Abstract
A clinical isolate (Candida albicans) was collected from diagnosed cases as vaginal candidiasis. Identification of this isolate was previously reported [Citation11] using both API 20C kit and real-time PCR assay. Adherence of C. albicans to the vaginal epithelium was evaluated. C. albicans recorded the highest rate (63.00%) of adherence among all collected isolates and recorded the highest record for the number (131.00) of adhered yeast cells (data not shown). The evaluation of the critical factors affecting the adherence process revealed that the maximum adherence values were recorded for a logarithmic phase-culture grown at 25 °C, incubation temperature of 37 °C in phosphate buffer saline adjusted at pH 5. Moreover, the adherence pattern of C. albicans cells to the vaginal epithelium was visualized by the scanning electron microscopy (SEM).
1 Introduction
The inflammation of the vagina as a result of infectious diseases is very common [Citation8]. Vaginal symptoms are usually related to one of three conditions: bacterial vaginosis, trichomoniasis and yeast vaginitis [Citation24,Citation25,Citation34,Citation37]. Yeast vaginitis is a very common problem. Theories explain the repeated yeast infections have focused on vaginal reinfection, either from a gastrointestinal source or from sexual transmission, or on vaginal relapse, which hypothesizes that recurrent infections are due to the same infecting organism, which is only temporarily suppressed by antifungal therapy [Citation24,Citation33].
Adherence has been shown to play a central role in the pathogenesis of many microbial infections [Citation2,Citation3,Citation9,Citation10,Citation12,Citation29]. The adherence to various surfaces represents the first step in the mechanisms of pathogenesis and suggests means of controlling infection at an early stage [Citation9,Citation10]. The definition of the mechanisms of attachment of Candida albicans may have important therapeutic implications. New therapeutic strategies for the treatment of candidiasis may involve inhibition of the attachment of the organism to the host cell [Citation16,Citation36].
The present study was conducted to focus on the characterization of the adherence of the C. albicans to the epithelium cells and to determine the critical factors and their statistical significance that affect the adherence process and consequently have an impact on the pathogenesis process.
2 Materials and methods
2.1 Microorganism and culture conditions
C. albicans was previously isolated and identified [Citation11]. C. albicans isolate was sub-cultured onto a set of three tubes of Sabouraud’s dextrose broth (10 ml) that sequentially had 1, 2 and 3 drops of 1 N HCl added to them. These broth cultures were incubated for 48–72 h at 25 °C. A loop of the inoculated Sabouraud’s dextrose broth (supplemented with 2 drops 1 N HCl) was streaked on Sabouraud’s dextrose agar plates, the plates were then incubated at 25 °C for 72 h. The developing colonies of yeasts were maintained on culture slants for further use.
2.1.1 Morphological characteristics
A plate containing either corn meal agar with Tween 80 was inoculated and incubated at 25 °C for 48–72 h. The growth was examined for the presence of pseudohyphae, true mycelium, blastospores and chlamydospores [Citation1] ().
2.1.2 Germ tube test
A tube containing 0.25 ml of yeast peptone dextrose medium (YPD) was inoculated with the C. albicans for the development of germ tubes [Citation18]. The cultures were incubated at 39 °C and examined microscopically after 4 h of incubation for the development of germ tubes and blastoconidia [Citation1].
2.1.3 Adherence of C. albicans to the vaginal cells
C. albicans was incubated overnight in yeast extract broth at 25 °C under shaking conditions at 60 rpm. Yeast cells were harvested by centrifugation after 18 h and washed in PBS twice. The yeasts were enumerated using haemocytometer, and then diluted to a density of 108 cells/ml in phosphate buffer saline (PBS) adjusted to pH 7.0 [Citation31]. The adherence of C. albicans to the vaginal epithelium was determined according to King et al. [Citation20] and Segal et al. [Citation30]. The freshly obtained vaginal epithelial cells (used within 4 h) were washed twice in 0.9% saline and resuspended in PBS adjusted at pH of 7.0. The concentration of the epithelial cells was adjusted to 105 cells/ml using haemocytometer.
2.1.4 Assay of C. albicans adherence
Equal volumes (0.25 ml) of epithelial cells (105) and yeast cells (108) were mixed and incubated for 30 min in a shaking incubator (50 rpm) at 37 °C and pH 7.0. After incubation, the suspensions were centrifuged and washed twice with saline to separate the non-adhering yeast cells. Several drops of the sediment were placed on glass slide, fixed and stained with crystal violet, and then the percentage of adherence was determined by counting the number of epithelial cells with 20 or more adhered yeasts out of a total of 100 cells. The number of yeast cells attached to each of 100 epithelial cells was also counted and the mean value was calculated [Citation30]. The adherence assay was performed in triplicates.
2.2 Factors affecting the adherence of C. albicans to the vaginal epithelial cells
2.2.1 Effect of the pH of the assay medium
The phosphate buffer was prepared to the following different pH values 5–9 to test the optimum pH value for the adherence [Citation26]. The cells were resuspended to the desired concentration and used for the application of the adherence assay.
2.2.2 Effect of the assay temperature
Equal volumes of epithelial cells and yeast cells (0.25 ml) suspended in PBS adjusted at pH 7.0 were mixed and incubated on a rotator individually at 25, 37 and 40 °C for 30 min [Citation19].
2.2.3 Effect of the yeast culture age
C. albicans was grown in yeast extract broth at 25 °C. The yeast cells were harvested by centrifugation after 8, 16, 24, 32, 44 and 56 h, washed twice and resuspended in PBS adjusted at pH 7.0 to the desired concentration [Citation28]. The cells were then ready for the application of the adherence assay.
2.2.4 Effect of the incubation temperature
C. albicans was grown in yeast extract broth at 25, 30, 35 and 40 °C. The yeast cells were harvested by centrifugation after 18 h (Standard growth curve was constructed to determine the timing of each of the growth phases), washed twice and resuspended in PBS (pH 7) to the desired concentration [Citation28]. The cells were then ready for the application of the adherence assay.
2.3 Scanning electron microscopy
The samples for electron microscopy were prepared as described by El-Naggar et al. [Citation10]. The processed samples were visualized using scanning electron microscope (S 800 Hitachi Co., Ltd., Tokyo, Japan) with an accelerating voltage 15–52 kV at magnification varied from 1000× to 12,000×.
2.4 Statistical analyses
Statistical analyses of the adherence data were carried out using SPSS program, version 12. One-way ANOVA test was used to evaluate the differences in the adherence values. A p-value of ≤0.05 was considered significant. Pearson’s correlation coefficient was also used.
3 Results and discussion
The incidence of the various fungal pathogens has increased dramatically over the past few decades. Candida species are the most common of these pathogens [Citation5,Citation27]. Vulvovaginitis is one of the commonest presentations of Candida infection. Despite therapeutic advances, vulvovaginal candidiasis remains a common problem worldwide, affecting all strata of society [Citation2,Citation29,Citation32].
3.1 The adherence of the C. albicans to the vaginal epithelial cells
The adherence of the C. albicans to the vaginal epithelial cells was observed under optimal temperature of 37 °C and pH 7. The adherence ability is expressed as the percentage of epithelial cells having more than 20 C. albicans cells attached and the number of adhered yeast cells attached to each of 100 epithelial cells. C. albicans showed more remarkable and potent adherence percentage to the vaginal epithelial cells (63.00%). Regarding the number of adhered yeast cells, this isolate recorded the highest number of adhered yeast cells (131.00).
C. albicans strains produced different and high levels of adherence to vaginal epithelial cells among other isolates (data not shown). Genomic variability may account for varied adherence abilities among the tested C. albicans strains. Kennedy [Citation17] reported that the extent of adherence to mucosal epithelium appears to be a strain-dependent property. However, Vidotto et al. [Citation35] indicate that all C. albicans strains appear to adhere equally well to exfoliate vaginal and buccal epithelial cells. The high recorded adherence abilities of the tested C. albicans strains should not surprising us if we consider that adherence to cell surfaces is an important virulence factor and that C. albicans is usually considered more virulent than other Candida species.
3.2 Factors affecting the adherence of C. albicans cells to vaginal epithelial cells
The adherence of C. albicans cells to vaginal epithelial cells under certain environmental conditions is most likely a pivotal point in the pathophysiology of vaginal candidiasis [Citation26]. In the present study, we have sought focus on how pH, the temperature of the adherence assay, the growth phase and the growth temperature of the yeasts affect its adherence to vaginal epithelial cells.
3.2.1 Effect of the pH of the assay medium
The results in showed that although the pH 5 allowed a maximal adherence, attachment could still be detected under both neutral and alkaline conditions. Comparing the adherence ability at different pH values (5–9), the highest value was recorded at pH 5 (84.50%, 157.50) and pH 6 (81.00%, 150.50), followed by pH 7 (62.00%, 133.50), while at pH 8 and pH 9 the adherence ability was decreased (32.00%, 65.00 and 25.50%, 55.50, respectively). Dostal et al. [Citation7] reported that the pH values were the critical factors in proteolytic activity because relatively small shifts can cause changes in extracellular proteolytic activity. Modification of the host cell membrane could occur in an acidic environment, but under such conditions general proteolytic activity and dissolution of host-cell membranes may be more important than adherence considerations. Modification of the cell surface of C. albicans, however, may proceed if the surface pH is lowered because of carbohydrate utilization and production of acidic by-products. Proteolytic action on the Candida cell surface alters hydrophobicity, which would also affect adherence [Citation6] ().
Table 1 Effect of the initial pH values on the adherence of C. albicans to the vaginal epithelial cells.
Table 2 Statistical analysis (one-way ANOVA test) for the data obtained in .
Variation of the pH of the adherence medium gave highly significant differences (p ≤ .001) in the adherence ability. The statistical analysis indicated that no significant difference (p > .05) in the adherence ability neither between pH values (5 and 6), nor between (8 and 9), but the differences in the adherence ability of the isolate at pH (5 and 6) and that at pH (8 and 9) were highly significant (p ≤ .001). Significant difference (p ≤ .05) in the adherence percentage between pH 7 and the other tested pH values was observed, whereas the number of adhered yeast cells at pH 7 differed significantly (p ≤ .05) from that at pH (5 and 6) and highly significantly (p ≤ .001) from that at pH (8 and 9). The results () revealed a negative correlation between the adherence ability of the tested isolate to the vaginal epithelial cells and pH, as the pH increased the adherence ability decreased, and this correlation was highly significant (r = −.952, p ≤ .001 and r = −.940, p ≤ .001).
Table 3 Statistical analysis (bivariate correlations test) for the data obtained in .
The results of the adherence assays of C. albicans to vaginal epithelial cells at different pH values showed that there was strong, statistically significant association between the adherence ability of the tested isolate and pH. The adherence at the neutral pH was slightly less than that at the acidic conditions. Under alkaline conditions the adherence was much less that under both neutral and acidic conditions. Our data thus differ from that of Lehrer et al. [Citation23] who found that adherence was not influenced by pH or pre-treatment of yeast cells with salts, divalent cations, detergents, urea, lipase or α-mannosidase.
3.2.2 Effect of the assay temperature
showed that the assay mixture incubated at 37 °C gave the maximal adherence (62.50%, 132.00), followed by those incubated at 25 °C (36.00%, 89.50) and the least at 40 °C (32.50%, 70.50). Statistically, there were significant differences (p ≤ .05) in the adherence ability of the tested isolate to the vaginal epithelial cells obtained at the different temperatures tested (). The adherence ability of C. albicans at 37 °C was significantly (p ≤ .05) greater than that at 25 and 40 °C (). Interestingly, the adherence percentage was almost twofold greater when the assay was performed at 37 °C compared to 25 and 40 °C. The adherence percentage was affected equally by the two temperatures 40 and 25 °C. There was no significant difference (p > .05) in the adherence ability at 25 and 40 °C. Data in indicated a weak and not significant correlation between the adherence ability of the tested isolate and the temperature of the adherence assay (r = .221, p > .05 and r = .026, p > .05).
Table 4 Effect of the assay temperature on the adherence of C. albicans to the vaginal epithelial cells.
Table 5 Statistical analysis (one-way ANOVA test) for the data obtained in .
Table 6 Statistical analysis (bivariate correlations test) for the data obtained in .
3.2.3 Effect of the yeast culture age
Yeast cell adherence is also influenced by the growth phase of the culture. In the present study, it was found that logarithmic-phase-yeasts had greater adherence ability than stationary-phase-yeasts. Maximal adherence ability was obtained with culture which has been incubated for 16 h while the minimal obtained with culture incubated for 56 h. This finding may be due to that the factor(s) present on the surface of logarithmic-phase-cells than on cells in the stationary phase of growth. In addition, enzymes such as phospholipase and proteinase which play a critical role in the adherence process are more active in cultures in the exponential growth phase than in the stationary phase.
C. albicans cultures grown at logarithmic phase for periods 8, 16 and 24 h had greater adherence ability than those grown at stationary phase for periods 32, 44 and 56 h. Maximal adherence ability was obtained with culture grown for 16 h (62.50%) with number of adhered cells reached (130.00), while the minimal obtained with culture grown for 56 h (31.50%) with number of adhered cells reached (60.50) (). Highly significant differences (p ≤ .001) in the adherence ability of the isolate were obtained at the different growth phases tested. It was obvious that adherence ability of C. albicans grown for 16 h of the logarithmic phase differed significantly (p ≤ .05) from those grown for 8 and 24 h. Although the adherence ability of the 24-h-culture was higher than that of the 8-h-culture, statistically, this difference was not significant (p > .05). Regarding the adherence ability of the stationary-phase-yeasts, no marked differences (p > .05) in the adherence ability were observed when the 32-h- and 44-h-cultures were compared. The adherence percentage of the 32-h- and 44-h-cultures differed significantly (p ≤ .05) from that of the 56-h-culture. The number of adhered yeast cells of the 56-h-culture recorded a high significant difference (p ≤ .001) from that of 32-h-culture, while the difference was significant (p ≤ .05) with that of 44-h-culture ().
Table 7 Effect of the age of C. albicans on its adherence to the vaginal epithelial cells.
Table 8 Statistical analysis (one way ANOVA test) for the data obtained in .
The results of the two phases of growth (logarithmic and stationary) showed that high significant differences (p ≤ .001) in the adherence ability were recorded between the 16-h-culture and all cultures of the stationary phase. The difference in adherence ability of the 8-h-culture was highly significant (p ≤ .001) with that of the 56-h-culture and significant differences (p ≤ .05) with those of the 32-h- and 44-h-cultures were recorded. There was a significant difference (p ≤ .05) in the adherence ability of the 24-h-culture and that of the 32-h-culture, but high significant differences (p ≤ .001) were obtained with those at 44-h- and 56-h-cultures. The results in revealed that there was a negative and highly significant correlation between the growth phase of the selected isolate and its adherence ability to the vaginal epithelial cells (r = −.866, p ≤ .001 and r = −.868, p ≤ .001).
Table 9 Statistical analysis (bivariate correlations test) for the data obtained in .
3.2.4 Effect of the incubation temperature
Our data also indicated that strong correlation exists between the growth temperature of the tested isolate and its adherence to vaginal epithelial cells. Yeast cells grown at 25 °C gave the highest adherence ability and as the growth temperature increased the adherence ability decreased. These data are supported by other investigators who reported that blastospores harvested from cultures grown at 25 °C adhered to vaginal epithelial cells in significantly greater numbers than did blastospores isolated from cultures grown at 37 °C [Citation22,Citation30].
Variation in growth temperature is one of the factors that are known to affect yeast cell surface hydrophobicity (CSH) [Citation4,Citation14] which has been shown to be involved in the adherence process of the yeast. Cell surface hydrophobicity is characterized by the presence of hydrophobic proteins embedded in the yeast cell wall matrix beneath an outer fibrillar layer [Citation13]. Exposure of these hydrophobic proteins results in CSH; therefore, CSH is subject to cell surface variations such as a shortened or rearranged fibrillar layer [–Citation13 Citation14 Citation15 ]. In fact, C. albicans cells are hydrophobic when grown at 25 °C and hydrophilic at 37 °C [Citation13,Citation14] this may explain why cells grown at 25 °C gave the highest adherence capacity.
Comparing the adherence ability at different growth temperatures (25, 30, 35 and 40 °C), the culture that incubated at 25 °C had a greater adherence ability (63.50%, 130.50) than the others, whereas that incubated at 40 °C had the lowest adherence ability (25.00%, 59.00). It is worth mentioning that the percentage of adherence at 30 °C was twice that recorded at 40 °C (). The results in suggested that among the tested growth temperatures, there was a statistically significant difference (p ≤ .05) in the yeast adherence ability to the vaginal epithelial cells. The percentage of adherence at 25 °C differed significantly (p ≤ .05) from that at 35 and 40, but not at 30 °C. The number of adhered yeast cells at 25 °C differed significantly (p ≤ .05) from that at 30 and 35 °C, while the difference was highly significant (p ≤ .001) from that at 40 °C. Although there was a significant difference (p ≤ .05) in the adherence ability of the isolate at 35 and 40, also at 30 and 40 °C, but there was not (p > .05) at 30 and 35 °C (). The results in revealed that the adherence ability of the isolate to the vaginal epithelial cells and its growth temperature were negatively correlated and this correlation was highly significant (r = −.937, p ≤ .001 and r = −.959, p ≤ .001).
Table 10 Effect of the incubation temperature on the adherence of C. albicans to the vaginal epithelial cells.
Table 11 Statistical analysis (one way ANOVA test) for the data obtained in .
Table 12 Statistical analysis (bivariate correlations test) for the data obtained in .
3.3 Scanning electron microscopy
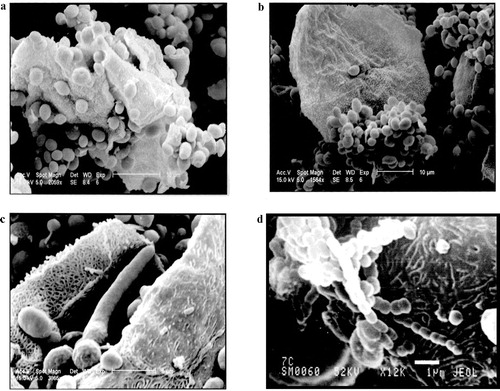
The morphological characteristics related to adherence of C. albicans to vaginal epithelial cells were observed using scanning electron microscope. The large numbers of adhered yeast cells noted were a reflection not only of yeast cell-to-epithelial cell adherence, but also of yeast cells co-adherence. The latter type of interaction resulted in the formation of aggregates of yeasts on the epithelial cell surface which could be observed both by light microscopy and by scanning microscopy. Klotz and Penn [Citation21] showed that the addition of a highly concentrated blastospore inoculum led to blastospore aggregation (self-adherence) and to a greater destruction of the intestinal cells used in the adherence study than to a normal random distribution of yeast cells. We suggested that formation of yeast aggregates through co-adherence may allow C. albicans to effectively amplify its cell numbers in vivo. Such amplification may localize aggregates of organisms in sufficient numbers to overcome host defences resulting in numerous foci of infection.
The features of the adherence of C. albicans to vaginal epithelial cells are shown in a–d. Blastospores with germ tubes and pseudomycelium were also seen on the surface of the vaginal epithelial cells after C. albicans was allowed to interact with them (c and d). Although many of the yeasts adhered directly to the mucosal cells, some yeast cell-to-yeast cell attachment was also noted. This latter type of interaction, co-adherence, appeared to result from yeasts attaching to those yeasts which had already established contact with the epithelial cells. Co-adherence was not noted except at the epithelial cell surface. Thus, preaggregation of the yeasts before their attachment to the epithelial cell apparently does not occur.
Fig. 2 Scanning electron micrograph showing (a) the adherence of C. albicans to the surface of the vaginal epithelial cells. (b) A direct attachment of the yeast cells of C. albicans to the vaginal epithelial cell and yeast cells co-adherence. Scale bar: 10 μm. (c) The formation of germ tubes by C. albicans. Scale bar: 5 μm. (d) the formation of pseudomycelium by C. albicans. Scale bar: 1 μm.
4 Conclusion
Since the adherence of C. albicans to the mucosal epithelial cells is considered as the initial step in the mucocutaneous candidiasis. So understanding of Candida–host cell interaction will have important therapeutic implications to figure out therapeutic strategies for the treatment of candidiasis, especially with the availability of some effective and safe antifungal agents.
Notes
Peer review under responsibility of Taibah University.
References
- D.G.AhearnMedically important yeastsAnnual Review of Microbiology3219785968
- R.Alonso-VargasL.ElorduyE.EvasoJ.F.CanoJ.GuarroJ.PontonG.QuindósIsolation of Candida africana, probable a typical strains of Candida albicans, from a patient with vaginitisJournal of Medical Mycology462008167170
- C.M.BendelColonization and epithelial adhesion in the pathogenesis of neonatal candidiasisSeminars in Perinatology272003357364
- R.A.CalderoneP.C.BraunAdherence and receptor relationships of Candida albicansMicrobiological Reviews551991120
- M.R.CapoorD.NairM.DebP.K.VermL.SrivastavaP.AggarwalEmergence of non-albicans Candida species and antifungal resistance in a tertiary care hospitalJapanese Journal of Infectious Diseases582005344348
- J.E.CutlerDifferential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissuesInfection and Immunity591991907912
- J.DostalP.HamalL.PavlicovaM.SoucekM.T.RumlI.PichovaO.Hrušková-HeidingsfeldováSimple method for screening Candida species isolates for the presence of secreted proteinases: a tool for the prediction of successful inhibitory treatmentJournal of Clinical Microbiology412003712716
- L.EdwardsThe diagnosis and treatment of infectious vaginitisDermatologic Therapy172004102110
- M.Y.El-NaggarM.A.AkeilaA.M.El-KenanyImmuno-modulatory role of two commercial probiotics in poultry industryAdvances in Food Sciences (CMTL)2720052226
- M.Y.El-NaggarM.A.AkeilaA.M.El-KenanyInfluence of two probiotic consortia on the colonization of enteroinvasive pathogens in the intestinal tract of chicken in poultry farmsAdvances in Food Sciences (CMTL)2720058593
- M.Y.El-NaggarH.M.Al-BasriA.A.Karam El-DinMolecular diagnosis of Candida albicans using real-time polymerase chain reaction of a CaYST1 geneJournal of Taibah University for Science32010813
- N.A.R.GowF.L.van de VeerdonkA.J.P.BrownM.G.NeteaCandida albicans morphogenesis and host defence: discriminating invasion from colonizationNature Reviews102012112122
- K.C.HazenParticipation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cellsInfection and Immunity57198918941900
- K.C.HazenD.L.BrawnerM.H.RiesselmanM.A.JutilaJ.E.CutlerDifferential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissuesInfection and Immunity591991907912
- F.Jabra-RizkJr.W.G.MerzJ.I.KelleyA.A.BaquiT.F.MeillerCandida dubliniensis and Candida albicans display surface variations consistent with observed intergeneric coaggregationRevista Iberoamericana de Micología161999814
- I.D.JacobsenD.WilsonB.WächtlerS.BrunkeJ.R.NaglikB.HubeCandida albicans dimorphism as a therapeutic targetExpert Review of Anti-infective Therapy1020128593
- M.J.KennedyAdhesion and association mechanisms of C. albicansM.R.McginnisCurrent Topics in Medical Mycology1988Springer-VerlagNew York73169
- D.KimW.S.ShinK.H.LeeK.KimJ.Y.ParkRapid differentiation of Candida albicans from other Candida species using its unique germ tube formation at 39 °CYeast192002957962
- L.H.KimuraN.N.PearsallAdherence of Candida albicans to human buccal epithelial cellsInfection and Immunity2119786468
- R.D.KingJ.C.LeeA.MorrisAdherence of Candida albicans and other Candida species to mucosal epithelial cellsInfection and Immunity271980667674
- S.A.KlotzR.L.PennMultiple mechanisms may contribute to the adherence of Candida yeasts to living cellsCurrent Microbiology161987119122
- J.C.LeeR.D.KingCharacterization of Candida albicans adherence to human vaginal epithelial cells in vitroInfection and Immunity41198310241030
- N.LehrerE.SegalL.CihlarR.CalderonePathogenesis of vaginal candidiasis: studies with a mutant which has reduced ability to adhere in vitroJournal of Medical and Veterinary Mycology241985127131
- R.McCathieVaginal discharge: common causes and managementCurrent Obstetrics & Gynaecology162006211217
- M.K.OwenT.L.ClenneyManagement of vaginitisAmerican Family Physician70200421252132
- A.M.PersiC.J.BurnhamL.J.DuhringEffect of carbon dioxide and pH on adhesion of Candida albicans to vaginal epithelial cellsInfection and Immunity5019858290
- M.A.PfallerR.N.JonesG.V.DoernH.S.SaderS.A.MesserS.CoffmanR.J.HollisBlood stream infection due to Candida species. SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998Antimicrobial Agents and Chemotherapy442000747751
- C.M.PiresB.CorreaW.GambaleC.R.PaulaExperimental model of Candida albicans (serotypes A and B) adherence in vitroBrazilian Journal of Microbiology322001110
- M.V.PirottaS.M.GarlandGenital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibioticaJournal of Clinical Microbiology44200632133217
- E.SegalN.LehrerI.OfekAdherence of Candida albicans to human vaginal epithelial cells: inhibition by amino sugarsExperimental Cell Biology5019821317
- E.SegalO.TrygermanY.GovH.Sandovsky-LosicaI.BerdicevskyAdhesion of Candida albicans to epithelial cells: effect of antimycoticsJournal of Medical Mycology719977176
- J.D.SobelVulvovaginal candidiasisLancet369200719611971
- J.D.SobelH.C.WiesenfeldN.MartensMaintenance fluconazole therapy for recurrent vulvovaginal candidiasisNew England Journal of Medicine3512004876883
- D.E.SoperTaking the guesswork out of diagnosing and managing vaginitisContemporary Obstetrics & Gynecology5020053239
- V.VidottoB.MantoanA.PuglieseJ.PontónG.QuindósS.Aokiet alAdherence of Candida albicans and Candida dubliniensis to buccal and vaginal cellsRevista Iberoamericana de Micología2020035254
- B.WächtlerD.WilsonB.HubeStage-specific inhibition by clotrimazole and damage of vaginal epithelial cells: Candida albicans adhesion to and invasionAntimicrobial Agents and Chemotherapy55201144364439
- H.XiangL.XiongX.LiuZ.TuRapid simultaneous detection and identification of six species Candida using polymerase chain reaction and reverse line hybridization assayJournal of Microbiological Methods692007282287