Abstract
Nitrofurantoin (NIT) is used as an antibiotic for the treatment of urinary tract infection. To determine the concentration of NIT in pharmaceutical preparations, two cost-effective extraction-free flow injection (FI) spectrophotometric methods (direct and indirect) were studied. The direct FI method was based on the reaction of NIT with potassium hydroxide and the formation of a red/orange coloured product with absorbance measured at 471 nm. The indirect method used a reduction reaction of NIT and involved an oxidative coupling step with 3-methyl-2-benzothiazolinone hydrazone hydrochloride (MBTH). The second method resulted in a green coloured product with absorbance measured at 610 nm. Both direct and indirect FI methods were in agreement with Beer’s law over a concentration range of 5–300 μg/mL. In addition, the limit for detection of the reaction product for direct and indirect method was 1.9 and 4.8 μg/mL, respectively. FI spectrophotometric methods used in the present study can be applied for the analysis of NIT in different pharmaceutical dosage forms.
1 Introduction
Nitrofurantoin (NIT) [N-(5-nitro-2-furyldine)-1-aminohydantoin] has the chemical formula C8H6N4O5 (). NIT is an antibiotic against both gram-positive and gram-negative bacteria in the body. It is used for the treatment of uncomplicated urinary tract infections (UTI) and as a prophylactic drug for recurrent UTI. There is increased interest nowadays in the use of NIT due to bacterial resistance to other commonly used antibiotics such as fluoroquinolones and trimethoprim/sulfamethoxazole (McKinnell et al., Citation2011; Ingalsbe et al., Citation2015").
The determination of NIT concentration in pharmaceutical preparations was previously done using spectrophotometry (Mahedero et al., Citation2002; Tubino et al., Citation2011"), spectrofluorimetry (Belal, Citation2008; Zhang et al., Citation2008"), cyclic and square voltammetry (Aydoğdu et al., Citation2014; de Lima-Neto et al., Citation2010"), and high-performance liquid chromatography (Du et al., Citation2014; Wilasinee et al., Citation2015"). On the other hand, FI analysis methods provide a fast, simple and cost-effective analysis of trace amounts of chemicals in pharmaceutical dosage forms. Moreover, FI techniques have the advantage of being more sensitive yet have a wide measuring range compared to the currently available chemilumenscense techniques (CitationThongsrisomboon et al., 2010). This study describes fast and simple FI methods (direct and indirect) that utilize a colorimetric reaction for the quantitative determination of NIT in pharmaceutical preparations. The methods were carried out using two different reaction setups. NIT was determined in both methods following a reduction reaction of its nitro group into an amino group.
2 Experimental
2.1 Apparatus
A digital double beam spectrophotometer (UV–visible, Shimadzu, Kyoto, Japan) was used for the measurement of absorbance of solutions for the described FI procedures. This was performed using a 1 cm length quartz cell (Cecil, 50 μL internal volume). The reaction reagents were pumped through the system using flexible vinyl tubing (0.5 mm internal diameter) attached to a six-channel peristaltic pump (Ismatec, Labortechnik Analytic, Zurich, Switzerland). Moreover, a 6-way loop valve (Rheodyne, Altex, Supelco, USA) was used for injecting the NIT containing samples. Finally, a reaction coil made of Teflon material (0.5 mm internal diameter) was attached to the loop valve.
The present study described two FI setups as shown in . The first setup was comprised of a single channel and was used for the direct FI determination of NIT (Method A) (a). The NIT-containing samples were injected into a stream of alcoholic potassium hydroxide (KOH) solution. KOH was propelled by the peristaltic pump at a flow rate of 3.25 mL/min. The resultant solutions were then mixed in the reaction coil before being pumped through the system into the detector. A red/orange coloured product was formed and absorbance was measured at 471 nm.
Figure 2 FI setups used for FI-spectrophotometric determination of NIT. (a) Method A, (b) Method B. I.V, injection valve; R.C, reaction coil; P, peristaltic pump; F.C, flow cell; D, detector; W, waste; ACS, ammonium cerric sulphate; NIT, nitrofurantoin.
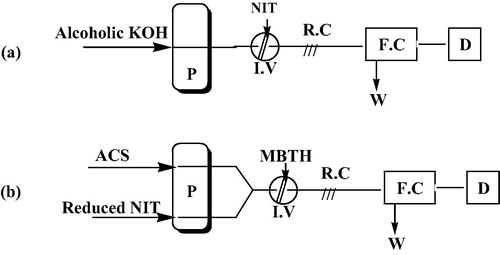
The second setup was comprised of two channels and was used for the indirect FI determination of NIT (Method B) (b). The determination procedure involves a reduction reaction that proceeds sample injections into the system. The reduced product was transported through one of the two channels in this setup. On the other hand, the second channel was used to transport ammonium cerric sulphate (ACS) solution. The reagent solution of methyl-2-benzothiazolinone hydrazone hydrochloride (MBTH) was injected through the system valve into a mixed stream from both channels in this setup. After proper mixing of reagents in the reaction coil, the resultant solutions were propelled at a flow rate of 1.25 mL/min into the detector. A green coloured product was formed and absorbance was measured at 610 nm.
2.2 Chemicals and materials
All reagents and chemicals used in this study were of analytical grade. Sample NIT was obtained from the State Company for Drug Industries and Medical Appliance (SDI, Samara, Iraq). The NIT-containing pharmaceutical dosage forms were purchased from local commercial sources. Two generic versions each contains NIT as its active constituent were used for analysis in this study (Uvamin® retard 100 mg, Basel, Switzerland and Furantil®, 50 mg, BioActive, UK).
2.3 NIT solution (500 μg/mL)
For method A (direct method), NIT standard solution was freshly prepared by dissolving 0.05 g of pure NIT powder in dimethyl sulfoxide (DMSO) and diluting the final mixture to a total volume of 100 mL with the same solvent. For method B, the proceeding reduction solution of NIT was prepared by dissolving 0.05 g of pure NIT powder in 50 mL of ethanol as previously described (CitationHadi, 2015). This solution was transferred into a 125 mL beaker before adding 20 mL of each distilled water and concentrated hydrochloric acid (11.64 N), and 3 g of zinc powder. In order to complete the reduction procedure, the solution was allowed to stand for 15 min at room temperature (25 °C). The solution was then filtered into a 100 mL volumetric flask and any residue was washed with distilled water. Finally, the mixture was diluted to the 100 mL mark with distilled water. The outcome from this procedure was a reduced NIT solution (500 μg/mL) which was then transferred into a brown bottle and can stand for one week at room temperature. More dilute solutions can be prepared freshly by appropriate dilution using distilled water.
2.4 Alcoholic KOH solution (0.1 M)
This solution was prepared by dissolving 1.40 g of KOH in a small volume of distilled water and by adding a sufficient volume of ethanol to complete the volume to 250 mL.
2.5 Methylbenzothiazoline-2-one hydrazone hydrochloride (0.05 M)
The acidic solution of this reagent was prepared by dissolving 1.08 g of methylbenzothiazoline-2-one hydrazone hydrochloride (Sigma Aldrich, Missouri, United States) in a 100 mL of 0.2 M HCl (Merck, Germany). This solution should be freshly prepared but more dilute solutions can be prepared daily by appropriate dilution using the same solvent.
2.6 Ammonium cerric sulphate (0.1 M)
This acidic solution was prepared by dissolving 6.60 g of ammonium cerric sulphate (BHD, UK) in 100 mL of 1% H2SO4 (Merck, Germany) solution. More dilute solutions can be prepared daily by appropriate dilution using the same solvent.
2.7 Solutions of pharmaceutical dosage forms
Method A: Ten Uvamin retard capsule (100 mg NIT) or fifteen Furantil capsules (50 mg NIT) were weighed and the contained powder was collected. An amount of resultant powder equivalent to 50 mg of NIT was dissolved in 30 mL of DMSO and the resultant solution was filtered into a 100 mL volumetric flask. The residue was washed with an additional volume of DMSO and the mixture was further diluted to the 100 mL mark with the same solvent to obtain a final solution of 500 μg/mL of NIT.
Method B: Similar to method A, the powdered NIT was obtained from the capsules and an amount equivalent to 50 mg of NIT was dissolved in 30 mL of ethanol. The solution was filtered into a 50 mL volumetric flask and the residue was washed with ethanol. A final concentration of NIT of 1000 μg/mL was obtained by diluting the mixture to the 50 mL mark with the same solvent. The resultant solution was transferred into a 125 mL beaker and was subjected to the reduction procedure as previously described. Further diluted solutions can be prepared from the stock solution using distilled water.
2.8 Recommended procedures and calibration curves
Method A: Series of solutions of NIT in a range of 5–250 μg/mL were prepared from the stock solution. A 200 µL portion of NIT was injected into a stream of 0.05 M of alcoholic KOH solution with a flow rate of 3.25 mL/min (a). The absorbance of the resultant red/orange coloured product was measured at 471 nm. Optimization of the conditions of this experimental setup was carried out using a concentration of 50 μg/mL of NIT.
Method B: A volume of 100 µL of MBTH (30 mM) was injected into an emerging stream from the two channels. The mixture stream was comprised of a solution of 50 mM of ammonium cerric sulphate and a reduced NIT solution of 5–300 μg/mL concentration range. The flow rate of the two emerging streams was at 1.25 mL/min in each channel (b). The mixture solution was further mixed in the reaction coil the resultant green coloured product absorbance was measured at 610 nm. Optimization of the conditions of this experimental setup was carried out using a concentration of 150 μg/mL of NIT.
3 Results and discussion
Both of the suggested NIT assay methods (direct and indirect FI) were designed to provide different reaction conditions in order to magnify the absorbance signal generated by the reactions. Different physical or chemical parameters were optimized individually.
3.1 Optimization of reaction conditions
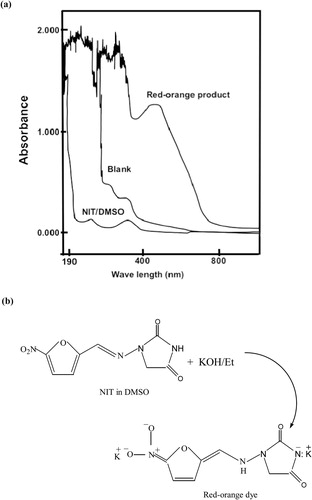
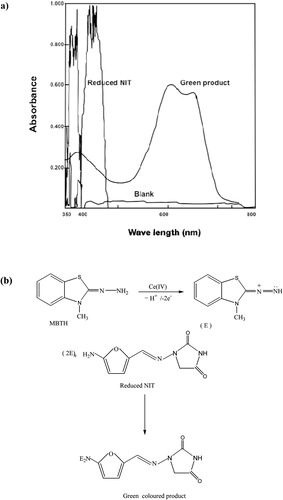
Method A: In line with the present study, a previous study used a colorimetric reaction in which an orange product is formed when nitro group containing compounds react with tetrabutyl ammonium hydroxide in a solution of dimethylformamide (CitationWalash et al., 1993). Similarly, the British Pharmacopeia reported an identification procedure for nitrofuran group containing drugs using alcoholic KOH in dimethylformamide (CitationBritish Pharmacopeia, 2009). The current study utilized a simple reaction (CitationSarker and Nahar, 2007) to develop the direct FI method for the determination of NIT without any pre-treatment steps. An orange coloured product (maximum absorbance at 471 nm) was formed immediately at room temperature after the addition of alcoholic KOH to NIT solution (NIT/DMSO). The absorption spectra of the coloured product with the blank and the proposed pathway of the reaction are shown in .
Figure 3 (a) Absorption spectra of the NIT/DMSO and the coloured product obtained from the reaction of 50 µg/mL of NIT with KOH/ethanol. The absorbance of the coloured product was measured against a reagent blank. The absorbance of the reagent blank was measured against ethanol. (b) Schematic representation of the reaction. KOH/Et, potassium hydroxide/ethanol; DMSO, dimethyl sulfoxide; NIT, nitrofurantoin.
A single channel setup was used for the direct determination of NIT (Method A) while a two-channel setup was used for the indirect method (Method B). Different setups were examined using different carrier solutions (DMSO or ethanol). The setup with more channels was found also to be the one with more dispersion. Moreover, in the single channel setup, the sample was injected directly into the stream of alcoholic KOH without pretreatment or extraction.
The effect of changing the concentration of the carrier was also investigated. Alcoholic KOH was used as a carrier reagent at a range of 0.02–0.1 M in this study. It was found that a concentration of 0.05 M of KOH gives the highest absorbance and was therefore selected as the optimum carrier concentration for further use (a). Physical parameters such as flow rate of solutions, length of the reaction coil and the volume of the injected samples were also studied. Regarding the flow rate, the effect of changing the flow rate on the absorbance of the reaction product was examined at a range of 1.65–10.5 mL/min. It was found that the maximum absorbance of the reaction product is reached at a flow rate of 3.25 mL/min. Hence, increasing the flow rate beyond this level results in decreased absorbance of the reaction product (b). The decrease in absorbance upon increasing the flow rate beyond 3.25 mL/min is due to increased dilution of the samples at higher flow rates.
Figure 4 The effects of different parameters on the absorbance of the reaction product. (a) the concentration of the carrier (KOH), (b) the total flow rate, (c) the reaction coil length, (d) the sample volume.
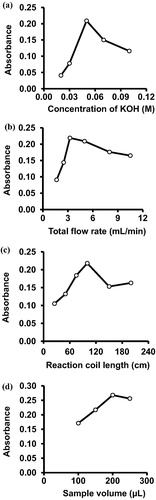
The effect of the length of the reaction coil on the absorbance of the coloured product in the FI system was also investigated. Increasing the length of the coil means that there is enough time for the reagent to mix. Different lengths of the reaction coil at a range of 25–200 cm were examined. The results indicated that a maximum absorbance of the reaction product was reached when the length is 100 cm (c).
Under the above-mentioned conditions, the volume of the injected sample was varied between 100 and 250 µL using different lengths of the sample loop. The results obtained showed that the maximum absorbance of the reaction product was reached at a sample volume of 200 µL (d).
Method B: NIT was found to react indirectly with MBTH in the presence of Ce (IV) to produce a green coloured product with absorbance measured at 610 nm. First, NIT is reduced using zinc powder in a concentrated HCl solution in order to convert the nitro group to an amino group. The reduction process was carried out outside the FI setup. MBTH has been utilized for the spectrophotometric determination of several other compounds (CitationMallikarjuna et al., 2016). A green coloured product was formed at room temperature after the addition of MBTH to reduced NIT solution in the presence of ammonium cerric sulphate as an oxidant agent. The absorption spectra of the coloured product and the proposed pathway of the reaction as described before (CitationRamachandra and Naidu, 2015) are shown in .
Figure 5 (a) Absorption spectra of reduced NIT and the green coloured product obtained from the reaction of 20 µg/mL of NIT with MBTH/ammonium cerric sulphate measured against the reagent blank. The reagent blank was measured against distilled water. (b) Schematic representation of the reaction as described by CitationRamachandra and Naidu (2015).
Three different reaction setups were used to conduct different pathways of the reaction. Similar to the direct FI method, the two-channel setup (b) was used for the indirect determination of NIT due to its high dispersion compared to the single-channel setup. Chemical and physical parameters were also optimized for higher sensitivity of the reaction. The optimum concentration of the coupling reagent (MBTH) was studied through the injection of different concentrations (ranging from 0.005 to 0.05 M) through the injection valve. The results indicated that a maximum absorption of the product can be obtained at a concentration of 0.03 M. Therefore, this concentration was chosen for further use. Different concentrations of ammonium cerric sulphate (oxidant) were also studied in a range of 0.01–0.1 M. The results showed that a maximum absorbance was obtained at a concentration of 0.07 M of ammonium cerric sulphate (b).
Figure 6 The effects of different parameters on the absorbance of the reaction product. (a) Concentration of MBTH, (b) Concentration of the oxidant, (c) Reaction coil length, (d) Total flow rate, (e) Sample volume.
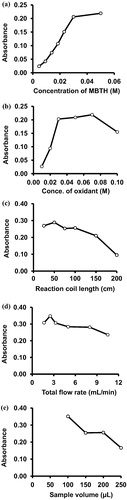
Different reaction coil lengths at a range of 25–200 cm were studied. The results obtained showed that the maximum absorbance was obtained at a length of 50 cm as shown in c and was therefore used in all subsequent experiments. Increasing the reaction coil length decreases the absorbance of the coloured product because of increased dilution of the samples. The effect of different flow rates on the absorbance of the reaction products was studied at a range of 1.7–10.5 mL/min. The results obtained showed that the maximum absorbance was reached at a flow rate of 2.5 mL/min then the absorbance decreased with increasing the flow rate. Higher flow rates result in shorter time available for mixing the reagents. Therefore, a flow rate of 2.5 mL/min (1.25 mL/min for each line) was chosen as the optimum value (d). The effect of the volume of the injected sample was investigated and the results obtained showed that the maximum absorbance was reached using a sample volume of 100 µL and was therefore used for further experiments (e). All optimum values obtained for both methods are summarized in .
Table 1 The optimum conditions for the determination of NIT using two FI methods. ACS, ammonium cerric sulphate.
3.2 Calibration graphs and analytical data
Under the optimum conditions described above (), a series of standard solutions of NIT were used and the calibration graphs for both FI methods were constructed. The resulting analytical data are shown in .
Table 2 Analytical features of the procedures developed for the determination of NIT.
3.3 Accuracy and repeatability
Five replicates at three different concentrations of NIT were studied. Accuracy and precision of the proposed FI methods were performed by calculating the relative standard deviation (RSD,%) and the relative standard error (RE,%) of NIT concentration (found concentrations) compared to theoretical values. The results indicated that the proposed methods for determination of NIT is accurate and reproducible ().
Table 3 Accuracy and precision of the proposed FI methods. *n = 5, Rec., recovery.
3.4 Pharmaceutical applications
The two proposed FI methods were applied for the analysis of NIT in two marketed dosage forms. The results obtained indicated an agreement between the total value as claimed by the manufacturer (100 and 50 mg) from both methods. Solutions of pharmaceutical dosage forms were prepared using the two methods and the accuracy of the methods was determined by performing recovery tests. An appropriate amount of NIT powder was weighed and spiked with known amount of the standard NIT and each sample was analyzed using optimum conditions (). The results are summarized in and compared with those obtained from CitationUSP standard method (2005) using HPLC. The obtained recovery values indicated no interference with the determination and suggested good selectivity of the FI methods. The results obtained from the two proposed FI methods were statistically compared, using the two statistical tests (CitationMiller and Miller, 2010) (Student t-test and variance ratio F-test) at a confidence interval of 0.95. The results in show that the calculated t- and F-values were comparable to theoretical values. The data also indicated no significant difference in accuracy and precision between the two proposed FI methods.
Table 4 Application of the proposed and official methods for the determination of NIT in different dosage forms. a, (n = 5); b, (n = 3); c, theoretical value; Conc., concentration; RSD, relative standard deviation.
4 Conclusion
The two proposed FI-spectrophotometric methods (direct and indirect) can be applied for accurate and precise determination of NIT concentration in pharmaceutical preparations. The methods described are simple, sensitive and cost-effective. They can be used over a wide range of NIT concentration with higher recovery and reproducibility.
Conflict of interest
The authors have no conflict of interest.
Acknowledgements
The authors would like to thank Dr Abdulla, M.H. for his help in preparing the manuscript. Technical assistance provided by the Department of Chemistry of Baghdad University to this study is also acknowledged.
Notes
Peer review under responsibility of University of Bahrain.
References
- G.AydoğduG.GünendiD.K.ZeybekB.ZeybekŞ.PekyardımcA novel electrochemical DNA biosensor based on poly-(5-amino-2-mercapto-1,3,4-thiadiazole) modified glassy carbon electrode for the determination of nitrofurantoinSensors Actuators B Chem.1972014211219
- T.S.BelalA simple and sensitive spectrofluorimetric method for analysis of some nitrofuran drugs in pharmaceutical preparationsJ. Fluoresc.182008771780
- British PharmacopoeiaThe Stationery office on behalf of the Medicines and Healthcare products Regulatory Agency (MHRA)2009 London
- P.De Lima-NetoA.N.CorreiaR.R.PortelaS.Juliao MdaG.F.Linhares-JuniorJ.E.De LimaSquare wave voltammetric determination of nitrofurantoin in pharmaceutical formulations on highly boron-doped diamond electrodes at different boron-doping contentsTalanta80201017301736
- N.N.DuM.M.ChenL.Q.ShengS.S.ChenH.J.XuZ.D.LiuC.F.SongR.QiaoDetermination of nitrofuran metabolites in shrimp by high performance liquid chromatography with fluorescence detection and liquid chromatography-tandem mass spectrometry using a new derivatization reagentJ. Chromatogr. A132720149096
- H.HadiSpectrophotometric determination of clonazepam in pure and dosage forms using charge transfer reactionIraqi J. Pharma. Sci.2420152532
- M.L.IngalsbeA.L.WojciechowskiK.A.SmithK.A.MergenhagenEffectiveness and safety of nitrofurantoin in outpatient male veteransTher. Adv. Urol.72015186193
- M.C.MahederoT.Galeano DiazS.Galan PascualResolution of ternary mixtures of nitrofurantoin, furaltadone and furazolidone by partial least-square analysis to the spectrophotometric signals after photo-decompositionJ. Pharm. Biomed. Anal.292002477485
- H.MallikarjunaK.H.ShivaprasadK.R.Venugopala ReddyK.S.LokeshSpectrophotometric determination of some nonsteroidal anti-inflammatory drugs by oxidative coupling reactionAustin J. Anal. Pharma. Chem.3201616
- J.A.MckinnellN.S.StollenwerkC.W.JungL.G.MillerNitrofurantoin compares favorably to recommended agents as empirical treatment of uncomplicated urinary tract infections in a decision and cost analysisMayo Clin. Proc.862011480488
- J.N.MillerJ.C.MillerStatistics and Chemometrics for Analytical Chemistry2010Ashford Colour Press Ltd.Gosport, UK
- B.RamachandraN.V.NaiduSpectrophotometric method for the determination of furazolidone in pharmaceutical formulations and human blood samples with MBTHInt. J. Sci. Res.4201510261031
- S.D.SarkerL.NaharChemistry for Pharmacy Students: General, Organic, and Natural Product Chemistry2007John Wiley and Sons Ltd.UK
- P.ThongsrisomboonB.LiawruangrathS.IawruangrathS.SatienperakuDetermination of nitrofurans residues in animal feeds by flow injection chemiluminescence procedureFood Chem.1232010834839
- M.TubinoL.F.BianchessiM.PalumboM.M.D.C.VilaGreen and simple uv-visible diffuse reflectance and transmittance methods for the determination in pharmaceutical preparationsEcletica Química3620116267
- United States PharmacopeiaThe National Formulary2005Pharmacopeial ConventionRockville, USA
- M.I.WalashA.M.El-BrashyM.A.SultanColorimetric determination of some aromatic nitrocompounds of pharmaceutical interestAnal. Lett.261993499512
- D.WilasineeP.SutthivaiyakitS.SutthivaiyakitDetermination of nitrofurans in chicken feed by high-performance liquid chromatography–tandem mass spectrometryAnal. Lett.48201519791987
- W.ZhangC.R.WilsonN.D.DanielsonIndirect fluorescent determination of selected nitro-aromatic and pharmaceutical compounds via UV-photolysis of 2-phenylbenzimidazole-5-sulfonateTalanta74200814001407