Abstract
The study investigated adsorption, leaching potential, and phytotoxicity of alachlor, bromacil, and diuron on melon, Molokhia, and wheat in Gaza strip. Plant height was used to estimate growth inhibition (phytotoxicity). Growth inhibition data were regressed versus concentrations of corresponding herbicide to estimate EC50 value. The lowest EC50 value indicates the highest phytotoxicity. Adsorption results indicated that alachlor, bromacil and diuron have different shapes. Leaching potentials indicated that alachlor totally disappeared from the top 5 cm and accumulated at a deeper depth whereas bromacil and diuron accumulated in the top 8 cm of soil layer with a decreasing intensity at deeper depth. Phytotoxicity tests showed that diuron has the lowest EC50 values on melon (1.64) and Molokhia (0.15) whereas bromacil has the lowest one on wheat (0.08), values are in (mg/kg soil). Results of binary mixtures showed that the mixture contained alachlor and diuron was the most toxic to melon, whereas mixture contained alachlor and bromacil was the most toxic to Molokhia and wheat. Tertiary mixture (alachlor + bromacil + diuron) was more toxic on Molokhia than melon and wheat, EC50 values were 3.02, 32.174, and 633.9 TU/kg soil on Molokhia, melon, and wheat respectively. An interesting outcome of the study is that Molokhia was the most sensitive plant and binary mixtures showed synergistic phytotoxicity.
1 Introduction
Alachlor, bromacil and diuron are herbicides widely used for weed control over the world. Alachlor is a chloroacetanilide herbicide, used to control annual grasses and certain broadleaf weeds in field corn, soybeans and peanuts. It inhibits protein synthesis in plant root (CitationWalker and Keith, 1992). Bromacil belongs to Uracil herbicide used for brush control on non-cropland areas. It is especially useful against perennial grasses. Diuron, one of the most commonly used herbicides, belongs to Urea derivatives that are applied as pre-emergence and post-emergence to control broadleaf weeds in a wide variety of annual and perennial broadleaf and grass weeds (CitationGooddy et al., 2002). Diuron is relatively persistent in the environment (with a half-life of over 300 days). Herbicide may enter freshwater ecosystems by different ways and pose potential risks for several aquatic organisms. Application of herbicides resulted in contamination of groundwater (CitationRiparbelli et al., 1996), food samples (CitationEl-Nahhal, 2004) and soil samples (CitationVryzas et al., 2012). This situation was associated with health disabilities (CitationAbu Mourad, 2000) and chronic diseases (CitationSafi, 2002). In addition applied herbicides in soil were adsorbed on clay minerals (CitationFranco et al., 1997), organoclay complexes (CitationNir et al., 2000), soil organic matter (Sánchez-Camazano et al., Citation2000; Rojas et al., Citation2013, Citation2014), and dissolved organic matter from a biodigester (CitationDal Bosco et al., 2012).
Moreover, the presence of herbicides as mixtures may create synergistic effects that can alter the balance of the ecosystem (CitationWendt et al., 2004). Farmers from Gaza strip claimed damaging growth of wheat, melon, and Molokhia plants cultivated in soils previously treated with alachlor, bromacil and/or diuron. Moreover, detailed information about phytotoxicity of herbicide mixtures to crops are not available elsewhere whereas in Palestine no reports are found. Accordingly, the authors designed this study to: (1) characterize the adsorption, leaching potential and phytotoxicity of alachlor, bromacil and diuron as individuals, binary and tertiary mixtures to, melon, Molokhia and wheat to satisfy the needs of the farmers, (2) characterize the synergistic or antagonistic effects of these herbicides.
2 Materials and methods
Technical material, purity 99% of alachlor, bromacil and diuron were purchased from Sigma Chemical Co., Germany. Their solubility in water is 170.3, 807, and 36.4 mg/l for alachlor, bromacil and diuron respectively. The applied rate of alachlor or bromacil is 2 kg/ha to control annual grasses and many broad leaved weeds in many crops, whereas for diuron it is 0.6 kg/ha for selective control of germinating grass and broad-leaved weeded in many crops including asparagus, tree fruit, sugar cane, cotton, alfalfa, cereals, sorghum and perennial grass seed crops, data were collected from CitationTomlin (2000). Melon, Molokhia, wheat seeds, and plastic pots were purchased from a local certified shop for agricultural products in Gaza.
2.1 Soil collection
Soil samples were collected from the 0 to 30 cm depth of an agricultural area has the following GPS information (N, 31°33′ 55.14″; E, 34°28′ 16.75″). It believed to be free of herbicide application at least 5 years. Soil sample was air-dried, sieved through 2 mm mesh and storied in plastic bags at laboratory conditions. Soil pH, salinity, organic mater content and soil texture were analyzed according to the Standard Method.
2.2 Adsorption experiments
The stock solutions of alachlor, bromacil and diuron were prepared by dissolving 30 mg in 1 L distilled water. The adsorption was measured at room temp (25 ± 2 °C), following the procedure previously reported (CitationEl-Nahhal and Lagaly, 2005). In this procedure appropriate aliquots of the aqueous stock solution of each herbicide was diluted with water to 25 mL and added to 50 mg Gaza soil in 30-mL centrifuge tubes. The final concentration of soil was 1 g/L. The dispersions were kept under continuous agitation during 48 h. The supernatant was separated by centrifugation at 20,000g for 0.5 h. Alachlor was determined by HPLC as described by CitationChen et al. (2011), in this procedure HPLC conditions were a reversed phase C-18 column was utilized to separate alachlor from other species using an acetonitrile/water mixture (1:1) containing 0.1 M phosphate buffer solution at pH 7.0 as the mobile phase. Detection was carried out with a UV-detector operated at 210 nm and at a flow rate of 0.1 μL/min. whereas bromacil and diuron concentrations were determined as in CitationEl-Nahhal and Lagaly (2005). In this procedure the concentration of bromacil in the supernatants was determined by Waters 717 HPLC with UV detector (detection wavelength 280 nm). Column: Nova-Pak C18 (inner diameter 3.9 mm, length 150 mm), flow rate: 20 μL/min. The mobile phase was methanol/water 50/50 (v/v). For the case of diuron, the same HPLC machine was used with changes in the detection wavelength to be 254 nm, flow rate: 1 mL/min and the mobile phase was methanol/water 70/30 (v/v).
The experiments were conducted at pH 7.33 ± 0.15.
The adsorbed amount of each herbicide was calculated from the depletion of the initial and remaining concentrations (CitationNir et al., 2000).
2.3 Leaching experiments
Leaching of herbicides in soil was performed using microcolumn techniques with slight modifications of those of CitationEl-Nahhal et al. (2014). In these techniques transparent plastic columns with 1 cm diameter and 15 cm long were filled with 2 mm mesh sieved sandy soils. Appropriate amount of the field rate of each herbicide which corresponds to 0.88 mg/kg soil for alachlor and bromacil, and 0.25 mg/kg soil for diuron was applied on surface areas of the plastic columns. Each column was irrigated with 25 ml water operated at 0.33 ml/1 min. The columns were held standing for 48 h for equilibrium then closed from the top, laid down and sliced along their length. One row of test plant (Molokhia) was sown along the columns and irrigated with 3 ml water after 1 day. Then irrigation continues every day. Plant height (cm) was taken as the indicator of herbicide leaching and phytotoxicity indicates the accumulation of herbicide concentration. The experiments were held in the growth chamber at 25 ± 2 °C and sprinkle irrigation was performed as needed. Plant height was determined 16 days after sowing and used as indicators to estimate the herbicide presence at different soil depths in the column. The percent of growth inhibition (%GI) (phytotoxicity) at a soil depth was calculated according to Eq. Equation(1)(1) (CitationEl-Nahhal et al., 2014):
(1) where Pc and Pt are the shoot height of the control and the treated samples at any soil depth.
2.4 Phytotoxicity of single tests
Phytotoxicity of alachlor, bromacil or diuron to wheat, melon, and Molokhia were determined as growth inhibition. The tested concentrations of alachlor and bromacil are 0, 0.06, 0.11, 0.22, 0.44, 0.88 and 1.76 mg/kg soil, whereas the tested concentrations of diuron were: 0.005, 0.01, 0.02; 0.075, 0.1 and 0.15 mg/kg. Following the procedure described by CitationEl-Nahhal et al. (2014), single toxicity tests were carried out using plastic pots under laboratory conditions. Plant heights were taken 2 weeks after germination and used to estimate %GI, and EC50 (the concentration required to inhibit 50% of plant growth), as mentioned above.
2.5 Phytotoxicity of binary mixtures
Alachlor, bromacil and diuron concentrations mentioned above were mixed together at even portions of solubility (50% each). Then transferred to one kg soil in a plastic bag, the soils were mixed thoroughly to insure homogenized distribution of mixtures. Then the soil was transferred to pot experiment for phytotoxicity evaluation as mentioned above. The following mixtures MX1 = (bromacil + Diuron), MX2 = (alachlor + bromacil) and MX3 = (Alachlor + Diuron) were prepared and tested. %GI and EC50, were estimated as mentioned above.
2.6 Tertiary mixture toxicity
One third of each single concentrations mentioned above of alachlor, bromacil and diuron were collected and mixed together to form a concentration of the tertiary mixture of alachlor + bromacil + diuron. Then concentrations were mixed in soil and used for phytotoxicity test. %GI and EC50 were estimated as mentioned above. For the binary or tertiary mixtures the phytotoxicity of the mixtures were compared using Toxic units (TUS), which is defined as the concentration of a chemical in the toxic mixture divided by its single toxic concentration for the end point measured (CitationIshaque et al., 2006). To estimate the synergetic and/or antagonistic effects of herbicides mixtures we calculate mixture toxicity index (MTI) as proposed by CitationKonemann (1981). MTI = 1 − (Log M/Log n), where M = ∑ c/EC50 at 50% effect in the mixture, and n = total number of compound in the mixture. Accordingly, phytotoxicity of mixture may be classified as antagonistic effect if MTI value is ⩽0; partial addition 0 < MTI < 1; and synergistic effect if the MTI ⩾ 1.
2.7 Statistical analysis
All experiments were performed in five replicates and each experiment was repeated three times during the project. Averages and standard deviation of the growth inhibition were calculated and fitted to the regression analysis. The averages of growth inhibition were compared by Tukey’s test and P-values were determined to evaluate the differences among treatments.
3 Results and discussion
3.1 Adsorption results
The relationships between peak area and low concentrations (0–6 ppm) of alachlor, bromacil and diuron in the standard curve were linear in all cases with values of regression coefficient (R2) in the range of 0.975–0.995, indicating a strong positive association. Accordingly the method was valid for determination of herbicide concentrations. In addition, results of blank sample of adsorption experiments indicated no changes in the initial concentration of alachlor, bromacil and diuron in the glass tube. Adsorption of alachlor, bromacil and diuron in Gaza soils are shown in . It is obvious that the adsorbed amounts of alachlor, bromacil and diuron increased nearly linearly at a low concentration whereas at a high concentration, only diuron had a curvature shape. This indicates different mode of interaction between herbicide and soil. However, the linearity of the curve suggests a high affinity of soil samples for alachlor, bromacil and diuron adsorption. Moreover, at the curvature section, the herbicides are adsorbed as bilayers. It appears that the adsorbed amount/added amount ratio is reduced as the added concentration increased. The explanation of these results is that soil has a hydrophilic nature due to the presence of high fractions of sand (72.5%), silt (17.%) and clay (10) and herbicides have a hydrophobic nature, KOW log P are 3.09, 1.88, and 2.85 for alachlor, bromacil and diuron (CitationTomlin, 2000). Moreover, soil pH was 7.33, Electric Conductivity 2.36 mS/cm, total organic matter content 0.57% and total organic carbon was 0.33%. Under this condition herbicide molecules tend to interact together through hydrogen bonding and form a larger molecule. Similar explanation was given to the adsorption of organic cations to clay (CitationLagaly, 2001). Under low organic matter content in soil as in our case (0.57%) small amount of alachlor was adsorbed in soil due to the hydrophobic nature of alachlor and hydrophilic nature of soil. Our results agree with Rojas et al. (Citation2013, Citation2014) who investigated the adsorption potential of several pesticides including alachlor on different soils with different organic matter and revealed that adsorption coefficient values increased with an increase in organic matter (OM) content of soil. Moreover, CitationDal Bosco et al. (2012) emphasized the influence of total and dissolved organic matter from a biodigester and a treatment lagoon of swine wastewater in the adsorption and desorption of alachlor. In the same context, CitationWang et al. (2015) revealed that the sorption behavior of diuron on Biochar (Enhanced Biochar, Hog Waste, Turkey Litter, Walnut Shell and Wood Feedstock) and an agricultural soil was well described by the Freundlich model (R(2) = 0.93–0.97) and the adsorbed amount was lower in soil than in the Biochar as in our case.
Figure 1 Adsorption of alachlor, bromacil and diuron in soils collected from agricultural land. Error bars represents standard deviations.
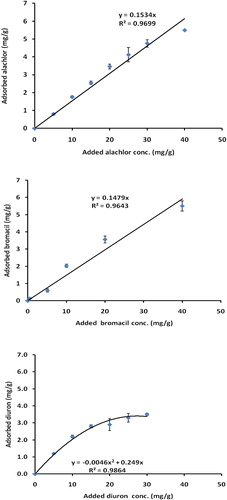
According to CitationGiles et al. (1960), isotherms of alachlor and bromacil can be classified L whereas adsorption of diuron can be classified as C types. Fitting the data in to a linear regression equation shows high values of R2 = 0.9699 for alachlor, 0.9643 for bromacil and 0.9864 for diuron indicating a positive association. It has been shown that soil organic matter plays a main role in adsorption of organic pesticides, which was proved in the study of CitationTang et al. (2009). Moreover, CitationLiu et al. (2010) found the adsorption of diuron on soils were rather high at low pH values and decreased with the increasing pH values of the suspension. At our case the soil pH was rather high (soil pH = 7.33 ± 0.15) accordingly low adsorbed amount of diuron was observed. Further supports for our results come from CitationAbadsa (2012), who found low adsorbed amounts of diuron and linuron in clayey soils in Gaza.
3.2 Leaching potential
Leaching depth of alachlor, bromacil and diuron in Gaza soils are shown in . It can be seen that alachlor leached out from the top 5 cm of soil and accumulated at deeper layers (6–10). Moreover, bioassay results indicated stronger phytotoxicity at layer of 6–8 cm more than 9–10 cm normal plant growth was observed. Our results agree with CitationVryzas et al. (2012), who investigated the leaching potential of alachlor under filed condition and found that alachlor was leached to 160 cm depth within 18 h following 40 mm irrigation at a concentration equal to 211 μg/L.
Table 1 Leaching potential of herbicides in soil. Positive symbols “++” and “+” indicate strong and weak phytotoxicity respectively, whereas negative symbol “−” indicates normal growth of test plant (Molokhia).
In addition, leaching depth of bromacil and diuron is nearly similar. Both herbicides accumulated in the top 0–8 cm with stronger phytotoxicity at the top soil layer (0–5 cm). Furthermore our leaching results on diuron agree with those of CitationImache et al. (2012) who found considerable leaching of diuron in sandy soil following elution at 124.5 mm. More support to our results comes from CitationFernández-Bayo et al. (2015) who investigated the leaching potential of diuron in sandy soils and soil amended by two winery vermicomposts and found the leached amounts of diuron were 75% and 53% in sandy loam and a silty-clay loam soil respectively, whereas in the vermicomposts-amended columns, the leaching of diuron was reduced 2–3-fold. Our leaching potential with bromacil agrees with that of CitationKim et al. (2007) who also emphasized the influence of water irrigation and carbon content of soil on the transport of bromacil in soil column.
A comparison of leaching potential of the three herbicides one can categorize alachlor as a mobile herbicide whereas bromacil and diuron are not. These differences in the leaching behavior can be explained by the fact that alachlor has nearly higher solubility in water (173.31 mg/l, at pH 7 and 20 °C) than diuron (36.4 mg/l, at 25 °C) (CitationTomlin, 2000). In contrast bromacil has a higher solubility (806 mg/l, at pH 5 and 25 °C) than alachlor but the leaching potential is similar to diuron. This is probably due to the fact that bromacil has a high pKa value (9.27). This enables bromacil to be ionized in soil solution (soil pH 7.33) and be adsorbed into the clay or organic matter fractions in soils. Moreover, one can realized that part of the threshold concentration of the three herbicides can be accumulated together in the soil depth 6–8 cm if the herbicides applied in the same time or in a sequence mode. Accordingly, the molecules of the herbicides may react together and form a larger size molecule that may enhance the phytotoxicity.
3.3 Phytotoxicity of single herbicide tests
Phytotoxicity of alachlor, bromacil and diuron on melon, Molokhia and wheat are shown in . The presented results clearly demonstrate that% growth inhibition on melon, Molokhia and wheat increased linearly as the concentration of alachlor, bromacil or diuron increased in the soil up to 0.5 mg/kg soil. A steady state increase of growth inhibition was observed in all cases above 0.5 mg/kg. However, % growth inhibition did not exceed 50% in melon and wheat, whereas it did in Molokhia. The explanation of these results is that at low concentrations, the herbicides are available in soil solution for plant uptake accordingly considerable growth inhibition of the tested plant was observed. At a high concentration (above 0.5 mg/kg soil) the herbicide tends to distribute in soil or may leach down the root zone, consequently a reduction of growth inhibition may be observed. This suggestion is supported by the leaching results () and recent work (CitationEl-Nahhal et al., 2014). Our results agree with those of CitationLiu et al. (2013) who found that wheat and some winter annual weeds in China were resistant to several acetolactate synthase (ALS) inhibitors. Moreover, alachlor may undergo biodegradation in soil systems due to the growth of cyanobacteria. Moreover, to compare the phytotoxicity of the three herbicides the EC50 value of each herbicide was calculated from the corresponding log scale of growth inhibition data presented in . It can be seen that Diuron has the lowest EC50 value (1.64 mg/kg) on melon, the most phytotoxic one, whereas alachlor has a value of 11.37 mg/kg which is nearly 7 times higher than diuron and 2.4 time higher than bromacil. These results indicated that alachlor is the safest herbicide among the tested compounds on the tested crops. These variations are probably due to the different mode of action of the tested herbicides since each of them represents a different chemical class, besides the fact that growth patterns of the test plants are also different. For the case of Molokhia, it appears that bromacil has the lowest EC50 value (0.08 mg/kg) indicating the most phytotoxic one and diuron has the highest EC50 value (0.24 mg/kg) indicating less phytotoxicity. Alachlor has nearly a half value of EC50 of diuron. Regardless of EC50 variations the three herbicides are still very toxic to Molokhia since the tested concentrations are far below the applied rate. For the case of wheat, the EC50 values have the same trend of that of melon. Diuron is the most toxic one to wheat, (EC50 = 1.83 mg/kg soil) and Alachlor is the safest one (EC50 3.91 mg/kg soil). Regression equations and R2 values are supporting our discussion. However, one can realize that EC50 values on Molokhia are the smallest among all indicating sensitivity to the tested herbicides. The sensitivity of Molokhia plant may come from the fact that it has a shorter period of growth than melon or wheat, accordingly it may not be able to develop resistant genotype for herbicides. Moreover, CitationAwad (2012) found that Molokhia seeds are sensitive test plants to diuron and acetochlor herbicides in soil and can be used as a good soil pollution indicator. In addition, CitationAbadsa (2012) revealed that diuron was highly adsorbed in soil profile accordingly it was available for plant uptake during the growth season, therefore more phototoxic effect on the test plant was observed. In addition, CitationSafi et al. (2014) reported that diuron was more resistant to biodegradation in soil and water systems, accordingly it was very toxic to the test plants. Furthermore, the regression values (R2) of the linear relationships of all tested compounds ranged from 0.909 to 0.993, indicating strong positive associations between % growth inhibition (y) and herbicide concentration (x) in all cases of single toxicity tests. These results agree with those of CitationChen et al. (2003) who found a similar trend for other cases. Moreover, the variation of EC50 values of the tested compounds may also be explained by two factors; the first factor is the value of KOW log P of each herbicide which is 3.09, 1.88, and 2.85 for alachlor, bromacil and diuron respectively, and adsorption results ().
Figure 2 Single toxicity test of herbicides on melon, Molokhia and wheat. Effect measured as growth inhibition (%). Error bars represent standard deviations.
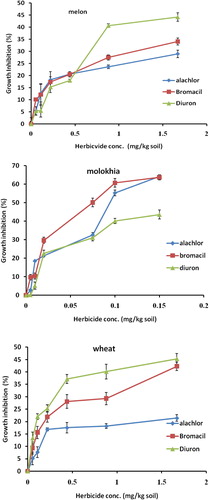
Statistical analysis showed no significant difference of alachlor and diuron on Molokhia and wheat (p = 0.09–0.1), whereas the effects on wheat are significantly different p-value less than 0.01. Furthermore alachlor and bromacil have a similar effect on wheat (p = 0.13), whereas the effects on Molokhia and melon were significantly different (p = 0.01).
3.4 Phytotoxicity binary mixtures
Phytotoxicity of binary mixtures is shown in . It is clearly demonstrated by the increased growth inhibition as the concentration of the herbicide mixture increased. The toxicity tests have similar trend but different magnitude of plant response. It can be seen that binary mixture that contained diuron as in MX1 (alachlor + diuron) has the lowest EC50 value on melon indicating the most toxic one (EC50 = 8.92 TU/kg soil) followed by MX2, (bromacil + diuron) mixture (EC50 = 28.52 mg/kg soil) whereas MX3 (alachlor + bromacil), the safest one among the mixtures (EC50 = 83.51 TU/kg soil). These results indicate that mixing diuron with alachlor or bromacil generate a high toxicity which can be referred to partial synergistic effect whereas mixing bromacil with alachlor produced a high value of (EC50 (83.51 TU/kg soil) which can be referred to the antagonistic effect. For the case on Molokhia, mixing diuron with alachlor or bromacil, nearly similar phytotoxicity was observed, (EC50 = 0.72 TU/kg soil) whereas mixture contained alachlor and bromacil provided less phytotoxicity (EC50 = 1.35 TU/kg soil).
Figure 3 Phytotoxicity of binary mixtures of herbicides to melon, Molokhia and wheat. Error bars represent standard deviations.
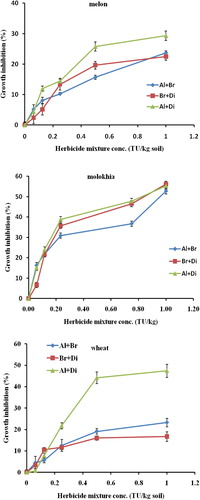
The antagonistic effects in these experiments can be explained by the fact that herbicide molecules in the mixture tests tend to interact to each other through hydrophobic interactions or π–π interactions (CitationNir et al., 2000) and may form a larger organic molecule that react with soil organic matter or clay mineral and form an organoclay complex that can strongly adsorb the herbicide molecules and slowly release them in soil environment accordingly less phytotoxic effect was observed. This explanation agrees with that of CitationEl-Nahhal and Safi (2004) who found that organic molecules dissolved with each other, formed a larger molecule that reacted with clay menial surfaces to produce organoclay complex able to maintain the slow release of the herbicide for the complex accordingly more toxic effect was observed.
For the case of wheat the trend is not similar, mixing diuron with alachlor produced a partially synergistic effect (EC50 = 0.98 TU/kg soil) whereas mixing diuron with bromacil produced an antagonistic effect (EC50 = 925.4 TU/kg soil). In contrast to the above cases mixing bromacil with alachlor produced (EC50 = 38.1 TU/kg soil) partial synergistic effects. Our results agree with CitationKerkez (2013) who found diuron and its mixtures were very toxic to cyanobacteria.
Moreover, the EC50 values of the binary mixtures on wheat () clearly demonstrated that mixture containing alachlor was the most toxic one and has the lowest TU, whereas mixture not containing alachlor had the highest EC50, indicating that alachlor is responsible for the toxicity of mixture against wheat. Furthermore, the phytotoxic effects of binary mixture can be visualized in . There were obvious gradual decreases in plant length as the concentrations of the tested herbicide mixtures increased in soil.
Figure 4 Visual rating of herbicide toxicity on the tested plants as single, binary and tertiary mixtures on melon, wheat and Molokhia, C0, C1–C6 represents the control sample and the tested concentrations respectively. A, B and C represent tertiary mixtures in melon, wheat and Molokhia respectively.

Table 2 Phytotoxicity parameters of alachlor, bromacil and diuron and their mixtures on melon, Molokhia and wheat.
Statistical analysis showed significant differences on melon (p < 0.01) whereas no significant differences were detected on Molokhia and wheat (p > 0.05).
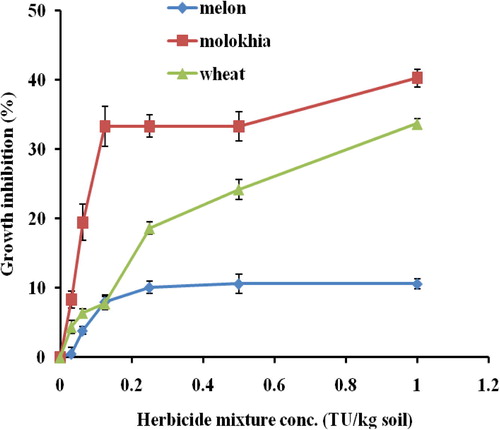
3.5 Tertiary mixtures
Phytotoxicity of tertiary mixtures () clearly demonstrated that mixtures containing alachlor and diuron were very toxic to wheat and Molokhia and less toxic to melon. These results suggest that melon either tolerant plant or can metabolize the herbicide to less toxic metabolites. Furthermore mixing the 3 herbicides evenly may enhance the interaction of the molecules to form a large size molecule that can penetrate the plant root and damage the cells. Comparing the EC50 values () one can realize that the value in Molokhia (1.93 TU/kg) is the lowest among all, the value in wheat is 9 TU/kg soil and the value in melon is the highest among all and reaches to 11060.65 TU/kg soil. Calculating MTI () showed negative values of binary and tertiary mixtures, indicating antagonistic effects according to CitationKonemann (1981). However, comparing the MTI values in melon showed extreme negative values comparing with those of Molokhia or wheat. Furthermore, it may be possible to consider MTI values of mixtures that are close to zero as partially synergistic effects. Accordingly, the tertiary mixture (Al + Br + Di) on Molokhia and wheat that have MTI values equal to −1.33 and −0.49 and binary mixture (Al + Di) in wheat has a value equal to −3.12 are in the synergistic effects. Moreover, the mixtures that have MTI values less than −3.22 can be categorized as antagonistic mixtures. These results agree with the data presented in Figs. and . In addition, it can be suggested that a mixture has synergistic effect if the EC50 value of single toxicity in mg/l is larger than value in the mixture. In the way around a mixture has EC50 value of the single toxicity test mg/l lower that value in the mixture can be regarded as antagonistic effect. It may be suggested that antagonistic effect of herbicides emerge from physiological bases and interaction between the mixture and test plan. This argument can be supported by the result of CitationFerreira et al. (1995) who revealed the physiological basis of antagonism among herbicide and plants.
3.6 Effects of herbicides on soil cyanobacteria
Our study revealed that diuron has toxic effect on cyanobacteria isolated from soil (data not shown). These results also agree with CitationKerkez (2013) who revealed that diuron was also very toxic to cyanobacteria isolated from waste water treatment plants and blue green algae. Moreover, CitationSafi et al. (2014) found partial degradation of diluted solutions of diuron treated with raw cyanobacterial mats collected from Wadi Gaza. In addition, CitationEl-Nahhal et al. (2013) found fast and rapid degradation of acetochlor (alachlor isomer) in water and soil systems. These results indicate the verity of herbicides’ effect on the eco-systems.
4 Conclusion
Adsorption and leaching potentials of bromacil and diuron in Gaza soil are quite similar whereas those of alachlor are not. The single toxicity test indicated that Molokhia was the most sensitive plant. EC50 value of single tests clearly demonstrates that diuron is more toxic than alachlor and bromacil. Binary mixture test indicted the highest toxicity of mixtures contained diuron. Phytotoxicity on melon and wheat follows this sequence: diuron > bromacil > alachlor whereas, on Molokhia follows this sequence bromacil > alachlor > diuron. Sensitivity of plants to tertiary mixtures follows the sequence: Molokhia > wheat > melon. Antagonistic effects were shown in all mixtures due to the negative values of MTI but mixtures having values close to zero were rated as partially synergistic. It may be recommended that a combination of herbicides application should avoid diuron from mixture. It can be concluded that the presence of herbicides in soil as mixtures may be toxic to the plant in the next growth season. Moreover, the study provides answers to the farmers and explains the leaching behavior and its effects on the crop.
Conflict of interest
There is no conflict of interest.
Acknowledgment
Dr. Y. El-Nahhal acknowledges Alexander von Humboldt Stiftung Foundation Fellowship Grant No IV-PAL/1104842 STP, Germany. Special thanks go to Prof Dr. G.lagaly at Kiel University, Germany.
Notes
Peer review under responsibility of University of Bahrain.
References
- M.AbadsaAdsorption and Leaching Potential of Diuron and Linuron in Gaza Soil Master thesis2012The Islamic University-Gaza
- A.T.Abu MouradHematological biomarkers of exposure in pesticide workers in Gaza Strip M.S. thesis2000Al-Quds UniversityPalestine
- Y.AwadBiodegradation of Herbicides by Cyanobaterial mats In Gaza Soils M.Sc. thesis2012Al-Azhar University Gaza
- Z.ChenH.MinW.X.WuM.ChenF.ZhangJ.ZhaoEffects of pesticide-contamination on population size and denitrification activity of denitrifying bacteria in paddy soilsChin. J. Appl. Ecol.14200317651769
- C.Z.ChenC.T.YanP.V.KumarJ.W.HuangJ.F.JenDetermination of alachlor and its metabolite 2,6-diethylaniline in microbial culture medium using online microdialysis enriched-sampling coupled to high-performance liquid chromatographyJ. Agric. Food Chem.5920118078808510.1021/jf201129j
- T.C.Dal BoscoS.C.SampaioS.R.CoelhoN.J.CosmannA.SmanhottoEffects of the organic matter from swine wastewater on the adsorption and desorption of alachlor in soilJ. Environ. Sci. Health B47201248549410.1080/03601234.2012.665338
- Y.El-NahhalContamination and safety status of plant food in Arab countriesJ. Appl. Sci.42004411417
- Y.El-NahhalG.LagalySalt effects on the adsorption of a pesticide on modified bentoniteColloid Polym. Sci.2832005968974
- Y.El-NahhalJ.SafiAdsorption behavior of phenanthrene on organoclays under different salinity levelsJ. Colloid Interface Sci.2692004265273
- Y.El-NahhalY.AwadJ.SafiBioremediation of acetochlor in soil and water systems by cyanobacterial MatInt. J. Geosci.42013880890
- Y.El-NahhalMohamedAbadsaSamirAffifiLeaching potential of diuron and linuron in Gaza soilsAm. J. Plant Sci.520144040404910.4236/ajps.2014.526422
- J.D.Fernández-BayoR.NogalesE.RomeroWinery vermicomposts to control the leaching of diuron, imidacloprid and their metabolites: role of dissolved organic carbon contentJ. Environ. Sci. Health B50201519020010.1080/03601234.2015.982423
- K.L.FerreiraJ.D.BurtonH.D.CoblePhysiological basis of antagonism of fluazifop-P by DPX-PE350Weed Sci.431995184191
- I.FrancoC.VischettiM.T.BacaM.De NobiliC.MondiniL.LeitaAdsorption of linuron and metamitron on soil and peats at two different decomposition stagesSoil Sediment Contam.61997307315
- C.H.GilesT.H.Mac EwanS.N.NakhwaD.A.SmithSystem of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solidsJ. Chem. Soc.196039733993
- D.C.GooddyP.J.ChiltonI.HarrisonA field study to assess the degradation and transport of diuron and its metabolites in a calcareous soilSci. Total Environ.29720026783
- A.E.ImacheS.DoussetA.SatrallahA.DahchourEffects of sewage sludge amendments on pesticide sorption and leaching through undisturbed mediterranean soilsJ. Environ. Sci. Health B47201216116710.1080/03601234.2012.632260
- B.A.IshaqueL.JonsonT.GeraldO.BoucaaudJ.GkohB.P.TchounwouAssessment of individual and combined toxicities of four non essential metals (As, Cd, Hg and Pb) in the micro toxic assayInt. J. Environ. Res. Public Health32006118120
- S.KerkezPotential toxicity of the Herbicides: Diuron, Diquat, Terbutryn and their Mixtures To Cyanobacterial Mats, Wadi Gaza, Palestine Master thesis2013The Islamic University-Gaza
- S.B.KimH.S.OnD.J.KimW.A.JuryZ.WangDetermination of bromacil transport as a function of water and carbon content in soilsJ. Environ. Sci. Health B422007529537
- H.KonemannFish toxicity tests with mixtures of more than two chemicals: a proposal for a quantitative approach and experimental resultsToxicology91981229238
- G.LagalyIntroduction: pesticide-clay interactions and formulationsAppl. Clay Sci.182001205209
- Y.LiuZ.XuX.WuW.GuiG.ZhuAdsorption and desorption behavior of herbicide diuron on various Chinese cultivated soilsJ. Hazard. Mater.1782010462468
- W.LiuY.BiL.LiG.YuanL.DuJ.WangTarget-site basis for resistance to acetolactate synthase inhibitor in water chickweed (Myosoton aquaticum L.)Pestic. Biochem. Physiol.1072013505410.1016/j.pestbp.2013.05.003
- S.NirT.UndabeytiaD.YaronY.El-NahhalT.PolubesovaS.SerbanG.RytwoG.LagalyB.RubinOptimization of adsorption of hydrophobic herbicides on montmorillonite preadsorbed by monovalent organic cations: interaction between phenyl ringsEnviron. Sci. Technol.34200012691274
- Riparbelli, C., Scalvini, C., Bersani, M., Auteri, D., Azimonti, G., Maroni. M., Salamana, M., 1996. Groundwater contamination from herbicides in the region of Lombardy – Italy. Period 1986–1993. The environmental fate of xenobiotics. In: Proceedings of the 10th Symposium Pesticide Chemistry, Castelnuovo Fogliani, Piacenza, Italy, pp. 559–566.
- R.RojasJ.MorilloJ.UseroL.Delgado-MorenoJ.GanEnhancing soil sorption capacity of an agricultural soil by addition of three different organic wastesSci. Total Environ.458–460201361462310.1016/j.scitotenv.2013.04.032
- R.RojasE.VanderlindenJ.MorilloJ.UseroH.El BakouriCharacterization of sorption processes for the development of low-cost pesticide decontamination techniquesSci. Total Environ.488–489201412413510.1016/j.scitotenv.2014.04.079
- J.M.SafiAssociation between chronic exposure to pesticides and recorded cases of human malignancy in Gaza strip (1990–1999)Sci. Total Environ.28420027584
- J.SafiY.AwadY.El-NahhalBioremediation of diuron in soil and by cyanobacterial MatAm. J. Plant Sci.5201410811089
- M.Sánchez-CamazanoM.J.Sánchez-MartínR.Delgado-PascualAdsorption and mobility of linuron in soils as influenced by soil properties, organic amendments, and surfactantsJ. Agric. Food Chem.48200030183026
- Z.W.TangW.ZhangY.M.ChenAdsorption and desorption characteristics of monosulfuron in Chinese soilsJ. Hazard. Mater.166200913511356
- C.D.TomlinThe Pesticides Manualtwelfth ed.2000Britich crop protection council
- Z.VryzasE.N.PapadakisE.Papadopoulou-MourkidouLeaching of Br−, metolachlor, alachlor, atrazine, deethylatrazine and deisopropylatrazine in clayey vadoze zone: a field scale experiment in north–east GreeceWater Res.4620121979198910.1016/j.watres.2012.01.021
- Mary M.WalkerLawrence H.KeithEPA’s Pesticide Fact Sheet Database1992Lewis PublishersChelsea, MI
- D.WangF.N.MukomeD.YanH.WangK.M.ScowS.J.ParikhPhenylurea herbicide sorption to biochars and agricultural soilJ. Environ. Sci. Health B50201554455110.1080/03601234.2015.1028830
- R.L.WendtP.J.Van den BrinkS.J.H.CrumP.WoinThe effective of pisticides mixture on aquatic ecosystems differing in trophic status: responses of the macrophite Myriophyllum spicatum and periphytic algal communityEcotoxicol. Environ. Saf.572004383398