Abstract
The number of diabetes mellitus (DM) patients is one of the major concerns worldwide. As one of the main mechanisms of DM pathology is the involvement of oxidative stress, here we investigate the antioxidant capacities of frankincense (FRN) to treat or reduce the DM complications in the brain cortices of DM rats. Animals were segregated into four groups, the control group, FRN group given a dose of 500 mg of FRN/kg for 5 weeks, DM group given a single dose of 150/kg i.p of alloxan to induce diabetes and DM + FRN group given a single dose of 150/kg i.p to induce DM then followed by FRN 500 mg/kg for 5 weeks. The animals were sacrificed; their cerebral cortices were removed and used for biochemical and histopathological analyses.
1 Introduction
The increasing patient’s number of diabetes mellitus (DM), expected to be 552 million by 2030, had lead the researchers to evaluate the capacity of natural products to either reduce or cure the complications of the disease (CitationWhiting et al., 2011). DM has been shown to be a risk factor in the brains, causing cognitive impairments and dementia because the brain is the main glucose consumers with a 60% use of the total body’s glucose (Biessels et al., Citation2006; Exalto et al., Citation2012; Gorelick et al., Citation2011; Wasserman, Citation2009). One of the main features of DM is high glucose level, which advances to glycation end-products and will subsequently produce reactive oxygen species (ROS) which will result in cell damage and apoptosis (CitationBrownlee, 2005). Two types of DM are known with at least 80% of cases having type II DM, also called non-insulin dependent DM, which can be induced in the animal models by injection of chemical compounds that have similarities with glucose in the body, such as alloxan yielding the main characteristics of DM include body weight loss, hyperglycemia, polyuria and dysregulation of insulin levels (CitationKing, 2012). Because of the lower side effects of the natural products, many medicinal plants have been used to cure different diseases. Frankincense (FRN) is one of those plants which is an aromatic resin obtained from species of the Burseraceae family. Boswellia sacra has been reported to possess a variety of pharmacological effects including anti-hyperglycemia effect, antioxidant effects, and anti-inflammatory effects (Akihisa et al., Citation2006; Banno et al., Citation2006; Gupta et al., Citation1998; Mothana, Citation2011; Mothana et al., Citation2007; Masoud et al., Citation2014). FRN extract has also been reported to possess antidiabetic action in streptozotocin- and alloxan-induced DM (Azemi et al., Citation2012; Kavitha et al., Citation2007; Masoud et al., Citation2014). The present study has been designed to study the possible antioxidant effects of FRN on rat’s brain cortices following DM development.
2 Materials and methods
2.1 Preparation of the FRN extract
The freshly obtained plant from Hadramout Governorate, Yemen was grinded, suspended in ethanol (96%), shacked for 4 h and the extract was prepared as described previously (Masoud et al., Citation2014; Chevrier et al., Citation2005). The selected dose of 500 mg/kg was given orally as it is reported as a safe dose (CitationDevi et al., 2012).
2.2 DM Induction
DM rats were given a single dose (150 mg/kg interaperitoneally) of freshly prepared alloxan monohydrate (Sigma- Aldrich, USA) dissolved in normal saline (0.9% w/v NaCl) to induce DM. The levels of blood glucose were estimated in blood sample collected by tail tipping method using Accu-Chek Glucometer (China). Animals of glucose levels greater than 250 g/dl were selected for the study.
2.3 Animals and treatments
Female Albino rats were obtained and housed in the animal house unit, Sana’a University, Yemen. They were divided randomly into four groups having 6–8 animals. The study was conducted for 5 weeks and followed the guidance of animal care and use, Sana’a University. The doses were given as follows:
Control Group: These animals were given DMSO solution (n = 6).
DM group: These animals injected with a single dose of 150/kg interaperitoneally of alloxan to induce diabetes (n = 8).
FRN group: Animals of this group received ethanolic extract of FRN (500 mg/kg) dissolved in DMSO solution for 5 weeks (n = 6).
DM + FRN group: Following DM induction by a single dose of 150/kg interaperitoneally of alloxan, the animals were dosed ethanolic extract of FRN (500 mg/kg) dissolved in DMSO solution for 5 weeks (n = 8).
2.4 Sample preparation and isolation
At the end of the study (5 weeks of FRN administration) animals were sacrificed by cervical dislocation, their brains were removed and the cortices were dissected and homogenized in phosphate buffered saline (pH 7.4). Part of the homogenates were centrifuged (3000g for 10 min) and the supernatants and rest of the homogenates were used for biochemical assays, also some cortices of each group were used for histopathological studies.
2.5 Biochemical analyses
2.5.1 Total thiols
The total thiol (T-SH) was spectrophotometrically quantified in the cerebral cortex homogenate according to the method of CitationEllman (1959) and as modified by CitationSedlak and Lindsay (1968). Tris–HCl (0.2 M) and EDTA (0.02 M, pH 8.2), homogenate and 0.01 M DTNB (in methanol) were incubated for 15 min at room temperature then followed by centrifugation at 1200g for 5 min. The absorbance was read at 412 nm and the results were expressed as nmoles of T-SH/mg protein using molar extension coefficient of DTNB (13,600 cm−1 M−1).
2.5.2 Glutathione contents
Glutathione (GSH) contents were measured in the cerebral cortex homogenate according to the method of CitationEllman (1959). Sulphosalicylic acid (4% w/v) was used to precipitate proteins, followed by centrifugation at 1200g for 5 min. 0.1 mM DTNB in 0.1 M phosphate buffer (pH 8.0) was added to the supernatant and the absorbance was read at 412 nm after 2 min. Results were expressed as nmoles of GSH/mg protein using molar extension coefficient of DTNB (13,600 cm−1 M−1).
2.5.3 Protein thiols
Protein thiols (P-SH) were measured by subtracting the GSH from T-SH and the results were expressed as nmoles of P-SH/mg protein using molar extension coefficient of DTNB (13,600 cm−1 M−1).
2.5.4 Catalase (CAT) activity
The activity of CAT was measured in the supernatant as described by CitationLuck (1971). The absorbance of a reaction mixture (supernatant and 12.5 mM H2O2 in 0.067 M phosphate buffer (pH 7.0) was followed at 240 nm for 3 min. Results were expressed as μmoles of H2O2 decomposed/min/mg protein using molar extinction coefficient of H2O2 (71 M−1 cm−1).
2.5.5 Protein, albumin and uric acid assays
The biochemical tests include assays of total protein, albumin, and uric acid (UA) were estimated following the instructions of commercial kits provided by Spinreact, Spain.
2.5.6 Histopathological studies
Histopathological studies were carried out by performing routine hematoxylin and eosin staining to evaluate the morphological and structural changes in the brain cortices and the slides were examined under light microscope.
2.5.7 Statistical analysis
Data were expressed as mean ± S.D. and were analyzed by one way ANOVA followed by Student–Newman–Keuls post hoc test. Differences between groups were considered significant when P < 0.05 and all analyses were performed using the sigma-stat software (version 3.5).
3 Results
Following DM development in rats, administration of FRN extract showed improvement in the antioxidants parameters assessed in the present study. The typical signs of DM were seen (e.g. increase in blood sugar, excessive urine etc.). The antioxidants of DM group showed sharp reduction in CAT activity by 4.8-fold as compared to control (), 3-fold decreases in GSH content, 1.5-fold reduction and T-SH and 1.34-fold decreases in P-SH levels (). Treatment with FRN increases significantly the activity of CAT in the brain cortex of DM + FRN animals as compared to DM group (p < 0.05, ). This improvement accompanied by increase in the levels of thiol contents, where T-SH, GSH and P-SH contents increased in the DM + FRN group as compared to DM group (p < 0.05, ). In the all above antioxidants no significant changes were seen among control and FRN group, however, the contents of all thiols were less in DM + FRN group as compared to both control and FRN groups.
Figure 1 CAT activity in the brain cortices of control, FRN, DM DM + FRN groups. Results expressed as mean ± S.D., p < 0.05 considered significant compared to control, n = 6 in each group.
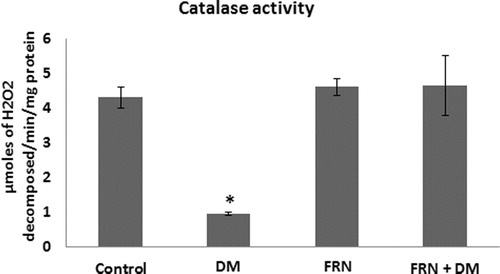
Table 1 Thiol contents in the brain cortex of control, FRN, DM, DM + FRN rats (nmoles/mg protein).
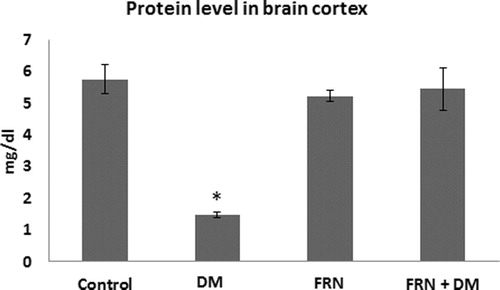
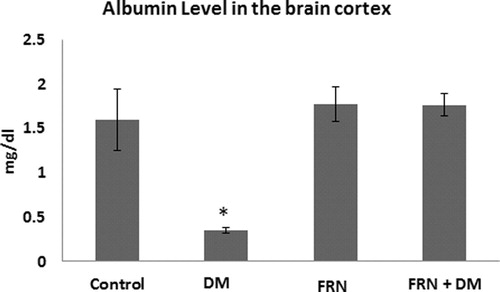
As a result of oxidative damage, proteins and albumin levels were affected in the brain cortices of DM group (decreased by 3.68 and 5.05 folds respectively, p < 0.05) when compared to control ( and respectively). However, administration of FRN extract brought back the levels of protein and albumin to almost the similar values of control.
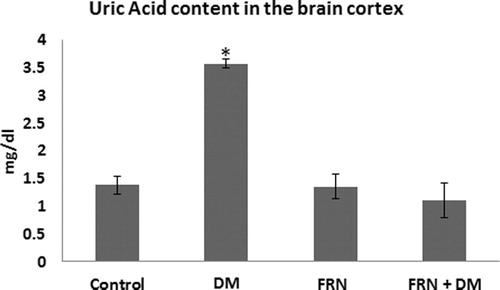
Our results showed 3.23-fold increase in UA in DM group compared to control, meanwhile, administration of FRN to DM rats reduces this increase to levels similar to those seen in control (p < 0.05, ).
Figure 4 UA levels in the brain cortices of control, FRN, DM and DM + FRN groups. Results expressed as mean ± S.D., p < 0.05 considered significant compared to control, n = 6 in each group.
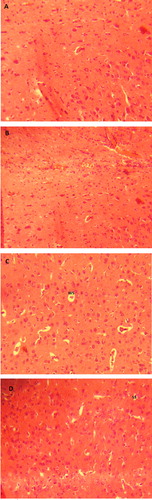
To evaluate the morphological change in the brain cortices and comparing them with the biochemical findings, the histopathological observations showed irregular and large spaces around neuron cell body in the DM group with cell congestion, however, these changes were slight spaces around neuron cell body in DM + FRN group (). No histopathological changes were seen in both control and FRN groups.
4 Discussion
The increasing evidences of oxidative stress which plays a major role in the pathogenesis of DM, had lead researchers looking for natural antioxidants that might play a role in reducing the damaged caused by DM. Here, we report the possible involvement of one of those natural antioxidants, FRN an aromatic resin from Boswellia species that used as anti-inflammatory, antibacterial, antifungal and anticancer (Banno et al., Citation2006; Chevrier et al., Citation2005; Weckesser et al., Citation2007) and as antidiabetic induced by alloxan (Kavitha et al., Citation2007; Masoud et al., Citation2014) or streptozotocin (Azemi et al., Citation2012; Shehata et al., Citation2011). Our results showed that administration of FRN (500 mg/kg for five weeks) to the DM rats increases the antioxidant capacities in their brain cortices, hence, reduces the oxidative stress caused by DM.
It is well established that oxidative stress due to the generation of free radicals, glucose auto-oxidation and protein glycation plays a role in the development of DM, where, over-production of free radical disturbs the natural balance between the production of free radicals and their antioxidant defense system (Maiese, Citation2015; Bonnefont-Rousselot, Citation2004; Desco et al., Citation2002; Lenzen, Citation2008; Muriach et al., Citation2014; Nowotny et al., Citation2015; Yan, Citation2014; Yang et al., Citation2011). This was evident from the data obtained here, where, CAT activity and thiol contents were reduced significantly in the brain cortex of DM rats. Concomitantly, there were decreases in the levels of protein and albumin accompanied by increase in UA indicating disturbance in the antioxidant defense system in the brain cortices of DM rats. The histopathological changes includes irregular and wide spaces around neuronal cell body along with congestion seen in the brain cortex of DM rats support the biochemical findings. However, FRN showed a relief in the antioxidants following 500 mg/kg body weight administration for 5 weeks. We have seen that CAT activity, thiol contents, protein level, albumin level and UA level came back to almost the similar levels as seen in the control animals, suggesting the potential antioxidant effect of this natural product. We reported previously that same dose of FRN helped rat’s red blood cells (RBCs) to recover from oxidative stress (induced by alloxan) by increasing the levels of antioxidant (CitationMasoud et al., 2014). Moreover, FRN showed that it possesses antioxidant effect (Azemi et al., Citation2012; Mothana, Citation2011; Mothana et al., Citation2007, Citation2009).
One of the metabolic abnormalities of DM is overproduction of superoxide radical, which is converted to H2O2 by the action of superoxide dismutase enzyme (CitationGiacco and Brownlee, 2010). The final product, H2O2, is then processed by CAT, it is also well documented that the production of xanthine oxidase (XO) is increased, which is an enzyme plays important role in the production of ROS in DM (Romagnoli et al., Citation2010; Desco et al., Citation2002; Matsumoto et al., Citation2003). Here, CAT activity was reduced in DM rats and the recovery following administration of FRN could be attributed to the antioxidant action of FRN which also helped in the recovery of other non-enzymatic antioxidants. Due to the presence of sulfhydryl groups (-SH) in thiols including GSH, they are one of the cellular non-enzymatic antioxidants acting as redox buffer (CitationMeister and Anderson, 1983) and quenching ROS and other oxygen-centered free radicals (CitationKidd, 1997). This could explain the reductions in thiols in our study, where, oxidative stress caused by DM overwhelms the presence of natural antioxidants in brain cortices; on the other hand, FRN helped cells to recover its thiol contents. Elevated UA levels might reflect the increasing activity of XO, FRN administration in DM + FRN group back up the UA level to the similar levels found in control animals. The administration of FRN results in the recovery of the antioxidant of brain cortices might be due to the inhibition of the activity of XO which is responsible for the production of ROS as other drugs reported to inhibit XO (CitationRomagnoli et al., 2010). We have reported that the changes in CAT, thiols and UA have been recovered in the RBCs of rats exposed to alloxan and treated with FRN extract (CitationMasoud et al., 2014). In addition to these changes, brain cortices of DM rats showed histopathological changes, where, irregular and large spaces around neuron cell body along with cell congestion have been observed in DM group. These observations were slight spaces around neuron cell body in DM + FRN group. Our findings are consistent with different studies reported histopathological changes following DM induction (Malone et al., Citation2006; Edwards et al., Citation2010; Francis et al., Citation2008), however, these findings in contrast to those reported by Guven et al., who did not observe any changes in neuron following 4 weeks of STZ-induced diabetes in rats (CitationGuven et al., 2009).
5 Conclusion
In Conclusion, our findings in favor of using FRN to reduce the oxidative damage caused by DM. Administration of FRN extract to DM rats showed significant increase in the antioxidant contents which was confirmed by histopathological studies suggesting the antioxidant beneficiary of FRN as a good candidate in the treatment of conditions that causes oxidative stress include DM with a suggestion for future study to reduce the dose less than 500 mg/kg for long period.
Conflict of interest
No financial, personal or other conflict of interest.
Acknowledgements
The authors are greatly acknowledged Dr. Ahmad Saif Muharram, Al-Nasser Board of Trustees Chairman, for his valuable assistance.
Notes
Peer review under responsibility of University of Bahrain.
References
- T.AkihisaK.TabataN.BannoH.TokudaR.NishimuraY.NakamuraY.KimuraK.YasukawaT.SuzukiCancer chemopreventive effects and cytotoxic activities of the triterpene acids from the resin of Boswellia carteriBiol. Pharm. Bull.29200619761979
- M.E.AzemiF.NamjoyanM.J.KhodayarF.AhmadpourA.Darvish PadokM.PanahiThe antioxidant capacity and anti-diabetic effect of Boswellia serrata triana and planch aqueous extract in fertile female diabetic rats and the possible effects on reproduction and histological changes in the liver and kidneysJundishapur J. Nat. Pharm. Prod.72012168175
- N.BannoT.AkihisaK.YasukawaH.TokudaK.TabataY.NakamuraR.NishimuraY.KimuraT.SuzukiAnti-inflammatory activities of the triterpene acids from the resin of Boswellia carteriJ. Ethnopharmacol.1072006249253
- G.J.BiesselsS.StaekenborgE.BrunnerC.BrayneP.ScheltensRisk of dementia in diabetes mellitus: a systematic reviewLancet Neurol.520066474
- D.Bonnefont-RousselotThe role of antioxidant micronutrients in the prevention of diabetic complicationsTreat Endocrinol.320044152
- M.BrownleeThe pathobiology of diabetic complications: a unifying mechanismDiabetes54200516151625
- M.R.ChevrierA.E.RyanD.Y.LeeM.ZhongzeZ.Wu-YanC.S.ViaBoswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitroClin. Diagn. Lab. Immunol.122005575580
- M.C.DescoM.AsensiR.MarquezJ.Martinez-VallsM.VentoF.V.PallardoJ.SastreJ.VinaXanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinolDiabetes51200211181124
- P.R.DeviK.AdilaxmammaG.S.RaoCh.SrilathaM.A.RajSafety evaluation of alcoholic extract of Boswellia ovalifoliolata Stem-bark in RatsToxicol. Int.192012115120
- J.L.EdwardsA.QuattriniS.I.LentzC.Figueroa-RomeroF.CerriC.BackusY.HongE.L.FeldmanDiabetes regulates mitochondrial biogenesis and fission in mouse neuronsDiabetologia532010160169
- G.L.EllmanTissue sulfhydryl groupsArch. Biochem. Biophys.8219597077
- L.G.ExaltoR.A.WhitmerL.J.KappeleG.J.BiesselsAn update on type 2 diabetes, vascular dementia and Alzheimer’s diseaseExp. Gerontol.472012858864
- G.J.FrancisJ.A.MartinezW.Q.LiuK.XuA.AyerJ.FineU.I.TuorG.GlaznerL.R.HansonW.H.Frey2ndC.TothIntranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathyBrain131200833113334
- F.GiaccoM.BrownleeOxidative stress and diabetic complicationsCirc. Res.107201010581070
- P.B.GorelickA.ScuteriS.E.BlackC.DecarliS.M.GreenbergC.IadecolaL.J.LaunerS.LaurentO.L.LopezD.NyenhuisR.C.PetersenJ.A.SchneiderC.TzourioD.K.ArnettD.A.BennettH.C.ChuiR.T.HigashidaR.LindquistP.M.NilssonG.C.RomanF.W.SellkeS.SeshadriCouncil on Epidemiology American Heart Association Stroke Council, Council on Cardiovascular Nursing Council on Cardiovascular Radiology Prevention, Intervention, Surgery Council on Cardiovascular, and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke associationStroke42201126722713
- I.GuptaV.GuptaA.PariharS.GuptaR.LudtkeH.SafayhiH.P.AmmonEffects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical studyEur. J. Med. Res.31998511514
- A.GuvenO.YavuzM.CamC.ComunogluO.Sevi´ncCentral nervous system complications of diabetes in streptozotocin-induced diabetic rats: a histopathological and immunohistochemical examinationInt. J. Neurosci.119200911551169
- J.V.KavithaJ.F.RosarioJ.ChandranP.AnbuBakkiyanathanHypoglycemic and other related effects of Boswellia glabra in alloxan-induced diabetic ratsIndian J. Physiol. Pharmacol.5120072939
- P.M.KiddGlutathione: systemic protectant against oxidative and free radical damageAlternat. Med. Rev.21997155176
- A.J.KingThe use of animal models in diabetes researchBr. J. Pharmacol.1662012877894
- S.LenzenThe mechanisms of alloxan- and streptozotocin-induced diabetesDiabetologia512008216226
- Luck, H. 1971. Catalase. In: Bergmeyer, H.U. (Ed.). Academic Press, New York.
- K.MaieseNew insights for oxidative stress and diabetes mellitusOxid. Med. Cell Longev.20152015875961
- J.I.MaloneS.K.HannaS.SaportaHyperglycemic brain injury in the ratBrain Res.10762006915
- A.MasoudM.Al-GhazaliF.Al-FutiniS.AlzagruriE.Al-AnsiA.SalamaH.Al-SelwiAmeliorating effect of frankincense on red blood cells of Alloxan induced-diabetes in ratInt. J. Pharmaceut. Sci. Invent.320142327
- S.MatsumotoI.KoshiishiT.InoguchiH.NawataH.UtsumiConfirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabeticmiceFree Radic. Res.372003767772
- A.MeisterM.E.AndersonGlutathioneAnnu. Rev. Biochem.521983711760
- R.A.MothanaAnti-inflammatory, antinociceptive and antioxidant activities of the endemic Soqotraen Boswellia elongata Balf. f. and Jatropha unicostata Balf. f. in different experimental modelsFood Chem. Toxicol.49201125942599
- R.A.MothanaR.GrunertU.LindequistP.J.BednarskiStudy of the anticancer potential of Yemeni plants used in folk medicinePharmazie622007305307
- R.A.MothanaU.LindequistR.GruenertP.J.BednarskiStudies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island SoqotraBMC Complement. Altern. Med.920097
- M.MuriachM.Flores-BellverF.J.RomeroJ.M.BarciaDiabetes and the brain: oxidative stress, inflammation, and autophagyOxid. Med. Cell Longev.20142014102158
- K.NowotnyT.JungA.HohnD.WeberT.GruneAdvanced glycation end products and oxidative stress in type 2 diabetes mellitusBiomolecules52015194222
- M.RomagnoliM.Gomez-CabreraM.PerrelliF.BiasiF.PallardóJ.SastreJ.PoliJ.ViñaXanthine oxidase-induced oxidative stress causes activation of NF-κB and inflammation in the liver of type I diabetic ratsFree Radical Biol. Med.492010171177
- J.SedlakR.H.LindsayEstimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagentAnal. Biochem.251968192205
- A.M.ShehataL.Quintanilla-FendS.BettioC.B.SinghH.P.AmmonPrevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE)Phytomedicine18201110371044
- D.H.WassermanFour grams of glucoseAm. J. Physiol. Endocrinol. Metab.2962009E11E21
- S.WeckesserK.EngelB.Simon-HaarhausA.WittmerK.PelzC.M.SchemppScreening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevancePhytomedicine142007508516
- D.R.WhitingL.GuariguataC.WeilJ.ShawIDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030Diabetes Res. Clin. Pract.942011311321
- L.J.YanPathogenesis of chronic hyperglycemia: from reductive stress to oxidative stressJ. Diabetes Res.20142014137919
- H.YangX.JinC.W.Kei LamS.K.YanOxidative stress and diabetes mellitusClin. Chem. Lab. Med.49201117731782