Abstract
Antibiotics represent a global environmental problem due to their role in the increasing of antimicrobial resistance. Therefore, the removal of antibiotics from wastewater has received unrivalled attention in the recent years. Several technologies including the biodegradation process have been applied for this purpose. However, the potential of bacterial biomass in the biosorption of antibiotics has limited studies. The present study investigated cephalexin removal from aqueous solution by consortium bacterial cells (living and dead) which are tolerant for antibiotics. The factors including cephalexin, biomass, pH, temperature as well as presence of heavy metal ions were tested. The maximum biosorption efficiency was recorded at 0.4 mg L-1 (94.73% vs. 92.98% for living and dead cells respectively), dead cells exhibited more efficiency compared to living cells at 5 mg L-1 (82.36% vs. 46.66% respectively). Living cells are more effective at pH value between pH 4 and 6 (71.95–68.90%). The maximum removal of living cells was highest at 30 °C (80.26%), while was at 25 °C of dead cell biomass (63.81%). Remarkable percentage for cephalexin biosorption by living cells was recorded in the presence low concentrations of Ni2+ (0.21 mg L-1, 40% vs. 30% of living and dead cells, respectively). Living cells exhibited 27.42% and 25% of the removal with Cu2+ (1 mg L-1) and Pb2+ (0.4 mg L-1) respectively. In conclusion the bacterial cells biomass has a potential to remove cephalexin with some negative effects of heavy metals which can be overcome by the removal of these metal ions first and then removal of antibiotics in a second cycle.
1 Introduction
The efficiency of treatment processes to remove antibiotic compounds is limited due to the ability of these compounds to persist the degradation process (CitationLi et al., 2013). Therefore, the generated secondary effluents still contain detectable amounts of these antibiotics. Furthermore, antibiotics in the effluents even at minimum inhibition concentrations (MIC) may contribute into increasing the antimicrobial resistance due to transfer of resistance gene (CitationKlavarioti et al., 2009). Hence, further removal of antibiotics from secondary effluents is needed to protect human health.
Fig. 6a Efect of Ni2+ ions on the biosorption. (0.4 mg cephalexin mL−1, 0.1 mg dry wt. biomass mL−1, 125 rpm, 10 min, pH 5 and 30 °C for living cells and pH 4 at 25 °C for dead cells).
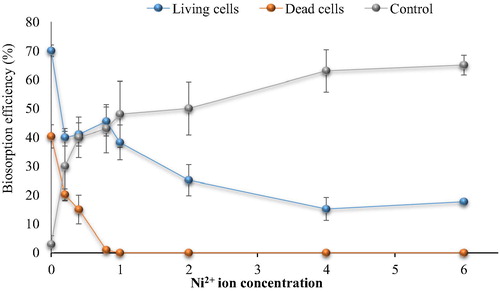
Fig. 6b Effect of Cu2+ ions on cephalexin biosorption. (0.4 mg cephalexin mL−1, 0.1 mg dry wt. biomass mL−1, 125 rpm, 10 min, pH 5 and 30 °C for living cells and pH 4 at 25 °C for dead cells).
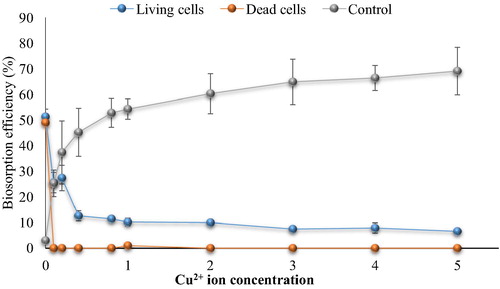
Fig. 6c Effect of Pb2+ ions on cephalexin biosorption. (0.4 mg cephalexin mL−1, 0.1 mg dry wt. biomass mL−1, 125 rpm, 10 min, pH 5 and 30 °C for living cells and pH 4 at 25 °C for dead cells).
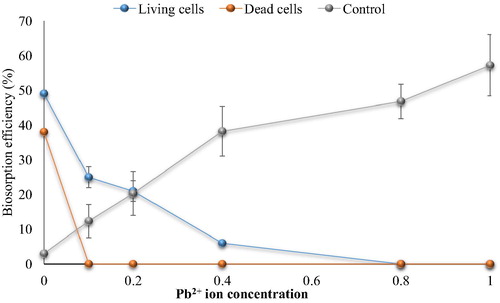
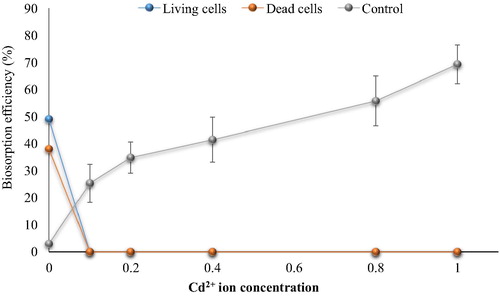
Fig. 6d Effect of Zn2+ ions on cephalexin biosorption. (0.4 mg cephalexin mL−1, 0.1 mg dry wt. biomass mL−1, 125 rpm, 10 min, pH 5 and 30 °C for living cells and pH 4 at 25 °C for dead cells).
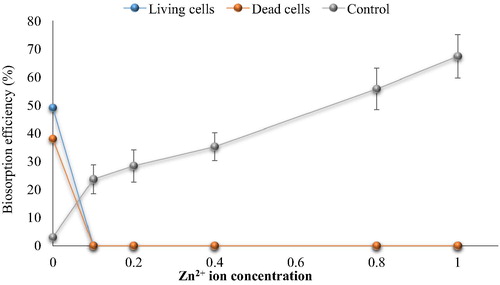
Fig. 6e Effect of Cd2+ on cephalexin biosorption. (0.4 mg cephalexin mL−1, 0.1 mg dry wt. biomass mL−1, 125 rpm, 10 min, pH 5 and 30 °C for living cells and pH 4 at 25 °C for dead cells).
Many advanced treatment processes have been evaluated for removal of antibiotic from sewage effluents such as oxidation (CitationLee et al., 2006), ozonation (CitationLee et al., 2014), ultrasonic irradiation (CitationNaddeo et al., 2009), UV irradiation and activated carbon adsorption (Aksu and Tunc, Citation2005; Rahim and Garba, Citation2016; Jodeh et al., Citation2016). However, the limitation of ozone application lies in the toxic by-products forms.
The removal of antibiotics by the biosorption process using bacteria has not been studied in depth yet. However, the biodegradation of antibiotics process has been reported in the literature (CitationAl-Gheethi and Norli, 2014). The biodegradation process depends on the ability of enzymes to degrade the structure of the active antibiotics to be inactive as in the case β-lactamase (CitationAl-Gheethi and Norli, 2014). In contrast, the biosorption process depends on the simple diffusion (passive uptake) of antibiotics through the bacterial cell membrane (CitationAl-Gheethi et al., 2015). It has been demonstrated that the microorganism cells have high potential for biosorption of heavy metals as well as the compounds such as dyes (Hanif and Bhatti, Citation2015; Mushtaq et al., Citation2016; Tahir et al., Citation2016; Rashid et al., Citation2016). Some of the authors focused on the nanotechnology application such as utilization of black phosphorus to remove the pollutants from the environments (Sun et al., Citation2015; Guo et al., Citation2015).
In previous work, the potential of β-lactamase produced from Bacillus subtilis strain to biodegrade of cephalexin was investigated, the maximum degradation was 25% (CitationAl-Gheethi and Norli, 2014). The biodegradation of cephalexin has increased at low concentrations of Ni2+, Cu2+ and Zn2+ but not in the presence of Cd2+ and Pb2+. However, the limitation of this technology lies in the efficiency where it is insufficient to totally remove the antibiotics. Therefore, this study focuses on the potential of bacterial cells biomass (living or dead) to removal of β-lactam antibiotics by biosorption process.
Cephalexin is a zwitterion antibiotic contains both an acidic and a basic group and for this reason it presented in a dissolved form (Connor et al., Citation1994; Nitzan et al., Citation2002). Thes properties might explain the high potential of cephalexin to diffuse through cell membranes of bacteria and then develop resistance. Meanwhile, the properties of antibiotics might facilitate their removal based on the bacterial biomass as biosorbent. The utilization of bacterial cell biomass might be efficient in the antibiotics removal without developments for antibiotics resistance because no new generation is produced. Moreover, the tolerant bacteria might have more potential to biosorp antibiotics, because the cell accumulates the antibiotics by diffusion process (passive transport where no energy is require) and prevents formation of toxic compounds that lead to death (CitationLewis, 2008). Therefore, the bacterial cell plays as a store for antibiotics without effects on the cell. In biosorption process, pure or mixed biomass cells might be used. However, the utilization of bacterial consortium might provide high diversity of bacterial cell wall surface which might enhance the efficiency of cephalexin removal and this emphasize the novelty of the current work. Moreover, the effect of cephalexin, biomass concentration, time, temperature, pH, as well as heavy metals on biosorption of cephalexin was tested.
2 Materials and methods
2.1 Experimental set-up
The experimental-setup in the current work consists of isolation, purification, identification, screening for the resistance of antibiotics (cephalexin, amoxicillin, ampicillin, cefuroxime and ciprofloxacin) and heavy metal ions included nickel (Ni2+), copper (Cu2+), zinc (Zn2+), cadmium (Cd2+) and lead (Pb2+). The flowchart of the experimental design is presented in Appendix A. The efficiency of living and dead biomass cells in the removal of cephalexin in a response for different factors was carried out. The effect of heavy metals on the biosorption efficiency was examined. Batch culture containing 100 mL of cephalexin solution was used to determine the removal efficiency by using living and dead cell biomass.
Factorial Complete Randomized Design (CRD) (3 * 5 * x) in triplicate was used to study the factors affecting biosorption process. Where: two (2) bacterial cell biomass (living and dead cells) and one (1) control for the biosorption process (cephalexin solution without inoculum) making a total of three (3) groups. Five (5) factors included cephalexin concentrations, biomass concentrations, time, pH and temperature, while x the values used for each factor (Appendix B). In order to examine the effect of heavy metals on the biosorption efficiency CRD (4 * 5 * x) was used. Where: two (2) bacterial cell biomass (living and dead cells) and two (2) control for the biosorption process (cephalexin solution without heavy metals, living and dead cells conducted at the optimal condition determined in this work) making a total of three (4) groups. Five (5) heavy metals (Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+), while x represent the concentration values for each heavy metals (Appendix C).
2.2 Selection of bacterial strains
The bacterial consortium used in this study was selected and identified as described in previous work (Al-Gheethi et al., Citation2014; Al-Gheethi and Norli, Citation2014). The pure bacterial isolates were subjected to the screening process to test the potential of these isolates to resist antibiotics (cephalexin, amoxicillin, ampicillin, cefuroxime and ciprofloxacin) and heavy metal ions (Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+) as described in previous work (CitationAl-Gheethi et al., 2014). The bacterial isolates which have exhibited resistance for both antibiotics and heavy metals were selected and then used to prepare bacterial consortium biomass. These strains included Burkholderia cepacia 103WTNC, B. cepacia 503WTNC, B. cepacia 903WTNC, Chryseomonas luteola 313WTNC, C. luteola 613WTNC, C. luteola 1113WTNC, Pseudomonas fluorescens 1353WTNC, B. subtilis 1556WTNC, B. subtilis 212WTNC, B. subtilis 1612WTNC, Bacillus megaterium 1558WTNC, Bacillus sterothermophilus 1050WTNC, Citrobacter freundii 1763WTNC and Kluyvera spp. 736WTNC as identified in previous work (CitationAl-Gheethi and Norli, 2014).
2.3 Preparation of bacterial biomass
Bacterial cell biomass, heavy metal and cephalexin solutions were prepared as mentioned by CitationAl-Gheethi et al. (2014). The bacterial strains were sub-cultured in BHI broth, and incubated at 125 rpm and at 35 °C for 24 h. The cells were separated by centrifugation at 4020 rcf for 10 min. The cells were washed twice with sterilized deionized water to remove culture media residues and then suspended in 10 mL. In order to prepare the bacterial consortium cells, 10 mL of each pure cell suspension was transferred into a sterilized glass bottle (200 mL) and mixed well using the shaker (100 rpm). After mixing, 5 mL was dried in an oven at 80 °C for 24 h to determine the dry mass. The bacterial consortium cell was divided into two parts. One part was used as living cells (untreated cells) while the second part (dead cells) was pre-treated at 100 °C for 15 min (CitationAbdel-Monem et al., (2010)). This treatment was enough to inactivate of bacterial cells. Moreover, to confirm the inactivation of cells the culture based method was used. A fixed amount (0.1 mL) of the bacterial suspension was sub-cultured in 10 mL of BHI broth as enrichment medium and then incubated at 35 °C for 24 h. The absence of growth confirmed the inactivation of bacterial cells.
2.4 Biosorption studies
The biosorption experiments were carried out with aqueous solutions (100 mL for each) based on the lab scale with triplicates. A series of cephalexin solutions were prepared with different concentrations (0.2, 0.4, 0.6, 0.8, 1, 2, 3, 4 and 5 mg cephalexin mL−1). pH of each solution was adjusted to pH 6 using (0.1 N) HCl and (0.1 M) NaOH. A fixed amount of the bacterial biomass (0.1 mg dry mass mL−1, living and dead cells) was mixed with each solution separately. The control experiments were conducted without biomass inoculum. In order to investigate the effect of biomass concentrations, 100 mL of cephalexin solutions with 0.4 mg L−1 of concentrations was prepared with pH 6. Each solution was inoculated 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 and 0.8 mg dry mass mL−1 of living and dead cells, respectively. One solution without biomass was used as a control. Both experiments of cephalexin and biomass concentrations factors were incubated in an incubator at 25 °C and 125 rpm for 10 min.
The time dependence studies for cephalexin antibiotic removal from aqueous solutions were tested by the incubation of cephalexin solution (0.4 mg mL−1) mixed with 0.1 mg dry wt. biomass mL−1 (living and dead cells) and pH 6 at 25 °C and 125 rpm for 10, 20, 30, 40, 50, 60, 70, 80 and 90 min. A control (cephalexin solution without biomass) was conducted for each time period. The effect of pH values on the biosorption efficiency was examined by adjusting the cephalexin solution (0.4 mg mL−1) to pH 4, 5, 6, 7 and 8 by adding drops of (0.1 N) HCl and (0.1 M) NaOH to get the desired pH value. The solutions were inoculated separately with 0.1 mg dry wt biomass mL−1. A control for each pH value was carried out. The mixtures were incubated at 25 °C and 125 rpm for 10 min. Five temperatures included 25, 30, 35, 40 and 45 °C were tested to investigate their effects on the biosorption of cephalexin using living and dead cells. The cephalexin solutions (0.4 mg mL−1) were inoculated separately with 0.1 mg dry wt. biomass mL−1, pH were adjusted to pH 5 for solutions with living cells and to pH 4 for solutions with dead cells. The pH values were adjusted to the desired values based on the experiments of pH factor. The solutions were then incubated at each desired temperature with 125 rpm for 10 min.
After each experiment, the biomass cells were separated from the solution by centrifugation at 6000 rpm for 10 min. The supernatant was used to determine the remaining soluble cephalexin.
2.4.1 Determination of cephalexin concentrations
The remaining cephalexin concentration in the supernatant was determined using a spectrophotometer (Model DR 2800, HACH, Germany) at room temperature (25 ± 2 °C) as described by CitationPriyanka and Suresh (2008) and CitationAl-Gheethi et al. (2014). For this purpose a fixed volume (one mL) of each supernatant was pipetted out into 10 mL volumetric flask. 0.5 mL of FeCl3·6H2O solution (0.1 M) and 1 mL of K3Fe(CN)6 solution (0.1%) (v/v) were added into each volumetric flask. The solution was shaking and then kept at room temperature for 10 min to ensure the completely reaction. The volumetric flask was filled up with deionized water to 10 mL. The mixture was used to determine the absorbance of green-colored complex using spectrophotometer with 791 nm of wavelength. Similarly, the absorbance of sample solution was measured and the amount of the cephalexin was determined by referring to the calibration curve. The calibration curve used was prepared with different cephalexin concentrations ranged from 0.1 to 5 mg mL−1. The solutions were subjected to the same procedure described above, aqueous solution without cephalexin was used as a reagent blank and the calibration curve was plotted (Appendix D). The biosorption efficiency (E%) of bacterial biomass toward cephalexin and the biosorptive capacity for gram of biomass was calculated using Eqs. (1) and (2) (CitationAl-Gheethi et al., 2014).
(1)
(2) where
is the amount of antibiotic biosorbed (mg g−1 biosorbent−1), V is the cephalexin solution volume (L),
is the initial cephalexin concentration (mg L−1),
is the remaining cephalexin concentration (mg L−1), and
is the amount of biomass cell biomass on dry basis (g).
2.5 Effect of heavy metal ions on the biosorption process
In order to investigate the presence or absence the competition between metal ions and cephalexin to the biosorption site on bacterial cell wall surface, different concentrations of metal ions ranged from the low concentrations which are available in sewage effluents to high concentrations which are presenting in the pharmaceutical industries were used. The concentrations used were 0.2, 0.4, 0.8, 1, 2, 4 and 6 mg L−1 Ni2+, 0.1, 0.2, 0.4, 0.8, 1, 2, 3, 4 and 5 mg L−1 Cu2+, 0.1, 0.2, 0.4, 0.8 and 1 mg L−1 Zn2+, 0.1, 0.2, 0.4, 0.8 and 1 mg L−1 Pb2+, and 0.1, 0.2, 0.4, 0.8 and 1 mg L−1 Cd2+. The cephalexin solutions (0.4 mg cephalexin mL−1) were mixed with each concentration separately. The solutions inoculated with living cell biomass (0.1 mg dry mass mL−1) were adjusted to pH 5 and incubated at 30 °C, while that inoculated with dead cell biomass were adjusted to pH 4 and incubated at 25 °C for 10 min. pH and incubation temperature were selected based on the previous experiments of these factors detected in this work. Two cephalexin solutions without heavy metals were inoculated with living and dead cells and used as control for comparison. The determination of remaining cephalexin after each experiment was carried out by using spectrophotometric method as described in Section 2.4.1.
2.6 Data analysis
The statistical package (EASE, M-STAT) was used in the computer to perform the analyses of least significance difference (LSD). Analyses of variance (ANOVA) were performed using software program of SPSS at 95% (P < 0.05) level of confidence in order to determine the significance of the differences between results. The data were expressed with standards division (±SD) of the mean for each experiments conducted in triplicate.
3 Results and discussion
Antibiotics in the environments represent real hazards for human health due to their role in the distribution of antimicrobial resistance. In the current study, bacterial consortium consists of fourteen bacterial strains (five Gram positive and nine Gram negative) which exhibited high tolerance for cephalexin used for removal of cephalexin from aqueous solution.
3.1 Factors affecting biosorption process
There are many factors affecting biosorption process, in this work substrate concentration, biomass concentration, time, pH and temperature, living or dead were investigated. The ability of the bacterial consortium biomass (living and dead cells) to biosorption of cephalexin was evaluated using the cephalexin concentrations range from 0.2 to 5 mg mL−1 (). The highest cephalexin removal by living cells was achieved at low external concentrations namely 0.4 and 0.6 mg mL−1 where removal efficiency was 94.73% and 92.22%, respectively. The efficiency of cephalexin biosorption decreased as antibiotic concentrations increased and it reached the lowest value at concentrations of 5 mg mL−1 (46.67%). The biosorption of cephalexin by dead cells was significantly different from that of living cells. At cephalexin concentrations 0.4 mg mL−1 both the living and dead cells removed 94.73% vs. 92.98% respectively. At higher cephalexin concentrations, (>3 mg mL−1), dead cells showed a significant increase in the amount of cephalexin removed, 75.51% vs. 69.04%; 80.27% vs. 51.94% and 82.36% vs. 46.66% in comparison to living cell at 3, 4 and 5 mg mL−1, respectively.
Fig. 1a Effect of cephalexin concentrations on the biosorption process (0.1 mg dry wt. biomass mL−1, pH 6, 125 rpm, 25 °C for 10 min). The control designed without biomass.
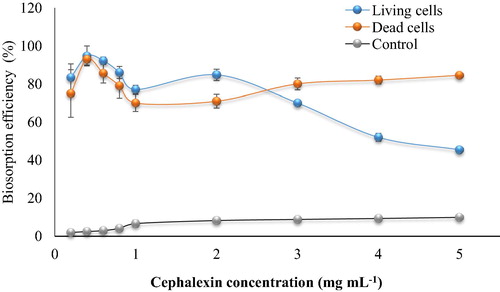
The wide range of the concentrations used here aimed to investigate the potential of bacterial cell biomass to be applied in different types of wastewater sewage effluents and pharmaceutical wastewater. The studies demonstrated that pharmaceutical wastewater contains high antibiotic concentrations ranging from 14 mg L−1 of ciprofloxacin to 44 mg L−1 of Penicillin G and in some cases reached 1065 mg L−1 of oxytetracycline (Qiting and Xiheng, Citation1988; Fick et al., Citation2009). Therefore, the potential of the bacterial biomass cells to remove detectable concentrations of cephalexin from high polluted solutions create it high applicable to be used in the field scale. In the current work, 46.66% and 82.36% of cephalexin were removed from aqueous cephalexin solution containing 5 mg mL−1 by living and dead cells respectively. The dead cells exhibited higher efficiency than living cells. However, the removal percentage by the living cells was also significant, since the cephalexin concentrations might reduce by more than 90% in the second cycle of the treatment with new biomass.
It has been demonstrated that cephalexin binds to specific proteins (PBPs) locating inside the bacterial cell wall, which does mean ability of bacterial cells to biosorp of cephalexin (CitationDisney et al., 1992). However, the explanation for the differences between living and dead cells might be related to the presence of proton ions in the living cells which play an important role in the equilibrium of diffusion process, while in the dead cells the absence of proton ions makes the cells as a store for cephalexin, this case is caused in high cephalexin concentration. Therefore, the removal percentage by living cell was less than dead cells. In the low concentration of cephalexin, the transmission of molecules across cell membrane of living cells are more faster than dead cells because the permeability of their cell membrane which acts as well, while the membrane cell in dead biomass was destructed due to the treatment process at 100 °C and thus the damage caused in the cell wall and cell membrane might effect negatively on the transport of cephalexin into the cell. For this reason the living cells exhibited high efficiency at low concentrations.
In comparison to the control samples (degradation in the cephalexin solution without bacterial inoculum), it can be noted that the natural degradation percentage ranged from 2% at low concentrations (0.2 mg mL−1) to 10% at 5 mg mL−1. The natural degradation might be due to the photo-catalysis. But it has to be mentioned that the natural degradation of cephalexin might be insignificant in the wastewater compared to the recorded here with aqueous solution. This is because the presence of total suspended solids in the wastewater would limit the penetration of light, as well as the settlement process of cephalexin at the bottom of wastewater tank due to absence of agitation.
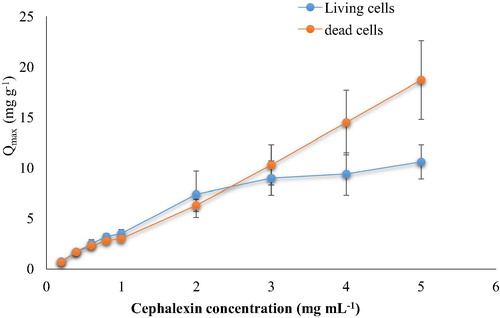
The biosorptive capacity was calculated based on the amount of cephalexin biosorped on each gram of bacterial biomass to know the capacity of bacterial cells in the remove of cephalexin. It can be observed that the
increased with increasing cephalexin concentration. The maximum biosorptive capacity was achieved at high concentration of 5 mg mL−1 where 10.61 mg g−1 was recorded (). These results are in agreement with CitationAksu and Tunc (2005) who found that the equilibrium uptake of penicillin G by Rhizopus arrhizus increased with increasing initial concentration up to 1000 mg L−1.
is dependent on the surface area of bacterial cell wall exposed to the cephalexin. In the current study the bacterial cell biomass used consisted of different species which have different cell shapes, this might provide more surface area available to adsorb of cephalexin from aqueous solution.
Fig. 1b Biosorptive capacity of living and dead cells at different cephalexin concentrations (0.1 mg dry wt. biomass mL−1, pH 6, 125 rpm, 25 °C for 10 min).
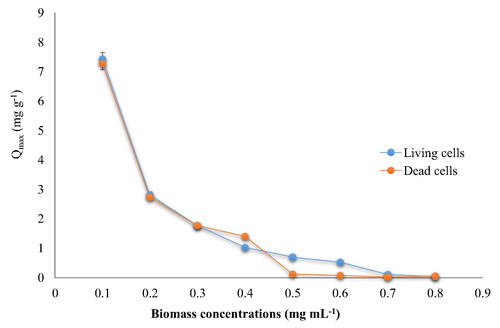
The biosorption efficiency of cephalexin decreased significantly as the cell biomass concentrations increased and attained minimum values at high concentrations (). These results are accepted by CitationAbdel-Monem et al. (2010) who reported that the biosorption efficiency (E%) increased with increasing of biomass doses but to a certain range due to the equilibrium limitations. Indeed, the removal percentage relies on the equilibrium between the concentrations of cephalexin in the solution and biomass cells. In high concentrations of cephalexin with low concentrations of bacterial biomass the removal is low because the active site on the bacterial cell wall is saturated, therefore, increasing of biomass cells would lead to increase in uptake process of cephalexin by the cells. However, the high concentration of bacterial cells would lead to agglomeration of these cells and then participate at the bottom as insoluble matter.
Fig. 2 Biosorption of cephalexin with different biomass concentrations (living and dead cells). (0.4 mg cephalexin mL−1, pH 6, 125 rpm, 25 °C for 10 min). The control sample was conducted without biomass.
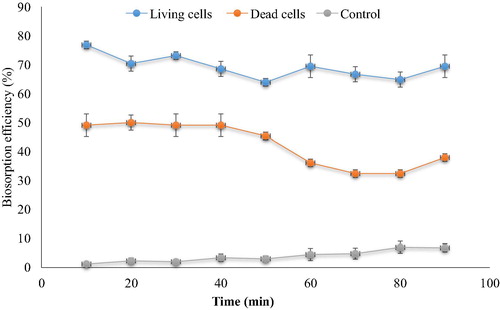
Removal of cephalexin did not differ significantly during the period from 10 to 50 min and from 50 to 90 min of dead cells biomass while, living cell occurred some differences in the removal process where biosorption percentage after 50, 70 and 80 min was less than that at other times (). However, efficiency of the removal process by living cell was significantly different and more than that by dead cell biomass at all times tested. The removal was 76.85% vs. 49.07% after 10 min and 69.44% vs. 37.96% after 90 min. Similar findings were reported by CitationCeribasi and Yetis (2001) who stated that that the biosorption process was very rapid during the first 15 min of contact time. CitationHabib-ur-Ruhman et al. (2006) revealed that the biosorption process is rapid in the initial 5 min, and further increase in contact time up to 20 min had a marginal positive effect on this process. In comparison with the sample control it was found that the natural degradation increased with time to reach 6.8% after 90 min, the removal process with the bacterial biomass cells is much better than the control, it has the ability to remove 76.85% and 49.07% by the living and dead cells within short time (10 min) which might be related to the nature of biosorption as a fast reaction process. This is an advantage of the application of bacterial biomass to remove of antibiotic from wastewater, where it can treat high quantities of wastewater within short period.
Fig. 3 Time dependence for cephalexin biosorption of cephalexin (living and dead cells) (0.4 mg cephalexin mL−1, 0.1 mg dry wt. biomass mL−1 pH 6, 125 rpm, 25 °C). The control samples were carried out without biomass.
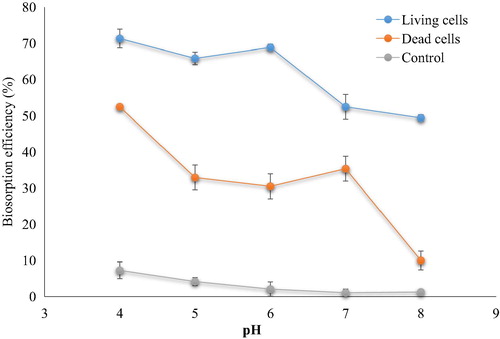
Biosorption efficiency of cephalexin by dead cell biomass decreased rapidly when pH increased from pH 4 to 8 (). Living cell biomass had the maximum uptake in pH ranging from 4 to 6, where percentage of removal ranged from 71.95% to 68.90%. After that, they decreased significantly when pH increased to pH 8 (51.58%). In the case of dead cell biomass, removal percentage remain non-significant in pH range 5–7 and decreased to reach the lowest value at pH 8 (5.59%). The pH range of 4–8 is known as optimal for the biosorption process for almost all types of bacterial biomass. However, effect of pH depends on other factors such as concentrations and type of pollution and physiological state of biomass and type of bacteria as gram positive or negative (CitationAl-Gheethi et al., 2015). The control samples at low pH exhibited a detectable removal percentage of cephalexin which indicate that the natural degradation of cephalexin might take place in wastewater. However, it has to be mentioned that the pH of wastewater is inconstant due to the chemical reaction of organic constitutes in these waste. These reactions might effect directly on the degradation process.
Fig. 4 Biosorption of cephalexin at different pH values (living and dead cells). (0.4 mg cephalexin mL−1, 0.1 mg dry wt. biomass mL−1, 125 rpm, 25 °C, 10 min). The control samples were carried out without biomass.
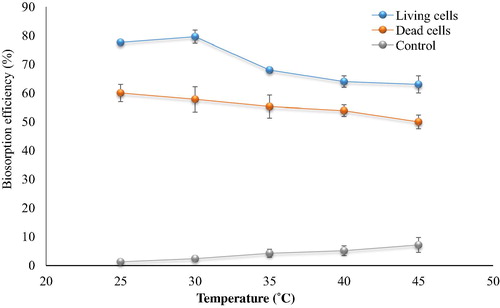
There were variations in the response of the bacterial biomass due to the change of temperature and physiological state of the cell biomass (). The maximum uptake of living cell biomass was at 30 °C, removing 80.26%. For dead cells biomass at 25 °C, the percentage removed being 63.81%. As temperature increased to 45 °C the reduction of cephalexin biosorption was observed. It has been demonstrated that the adsorption process generally decreases with the increase in temperature, because adsorption reactions are normally exothermic (CitationSelatnia et al., 2004). The high temperature (45 °C) induced the natural degradation of cephalexin (7.2% was recorded), the utilization high temperature might provide high degradation process. However, the thermal treatment of wastewater becomes unacceptable due to high cost and secondary products. The microbial process is more eco-friendly than the thermal treatment.
3.2 Effect of heavy metals on the biosorption process
The pharmaceutical wastewater is also contaminated with different types of heavy metals such as mercury, cadmium, zinc and nickel with concentrations might reach to 35.8 and 33.6 mg L−1 for Cd+2 and Ni2+ respectively. Meanwhile, these concentrations are in the range 1–100 mg L−1 which is less than that can be removed by adsorption and ion exchange processes (Wang and Chen, Citation2009; Al-Gheethi et al., Citation2015). Therefore, the effect of heavy metals at different concentrations Ni2+, Cu2+, Zn2+, Pb2+ and Cd2+ in biosorption of cephalexin from aqueous solutions by mixed bacterial biomass (living and dead cells) was tested for studying the effect of these metals on biosorption process (). The ranges of heavy metal concentrations were selected to simulate their concentrations in pharmaceutical wastewater. The concentrations used were 0.4 mg mL−1 where this concentration was the optimum based on the optimization experiments conducted in this study.
The results showed that the biosorption of cephalexin by bacterial consortium biomass decreased in aqueous solution contaminated with nickel. At 0.2 mg Ni2+ L−1 the removal efficiency of cephalexin was 40.33% vs. 30.94% (living and dead cells, respectively) compared to 70% vs. 40% in the control (without metal ions). At high concentrations more than 1 mg Ni2+ L−1, there is no removal of cephalexin by dead cell biomass was observed. The removal percentage by living cells biomass decreased significantly to reach minimum percentage at 4 mg L−1 (15.2%). There are no removal of cephalexin by dead cells was observed at Cu2+ (>1 mg L−1) and Pb2+ (0.4 mg L−1), while remarkable removal (27.42% and 25%) of cephalexin by living cells was shown at 1 Cu2+ mg L−1 and 0.4 Pb2+ mg L−1. Both living and dead cells failed to remove cephalexin in the presence of Zn2+ and Cd2+ even at low concentrations. Reduction in the removal or biosorption of cephalexin in aqueous solution contaminated with heavy metals may be related to that the heavy metals adsorb to function group of cephalexin to form complexes and thus prevent uptake process of antibiotics by bacterial cells, as in case of Al2+ and Fe2+ oxides that form ciprofloxacin-surface complexes (CitationGu and Karthikeyan, 2008). CitationLi and Zhang (2010) also indicated that divalent cations (Ca2+ and Mg2+) in saline sewage significantly decreased the adsorption of fluoroquinolones onto activated sludge.
In comparison with the control (cephalexin solution with heavy metal ions but no addition of bacterial biomass), it appeared that the removal of cephalexin was higher than that by bacterial biomass. The removal percentage reached 65, 69, 57, 67 and 69% in the presence of high concentrations of Ni2+, Cu2+, Pb2+, Zn2+ and Cd2+, respectively, while the maximum removal by the living cell biomass in the lowest concentrations of Ni2+, Cu2+, Pb2+ were 40%, 27.42% and 25% respectively with no removal process. These findings indicate the potential of heavy metals to remove cephalexin antibiotics which might be due to the chelation agents’ characteristics of the metal ions. Moreover, the reductions of cephalexin removal by this process in the presence of bacterial cell biomass might be related to the adsorption of the metal ions on the bacterial cell surface, where the bacterial cells have high affinity to adsorb of metal ions. Nevertheless, the chemical treatment of wastewater becomes non-acceptable due to the toxic by-products associated with these techniques and limits their application.
The application of biosorption of antibiotics from wastewater using bacterial biomass is applicable on the land scale due to their potential to remove these pollutants. Even in the presence of heavy metal ions these biomass are still active where the biomass cells can be used for the removal of metal ions and then regenerated and reused for the removal antibiotics in the consistency with the point of zero charge on biomass. The regeneration and recycle of bacterial biomass for several cycles of the biosorption process have been reported by authors (Abdel-Monem et al., Citation2010; Al-Gheethi et al., Citation2014).
4 Conclusions
The bacterial cell biomass exhibited a good potential to remove of cephalexin based on the biosorption process. The kinetic and equilibrium modeling might provide more details about the relationship between the removal percentage and time. Moreover, the current study focused on the potential of bacterial cells to biosorp of cephalexin. The kinetic study and the mechanism of the removal based on the analysis of cell wall structures and functional group which play the important role in the biosorption process need to be investigated in depth to best understand the biosorption efficiency and the applicability to be used on the site scale.
Conflict of interest
There is no conflict of interest.
Supplementary data 1
Download MS Word (81.5 KB)Supplementary data 2
Download MS Word (13.4 KB)Supplementary data 3
Download MS Word (13.6 KB)Supplementary data 4
Download MS Word (15.7 KB)Acknowledgements
The authors gratefully acknowledge TWAS and Ministry of Higher Education of Malaysia for the research project financial support under Fundamental Research Grant Scheme No. 1453.
Notes
Peer review under responsibility of University of Bahrain.
References
- M.O.Abdel-MonemA.H.Al-ZubeiryA.A.Al-GheethiBiosorption of nickel by Pseudomonas cepacia 120S and Bacillus subtilis 117SWater Sci. Technol.61201029943007
- Z.AksuO.TuncApplication of biosorption for penicillin G removal: comparison with activated carbonProcess Biochem.402005831847
- A.A.Al-GheethiI.NorliBiodegradation of pharmaceutical residues in sewage treated effluents by Bacillus subtilis 1556WTNCJ. Environ. Processes142014459489
- A.A.Al-GheethiI.NorliJ.LalungA.Megat-AzlanZ.A.Nur-FarehahM.O. Ab.KadirBiosorption of heavy metals and cephalexin from secondary effluents by tolerant bacteriaClean Technol. Environ. Policy162014137148
- A.A.Al-GheethiJ.LalungA.N.EfaqJ.D.BalaN.IsmailRemoval of heavy metals and β-lactam antibiotics from sewage treated effluent by bacteriaClean Technol. Environ. Policy178201521012123
- I.H.CeribasiU.YetisBiosorption of Ni2+ and Pb2+ by Phanerochaete chrysosporium from a binary metal system kineticsWater SA2720011421
- S.C.ConnorJ.R.EverettK.R.JenningsJ.K.NicholsonG.WoodnuttHigh resolution 1H NMR spectroscopic studies of the metabolism and excretion of ampicillin in rats and amoxicillin in rats and manJ. Pharm. Pharmacol.461994128134
- F.A.DisneyH.DillonJ.L.BlumerB.A.DuddingS.E.McLinnD.B.NelsonS.M.SelbstCephalexin and penicillin in the treatment of group A beta-hemolytic streptococcal throat infectionsAm. J. Dis. Child.146199213241327
- J.FickH.SoderstromR.H.LindbergC.PhanM.TysklindD.G.J.LarssonContamination of surface, ground, and drinking water from pharmaceutical productionEnviron. Toxicol. Chem.28200925222527
- C.GuK.G.KarthikeyanSorption of the antibiotic tetracycline to humic-mineral complexesJ. Environ. Qual.372008704711
- Z.GuoH.ZhangS.LuZ.WangS.TangJ.ShaoZ.SunH.XieH.WangX.F.YuP.K.ChuFrom black phosphorus to phosphorene: basic solvent exfoliation, evolution of Raman scattering, and applications to ultrafast photonicsAdv. Funct. Mater.25201569967002
- S.M.Habib-ur-RuhmanI.AhmadS.ShahSorption studies of nickel ions onto saw dust of Dalbergia sissooJ. Chin. Chem.53200610451052
- M.A.HanifH.N.BhattiRemediation of heavy metals using easily cultivable, fast growing, and highly accumulating white rot fungi from hazardous aqueous streamsDes. Water Treat.532015238248
- S.JodehF.AbdelwahabN.JaradatI.WaradW.JodehAdsorption of diclofenac from aqueous solution using Cyclamen persicum tubers based activated carbon (CTAC)J. Assoc. Arab Univ. Basic Appl. Sci.2020163238
- M.KlavariotiD.MantzavinosD.KassinosRemoval of residual pharmaceuticals from aqueous systems by advanced oxidation processesEnviron. Int.3522009402417
- C.LeeY.LeeJ.YoonOxidative degradation of dimethylsulfoxide by locally concentrated hydroxyl radicals in streamer corona discharge processChemosphere65200611631170
- Y.LeeL.KovalovaC.S.McArdellU.von GuntenPrediction of micropollutant elimination during ozonation of a hospital wastewater effluentWater Res.642014134148
- K.LewisMultidrug tolerance of biofilms and persister cellsT.RomeoBacterial Biofilms. Curr. Top. Microbiol. Immunol.vol. 3222008107131
- B.LiT.ZhangBiodegradation and adsorption of antibiotics in the activated sludge processJ. Environ. Sci. Technol.44201034683473
- W.LiY.ShiL.GaoJ.LiuY.CaiOccurrence and removal of antibiotics in a municipal wastewater reclamation plant in Beijing, ChinaChemosphere922013435444
- M.MushtaqH.N.BhattiM.IqbalS.NoreenEriobotrya japonica seed biocomposite efficiency for copper adsorption: isotherms, kinetics, thermodynamic and desorption studiesJ. Environ. Manage.17620162133
- V.NaddeoS.MeriçD.KassinosV.BelgiornoM.GuidaFate of pharmaceuticals in contaminated urban wastewater effluent under ultrasonic irradiationWater Res.4316200940194027
- Y.NitzanE.B.DeutschI.PechatnikovDiffusion of β-lactam antibiotics through oligomeric or monomeric porin channels of some gram-negative bacteriaCurr. Microbiol.452002446455
- P.PriyankaP.SureshDevelopment of colorimetric method for cephalexin in dosage formsAsian J. Pharm.22008120122
- J.QitingZ.XihengCombination process of anaerobic digestion and ozonation technology for treating wastewater from antibiotics productionWater Treat.31988285291
- A.A.RahimZ.N.GarbaEfficient adsorption of 4-Chloroguiacol from aqueous solution using optimal activated carbon: equilibrium isotherms and kinetics modelingJ. Assoc. Arab Univ. Basic Appl. Sci.2120161723
- A.RashidH.N.BhattiM.IqbalS.NoreenFungal biomass composite with bentonite efficiency for nickel and zinc adsorption: a mechanistic studyEcol. Eng.912016459471
- A.SelatniaA.BoukazoulaN.KechidM.Z.BakhtiA.CherguiY.KerchichBiosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomassBiochem. Eng. J.192004127135
- Z.SunH.XieS.TangX.YuG.ZhinanS.JundongH.ZhangH.HuangH.WangP.K.ChuUltrasmall black phosphorus quantum dots: synthesis and use as photothermal agentsAngew. Chem.12720151168811692
- M.A.TahirH.N.BhattiM.IqbalSolar red and brittle blue direct dyes adsorption onto Eucalyptus angophoroides bark: equilibrium, kinetics and thermodynamic studiesJ. Environ. Chem. Eng.4201624312439
- J.WangC.ChenBiosorbents for heavy metals removal and their future. Research review paperBiotechnol. Adv.272009195226
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jaubas.2016.09.002.