitude or height of 5 cm from 350 g of zirconium sand at the bottom of cups. The samples were saved in vertical position in tightly closed cups to prevent any particles from entering into the cups.
2.2 Etching process conditions
The samples of gamma irradiated CR-39 detector were etched in 6.25 N NaOH solution at temperatures 60, 65, 70, 75 and 80 ± 1 °C for 4 h as etching time duration.
2.3 Measurements
After etching process the bulk etch rate was measured using digital micrometer of accuracy 1 μm. The number of tracks was determined using optical microscope, to confirm the authenticity designate; an optical microscope attached with a video camera via a numerical interface card with the PC and specified software (Arc Soft Webcam Companion) was used. The track density was calculated while the samples were etched with 6.25 N NaOH for 4 h as etching duration. To resolve the tracks and to explain the change in the degree of crystallinity percentage for etched samples a FESEM was used. The surfaces of the etched pristine (un-irradiated sample) and etched irradiated samples with 50 and 200 kGy gamma doses were scanned with a high resolution field emission microscope (FESEM) in National Research Center of Egypt.
The degree of crystallinity percentage of different irradiated samples before and after etching were measured using X-ray diffraction patterns (XRD). A Philips X Pert MPD diffractometer type (Women’s College Ain Shams University) was used, where etch sample was subjected to X-ray analysis under working conditions of 40 kV and 25 mA. There are two different ways to determine the degree of crystallinity percentage from X-ray chart; the first way is by using the weight in grams and the second way is by calculating the area under the peaks. In present study the weight in grams (the first way) was used to calculate the degree of crystallinity percentage for different samples. To explain the change in the degree of crystallinity percentage for etched samples; FT-IR analysis was carried out for the pristine (un-irradiated sample) and the etched un-irradiated one. Jasco FT-IR 460 Pulse made in Japan with accumulation 16, resolution 4 cm−1, auto gain and auto scanning speed 2 mm/s in National Research Center of Egypt. FT-IR was used to determine the function side groups of the polymeric material of pristine and the etched one. The spectra were recorded in the wave number range of 4000–400 cm−1.
3 Results and discussion
The bulk etch rate was calculated for the pristine and irradiated samples with different gamma doses (10, 50, 100, 150, and 200 kGy). The bulk etch rate (Vb) was determined using the thickness measurement technique from the equation (CitationMalik et al., 2002):(1) where, L1 is the initial thickness, L2 is the final thickness after etching, ΔL is the removal thickness, Δt is the etching time duration. The corresponding activation energies for these calculated values of bulk etch rate at different temperatures, have been observed by using the following equation (Kissinger, Citation1959; Singh and Neerja, Citation2007").
(2) where,
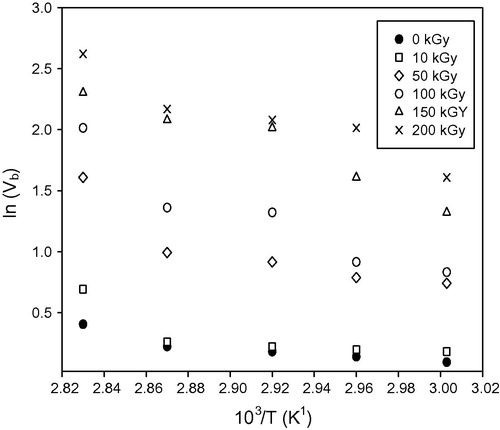
is the activation energy for bulk etch rate, K is Boltzmann constant, A is a constant and T is the absolute temperature. By taking a natural logarithm of the above equation and plotting the graph of
versus
, the value of bulk activation energy
gives by the slope of graph.
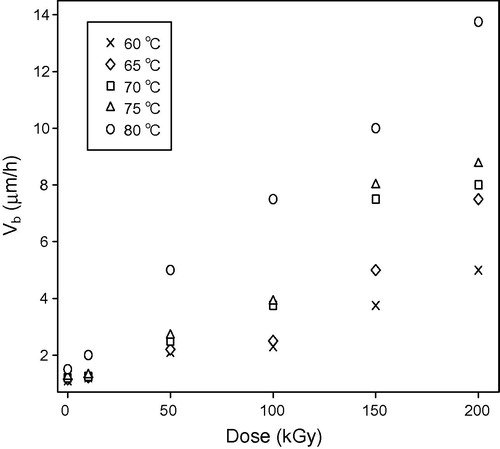
illustrates the small increase in bulk etch rate for pristine (un-irradiated samples) and irradiated samples with 10 and 50 kGy, where a large increment clears in the bulk etch rate at 100, 150, and 200 kGy. The general behavior of the bulk etch rate of pristine and irradiated samples with the increase of the etchant temperature is in agreement with the results carried out by Rana and Qureshi (Citation2002), Awad et al. (Citation2009)" and CitationHermsdorf et al. (2007). The increment of bulk etch rate may be due to the effect of hydrolysis process where the etching cuts the carbonate link and liberates the chains in the form of poly allyl alcohol from the polymer net work (CitationStejny, 1987). Also the large increment in bulk etch rate could be attributed to the decrease in the average molecular weight by scissioning of the molecular chains by irradiation (Kuleznev and Shershnev, Citation1990; Singh and Neerja, Citation2007"). Exposing polymers to high energy radiation appears in a number of physical and chemical changes, both temporary and permanent (CitationTager, 1978). The increment of bulk etch rate with increase in the gamma dose is in agreement with Abu-Jarad et al. (Citation1997), Hegazy et al. (Citation2013)" and CitationSingh and Prasher (2004). Abu-Jarad et al. and Hegazy et al. used a wide range of high gamma dose; where the bulk etch rate increases with the increase of gamma dose (Abu-Jarad et al., Citation1997; Hegazy et al., Citation2013"). Also Brahimi et al. results are in agreement with the present study results, Brahimi used gamma dose ranging between 1 and 105 Gy (CitationBrahimi et al., 2008). is plotted between the natural logarithm of bulk etch rate and temperature. The values of bulk activation energies for the pristine and gamma irradiated samples are mentioned in . These values have been found to decrease with increase in the gamma dose. The result behavior of the bulk activation energy in the present study is in agreement with the results carried out by Rana and Qureshi (Citation2002), Awad et al. (Citation2009)" and CitationHermsdorf et al. (2007). This decrement in the values of bulk activation energies might be due to the chain-scission of the polymeric material by gamma rays (CitationSingh and Neerja, 2007).
Table 1 Variation of the bulk activation energy of CR-39 detector with absorbed gamma dose.
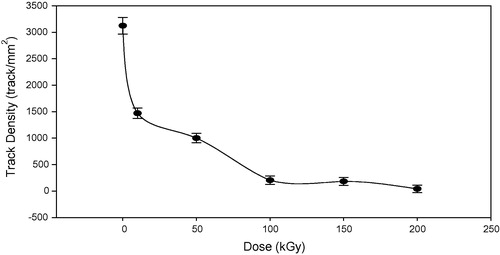
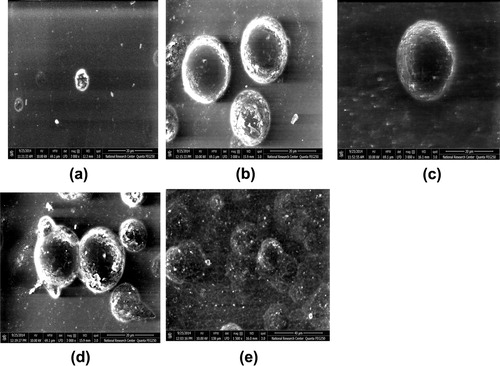
The track density of pristine and irradiated samples etched with 6.25 N NaOH solution at 70 °C was calculated. shows the relation between the track density and gamma dose, where the track density decreases with the gamma dose increasing. The decrease of track density is due to the overlapping of tracks while the tracks in the pristine were with small volume, then by increasing the gamma absorbed dose, the volume of track increased and tracks overlapped. These results were confirmed by using the high resolution field emission microscope FESEM. illustrates some typical images taken by high resolution field emission microscope FESEM to clarify the increase in track’s volume and the overlapping of tracks with increase in the gamma absorbed dose.
Figure 4 Typical images showing the increment in the track volume (a) pristine (b) 50 kGy (c) 200 kGy and illustrating the tracks overlapping with increase in the gamma absorbed dose (d) 50 kGy (e) 200 kGy.
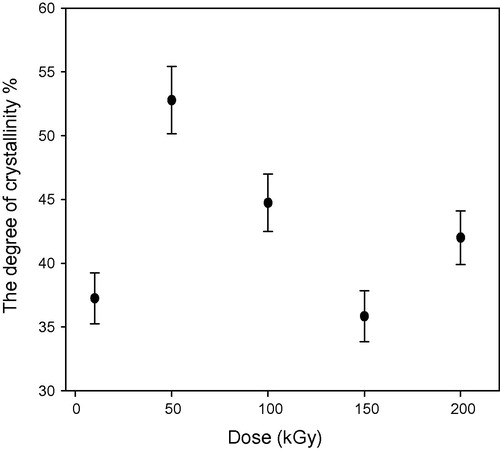
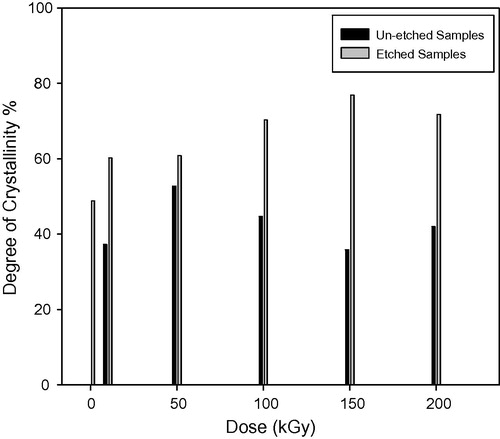
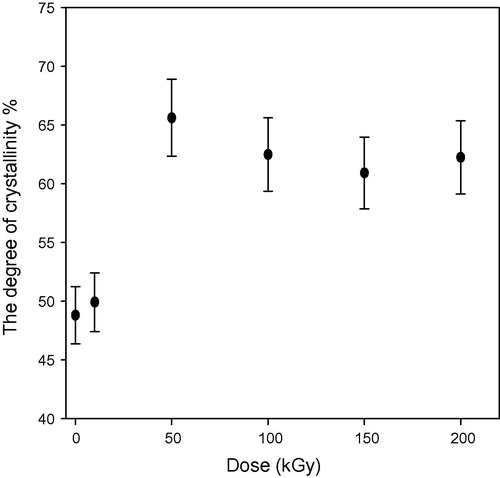
show the relation between the degree of crystallinity percentage and the gamma dose in kGy for un-etched and etched samples respectively. The degree of crystallinity Xc is obtained from the equation (CitationYoung and Lovell, 1991):(3) and
(4) where W: is the mass of the specimen, Wc and Wa: are the masses of crystalline and amorphous material in the sample respectively.
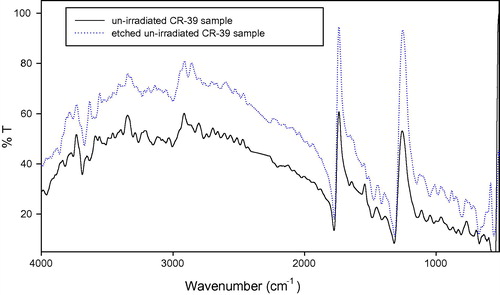
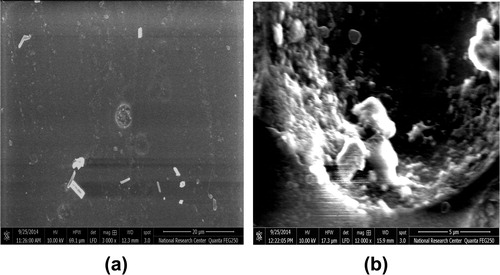
shows the degree of crystallinity percentage variation with the change of gamma absorbed dose where; the high-energy radiations cause degradation, cross-linking of polymers, an increase in the un-saturation of the molecular chains, and breaking up of the crystalline structures. The chains break into irradiation according to a random law, while the number of broken spots or cross-links is proportional to the irradiation dose and does not depend on the intensity of irradiation (CitationKuleznev and Shershnev, 1990). illustrates the relation between the gamma absorbed dose with the degree of crystallinity percentage of etched samples (pristine and irradiated samples). clarifies the effect of etching process at constant time, temperature and concentration on the degree of crystallinity percentage, where each un-etched sample was the pristine to the etched one. shows the relation between the γ-dose with the degree of crystallinity percentage of etched samples. clarifies the effect both of γ-dose and etching process (hydrolysis process) at the same time on the degree of crystallinity percentage, in this relation the un-etched pristine was the blank to all etched samples (un-irradiated and irradiated samples). shows the comparison between the degree of crystallinity percentage of un-etched and etched samples. illustrates the effect of etching process on the gamma irradiated samples with different doses, where the degree of crystallinity percentage of etched samples increases with increase in the gamma absorbed dose. This increment at different gamma absorbed doses (ranging from 0 to 200 kGy) is related to the increase in crystal percentage with respect to the amorphous percentage in polymeric material. On the other hand, it is well known that CR-39 is a partly crystalline polymer with dominant amorphous phase. So we can say that, the increment in the degree of crystallinity is a percentage with respect to the degree of crystallinity (minor phase) in the pristine. This increase in the degree of crystallinity percentage might be related to some traces of the carbonate group (as sodium carbonate) precipitates in polymeric material as a result of the hydrolysis process where in the hydrolysis process; the attack by the hydroxide ion results in hydrolysis of the carbonate ester bonds and the release of poly-allyl alcohol and carbonate from the polymer network (CitationBrydson, 1975). To make sure of the presence of Na2CO3 in an etched sample; FT-IR analysis was carried out for the pristine (un-etched and un-irradiated sample) and the etched pristine (un-irradiated sample). FT-IR of etched pristine is shown in , the figure illustrates appearance of new peaks with small transmittance intensity around at 1420, 880, and 700 cm−1, which indicate the presence of the carbonate group found as Na2CO3 salt in the spectrum of etched sample (CitationPretch, 1983). These results were confirmed by using FESEM to prove the appearance of Na2CO3 salt, (a) and (b) shows etched pristine and etched irradiated sample with gamma absorbed dose 200 kGy. clarifies crystals which refer to the accumulation of salt on CR-39 polymeric material. The increment in the degree of crystallinity percentage of etched irradiated samples at different gamma absorbed doses is in agreement with the bulk etch rate behavior where the crystallinity makes a material strong, but it also makes it brittle and the ability of breaking increases (CitationFried, 2005).
Figure 6 Relation between the degree of crystallinity (%) and the γ-dose (kGy) for pristine and irradiated samples after etching process (study the etching effect only).
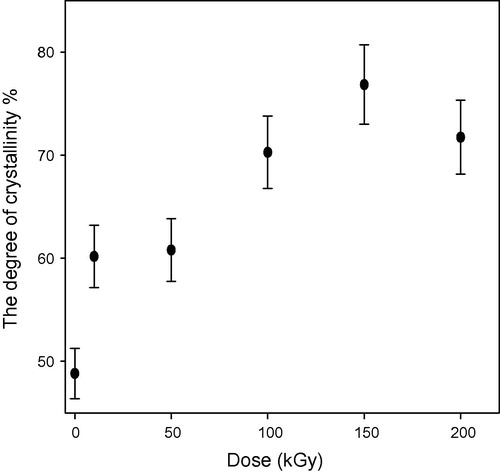
Figure 7 Relation between the degree of crystallinity (%) and the γ-dose (kGy) for pristine and irradiated samples after etching (study the effect of etching and dose).
4 Conclusion
Increase in the bulk etch rate with the increase of the gamma absorbed dose indicates the significant damage caused in the CR-39 by gamma ray. The decrement of track density with increase in the gamma absorbed dose is due to the overlapped tracks; which is related to the significant damage caused in the polymeric material by effect of gamma irradiation. The decrement of bulk activation energy with increase in the gamma absorbed dose might be the result of chain-scission in the polymeric material. Induced changes in the degree of crystallinity percentage may be due to the degradation and breaking up of the crystalline structure in polymeric material. The increment in the degree of crystallinity percentage might be due to the presence of small traces of carbonate on the polymeric material under the effect of hydrolysis process.
Acknowledgements
The authors are grateful to Prof. Dr. Essam Kishar and Prof. Dr. Samia Mokhtar at Women’s College, Ain Shams University for their continuous encouragement and support.
Notes
Peer review under responsibility of University of Bahrain.
References
- F.Abu-JaradA.M.HalaM.FarhatM.IslamEffect of high gamma dose on the PADC propertiesRadiat. Meas.281997111114
- V.K.AhluwaliaAnuradhaMishraPolymer Science2008CRC/Taylor and Francis, Ane Books IndiaBoca Raton, New Delhi ISBN: 9781420068191 – 1420068199
- E.M.AwadV.A.DitlovM.FrommD.HermsdorfDescription of the bulk etching rate of CR-39 by an extended Arrhenius-like law in increased intervals of temperature and etchant concentrationRadiat. Meas.442009813820
- S.BrahimiZ.Lounis-MokraniD.ImatoukeneA.BadreddineF.Z.AbdelazizM.AllabEffects of high gamma doses on the bulk etch rate of two grade CR-39 materialsRadiat. Meas.4320085661
- J.A.BrydsonPlastic Materials1975Butterworth and Co.
- Durrani, S.A., Bull, R.K., 1986. Solid State Nuclear Track Detector, vol. 11, p. 162.
- R.L.FleischerP.B.PriceR.M.WalkH.HubbardCriterion of registration in dielectric track recordersPhys. Rev.1561967353
- J.R.FriedPolymer Science and Technologysecond ed.2005Prentice Hall of IndiaNew Delhi
- T.M.HegazyM.Y.ShoeibG.M.HassanStudy on the effect of NaOH concentration and etching duration on some properties of γ-irradiated PADCBeni-Suef Univ. J. Appl. Sci.2120135258
- D.HermsdorfM.HungerS.StarkeF.WeickertMeasurement of bulk etch rates for poly-allyl-diglycol carbonate (PADC) and cellulose nitrate in a broad range of concentration and temperature of NaOH etching solutionRadiat. Meas.42200717
- IUPACCompendium of Chemical Terminologysecond ed.1997Royal Society of ChemistryCambridge, UK Compiled by Alan D. Mc Naught and Andrew Wilkinson, P.B. 75
- H.E.KissingerReaction kinetic in differential thermal analysisAnal. Chem.29195917021706
- Kuleznev, V.N., Shershnev, V.A., 1990. The Chemistry and Physics of Polymers. Printed in the Union Soviet Socialist Republics.
- F.MalikE.U.KhanI.E.QureshiS.N.HusainiM.SajidS.KarimK.JamilSwelling in CR-39 and its effect on bulk etch- rateRadiat. Meas.352002301
- A.R.MukhtarI.E.QureshiE.U.KhanS.ManzoorM.I.ShahzadH.A.KhanThermal annealing of fission fragment radiation damage in CR-39Nucl. Instrum. Method Phys. Res. B1702000149155
- C.PapachristodoulouD.PatirisK.G.IoannidesDetermination of bulk etch rate for CR-39 nuclear track detectors using an X-ray fluorescence methodNucl. Instrum. Method Phys. Res. B2642007177182
- C.S.S.PretchSpectral Data for Structure Determination of Organic Compounds1983Springer-VerlagBerlin Heidelberg
- M.A.RanaI.E.QureshiStudies of CR-39 etch ratesNucl. Instrum. Methods Phys. Res. B1982002129134
- SurinderSinghNeerjaThe effect of gamma irradiation on the activation energy of bulk and track etching in CR-39 plastic track detectorRadiat. Meas.42200715071509
- SurinderSinghSangeetaPrasherThe etching and structural studies of gamma irradiated induced effects in CR-39 plastic track recorderNucl. Instrum. Methods Phys. Res.2222004518524
- M.SolarzY.D.HeDependence of CR-39 etch rates on concentration of etched products in sodium hydroxide etchantNucl. Instrum. Method Phys. Res. B1001995188190
- J.StejnyThe polymer physics of CR-39 – the state of understandingRadiat. Prot. Dosimetry.201/2198731
- A.TagerPhysical Chemistry of Polymersecond ed.1978Mir PublishersMoscow
- K.C.C.TseD.NikezicK.N.YuComparative studies of etching mechanisms of CR-39 in NaOH/H2O and NaOH/ethanolNucl. Instrum. Method Phys. Res. B2632007300305
- R.J.YoungP.A.LovellIntroduction to Polymerssecond ed.1991Chapman and HallLondon ISBN 0-412-30640-9 (PB), ISBN 0-412-30630-1 (HB)