?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A previous investigation was carried out concerning the isolation of bacteria and fungi having ability to decompose cholesterol from different cholesterol-rich sources. Streptomyces fradiae was identified as the most potent degrading isolate. In a trail to increase the cholesterol decomposing potentiality of S. fradiae, low intensity Nd-YAG laser irradiation was evaluated. The exposure of the chlorophyllin-photosensitized bacterium to 210 mW Ne-YAG laser for 8 min induced significant increase in cholesterol degrading activity reaching 73.8% as compared with 54.2% in the case of non-irradiated, non-photosensitized culture. The optimization of environmental conditions and nutritional factors of the irradiated bacterium was carried out and aimed to maximize cholesterol decomposition. The following optimas were recorded for irradiated and non-irradiated S. fradiae: substrate concentration 1–1.5 as compared to 1.0 g/l; yeast extract, 4–6 as compared to 5–6 g/l; magnesium nitrate and ammonium nitrate as nitrogen source for both bacteria; glucose concentration, 10–20 as compared to 10 g/l; incubation period, 6 days for both; incubation temperature, 30–35 °C as compared to 30 °C; pH, 7–8 as compared to 7; and shaking speed, 40–120 as compared with 80 rpm, respectively. Under all optimized conditions, the cholesterol decomposing activity of irradiated photosensitized S. fradiae (88.5%) exceeds the activity of the non-irradiated one (78.7%). Moreover, the irradiated bacterium was able to keep its optimas over a wider range of substrate, yeast extract, glucose, temperature, pH, and shaking as compared the non-irradiated one.
1 Introduction
The major role of cholesterol in pathological process is as a factor in the genesis of atherosclerosis of vital arteries causing cerebrovascular, coronary and peripheral vascular diseases [Citation39].
Many bacteria have been described to degrade some steroid molecules such as cholesterol and its derivatives [Citation4,Citation28,Citation13,Citation45,Citation12]. The bacterial species involved in such degradation include lactic acid bacteria [Citation37,Citation40,Citation24], Bifidobacterium bifidum [Citation16], Bacillus licheniformis [Citation9], Rhodococcus equi [Citation11,Citation10,Citation46] and Mycobacterium tuberculosis [Citation7].
Radiation was tried to enhance the activity of microbial enzymes [Citation42,Citation14,Citation33]. Low intensity laser radiation with wavelength of 400–600 nm brought about accelerated cell division in various microorganisms and enhanced protein synthesis [Citation6]. Gewelly et al. [Citation15] reported enhancement of xylanase production on irradiation of 7.3 mW He–Ne laser irradiated Aspergillus terreus in the presence of crystal violet, toluidine blue O and hematoporphyrin as photosensitizers.
The optimization of the environmental and nutritional factors was the initial avenue pursuing the enhancement of cholesterol decomposition by microorganisms and cholesterol oxidase activity. The factors include substrate concentration [Citation27], incubation temperature [Citation35], pH [Citation10,Citation21], shaking [Citation31] and activators and inhibitors [Citation18].
This research is the continuation of previous work carried out by the authors [Citation29,Citation30]. In this part, the optimization of environmental and nutritional factors of the Nd-YAG laser irradiated Streptomyces fradiae was performed and aimed to induce maximum cholesterol degrading activity of the irradiated culture.
2 Materials and methods
2.1 Media used for bacterial isolation
The cholesterol-degrading bacteria were isolated using the medium described by [Citation4]. It consists of (g/l): NH4NO3, 1.0; K2HPO4, 0.25; MgSO4.7H2O, 0.25; yeast extract, 5; FeSO4.7H2O, 0.001; cholesterol, 1.0; agar-agar, 15. The medium with agar but without carbon source (cholesterol) was sterilized separately. The recovered colonies were purified by subculturing on the same cholesterol medium and incubated at 30 °C for 5 days. The purified bacterium was subcultured on slants and then kept in the refrigerator.
2.2 Quantitative determination of cholesterol decomposition by S. fradiae
S. fradiae was assayed for their cholesterol degrading activity. One ml bacterial suspension (107–108 bacterial cells/ml) was incubated with 150 ml of the corresponding cholesterol medium described before for 7 days at 30 °C under stationary condition. At the end of the incubation time, the percentage of cholesterol reduction was measured according to the colorimetric method of acetic anhydride/sulphuric acid [Citation34,Citation38] using the following general formula:
2.3 Irradiation of the test bacterium using Nd-YAG laser radiation
2.3.1 Source of radiation
The source of irradiation was located at the National Institute of Laser Enhanced Science (NILES), Cairo University (laser model BL-106C; Spectra-Physics Lasers, Inc., Mountain View, CA). Samples were irradiated by 210 mW, pulse duration 8–9 ns Nd:YAG at a wavelength of 1064 nm.
2.3.2 Photosensitizers
The photosensitizer used in this research was chlorophyllin (Sigma), used at a concentration of 0.5 mg/ml and was incubated with the test bacterium for 5 min before irradiation.
2.3.3 Bacterium treatment
The test bacterium was grown on plates containing the cholesterol medium and incubated at 30 °C for 5 days. Bacterial suspension (107–108 cells/ml) was prepared and incubated with the photosensitizers for 5 min at room temperature before irradiation. A photosensitizer-free sample was used for comparison. The samples were then irradiated with Ne-YAG laser for 8 min. The irradiated bacterial suspension was inoculated into 100 ml of the cholesterol medium [Citation4]. After 7 days of incubation at 30 °C, the bacterial cells were separated by centrifugation at 3000 rpm. The decomposition of cholesterol (mg/100 ml) was determined in the medium as stated before.
2.4 Environmental conditions and nutritional factors influencing the activity of cholesterol-degrading enzymes
Liquid growth media were prepared and supplemented with the following concentrations of cholesterol (g/l); 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5.0, or 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 glucose, in case of optimizing glucose concentration, or 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 yeast extract in case of testing yeast concentration. For nitrogen source, ammonium nitrate at the concentration of 1 g/l was replaced by equimolecular weight concentration of peptone, ammonium nitrate, potassium nitrate, ammonium chloride, sodium nitrate, magnesium nitrate, ammonium acetate, ammonium sulfate, ammonium formate or ammonium carbonate. For optimizing metallic ions, a concentration level of 0.2% of different metallic salts were supplied separately to the mineral salt basal liquid cholesterol medium free from MgSO4. The tested metallic salts were MgSO4 (control), BaCl2, CoCl2, NiCl2, CdSO4, CaCl2, CuSO4, ZnSO4 and HgCl2. For testing the incubation period, the flasks were incubated at 30 °C for two to ten days and the cultures were removed at different periods of incubation. The tested incubated temperatures were 10, 15, 20, 25, 30, 35, 40 and 45, 50, 55 °C. For optimization of pH, five types of buffers were applied at different pH values to cover the pH range of 2–11 according to Gomori [Citation17]. The buffers applied were citrate, citrate phosphate, phosphate, tris, and borate buffers. The test shaking speeds were 0, 40, 80, 120, 150, 200, 250 and 300 rpm.
The pH was adjusted to 7.0–7.2, before sterilization. After sterilization, each flask was inoculated with standardized 1.0 ml bacterial suspension (107–108 bacterial cells/ml) and incubated at 30 °C. By the end of incubation period, cholesterol decomposition was determined. A set of triplicate flasks were used for each particular case.
2.5 Statistical analyses
All data were subjected to statistical analyses of variance (F-test) “one way ANOV” [Citation5].
3 Results
3.1 Optimization of nutritional factors
3.1.1 Cholesterol concentration
For non-irradiated S. fradiae, the results indicate that the amount of decomposed cholesterol increases with elevating cholesterol concentration from 0.1 up to 1.0 mg/100 ml., then decreased with increasing concentration. Nd-YAG laser irradiated bacterium showed similar trend but could keep its maximum activity (73.8 and 72.8% cholesterol decomposition) within a broader range of cholesterol concentrations (1.0–1.5 mg/100 ml, respectively) ().
Fig. 1 Effect of different cholesterol concentrations on the percentage of cholesterol decomposition by non-irradiated and 8 min Nd-YAG laser-irradiated Streptomyces fradiae pre-inocubated with chlorophyllin as a photosensitizer for 5 min. Regression of the line above an X is significant different from that below an X.
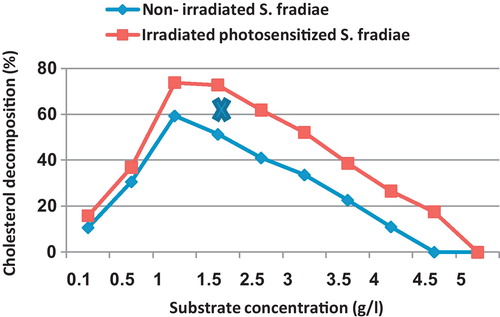
It is noticed that the concentration of 4.5 mg/100 ml induced complete inhibition of growth for non-irradiated culture, while the irradiated one showed slight cholesterol decomposition reaching 17.5%. A cholesterol concentration of 5% prevented the bacterial growth of non-irradiated and radiated bacteria.
3.1.2 Yeast concentration
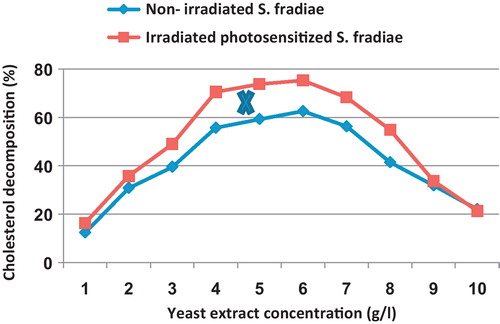
indicates that the percentage cholesterol decomposition of S. fradiae increased gradually with increasing the concentration of yeast extract and reached its maximum value (62.6%) at the concentration of 6 g/l in the case of non-irradiated culture. For irradiated bacterium, the maximum cholesterol decomposition (73.8–75.3%) was occurred at 5–6 g/l of yeast extract. At higher concentrations of yeast extract (9 and 10 g/l), the ability of cholesterol decomposition was sharply declined and became insignificantly varied in the case of non-irradiated and irradiated bacteria.
Fig. 2 Effect of different concentrations of yeast extract on the percentage of cholesterol decomposition by non-irradiated and 8 min Nd-YAG laser-irradiated Streptomyces fradiae pre-inocubated with chlorophyllin as a photosensitizer for 5 min. Regression of the line above an X is significant different from that below an X.
3.1.3 Nitrogen source
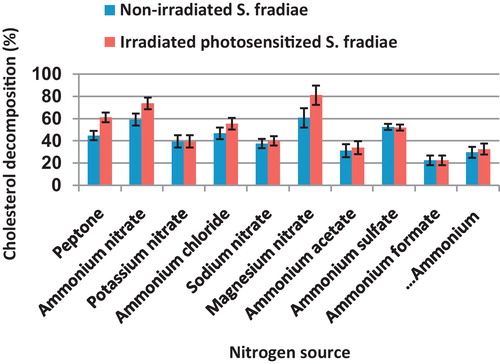
It is evident that the irradiated culture was more active in cholesterol decomposition than non-irradiated one under different sources of nitrogen (). Magnesium nitrate and ammonium nitrate were the most suitable nitrogen sources for cholesterol decomposition in the case of the non-irradiated S. fradiae (60.8 and 39.3%, respectively). For irradiated culture, magnesium nitrate was the best nitrogen inducing cholesterol decomposition (81.2%). This nitrogen source was followed by ammonium nitrate (73.8%), peptone (61.3%) and ammonium chloride (55.5%). There was insignificant difference in cholesterol decomposition between non-irradiated and irradiated S. fradiae grown on potassium nitrate, sodium nitrate, ammonium acetate, ammonium sulfate, ammonium formate and ammonium carbonate.
Fig. 3 Effect of different nitrogen sources, added in equimolecular amount to 0.10% ammonium nitrate, on the percentage of cholesterol decomposition by non-irradiated and 8 min Nd-YAG laser-irradiated Streptomyces fradiae pre-inocubated with chlorophyllin as a photosensitizer for 5 min. Bars represent standard deviation.
3.1.4 Glucose concentration
reveals that addition of glucose concentration up to 10 g/l to the growth medium enhanced the cholesterol degrading activity in the case of non-irradiated and irradiated S. fradiae reaching 65.8 and 85.1% as compared with 59.3 and 73.8%, respectively in the case of glucose-free medium.
Fig. 4 Effect of different glucose concentrations on the percentage of cholesterol decomposition by non-irradiated and 8 min Nd-YAG laser-irradiated Streptomyces fradiae pre-inocubated with chlorophyllin as a photosensitizer for 5 min. Regression of the line above an X is significant different from that below an X.
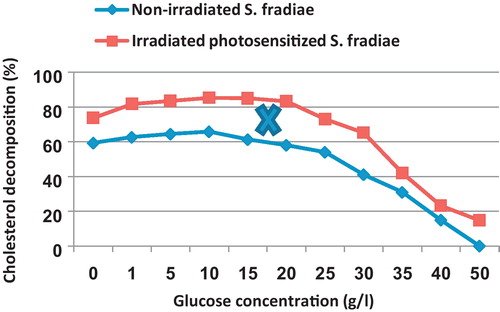
The elevation of glucose concentration from 10 g/l up to 20 g/l caused a slight decrease in cholesterol decomposition for the non-treated culture in contrast to the irradiated one which kept its activity. The decrease in cholesterol degrading activity of non-irradiated culture was more than that of non-irradiated one. The higher glucose concentrations (>25 g/l) was inhibitory for cholesterol decomposition of both non-irradiated and irradiated bacteria. The non-irradiated S. fradiae failed to grow on the growth medium provided with 50 g/l.
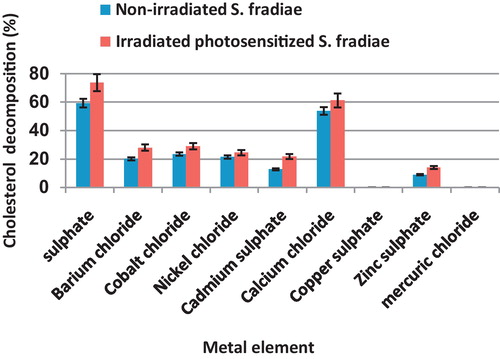
3.1.5 Metallic ions
The most suitable metallic ions for cholesterol degrading activity of non-irradiated and radiated S. fradiae was MgSO4 followed by CaCl2 (59.3 and 73.8%, respectively for MgSO4 and 53.9 and 61.3%, respectively for CaCl2). NiCl2, BaCl2 and CdSO4, and ZnSo4 induced moderate activity for non-treated and treated bacteria. HgCl2 and CuSO4 were toxic for the tested bacterium ().
3.2 Optimization of environmental factors
3.2.1 Incubation period
In this experiment and those ahead, the optimal medium composition for the production of maximum cholesterol activity by S. fradiae was adjusted according to the experiments previously carried out.
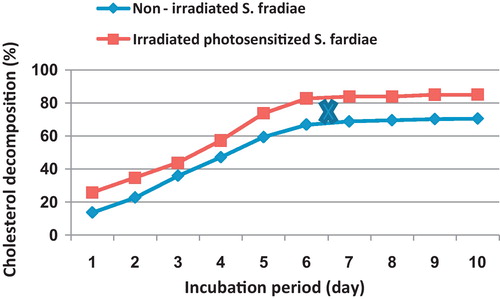
shows that the maximum decomposition of cholesterol was significantly achieved by the end of the sixth and seventh day of incubation, in the case of non-irradiated and irradiated bacteria, respectively. The percentage of cholesterol decomposition amounted 70.5 and 85.1% at the end of incubation period in the case of non-irradiated and irradiated culture, respectively. Generally, the decomposition of cholesterol, at any incubation time, by the irradiated S. fradiae significantly exceeded that recorded by non-irradiated culture.
Fig. 6 Effect of different incubation periods (days) on the percentage of cholesterol decomposition by non-irradiated and 8 min Nd-YAG laser-irradiated Streptomyces fradiae pre-inocubated with chlorophyllin as a photosensitizer for 5 min. Regression of the line above an X is significant different from that below an X.
3.2.2 Incubation temperature
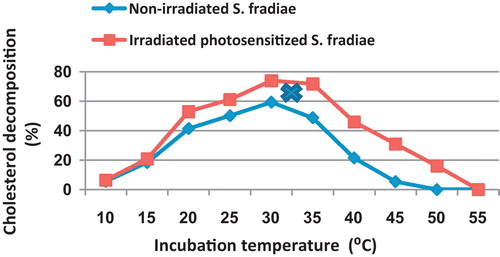
As shown in , the cholesterol decomposition significantly increased by increasing incubation temperature from 10 up to 30 °C, reaching 59.3 and 73.8 at 30 °C, then the cholesterol decomposing activity began to decrease with the elevation of temperature. The non-irradiated S. fradiae failed to grow at 50 °C, while the lethal temperature for the irradiated one was 55 °C.
Fig. 7 Effect of different incubation temperatures on the percentage of cholesterol decomposition by non-irradiated and 8 min Nd-YAG laser-irradiated Streptomyces fradiae pre-inocubated with chlorophyllin as a photosensitizer for 5 min. Regression of the line above an X is significant different from that below an X.
3.2.3 pH
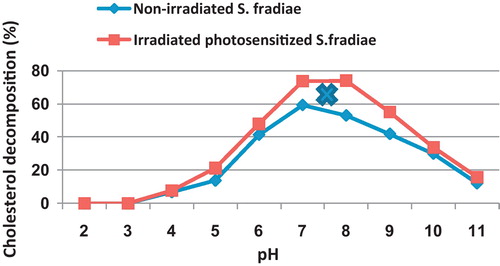
The neutral medium (pH 7.0) was the most favourable in decomposing cholesterol by non-irradiated and irradiated bacteria (). Shifting towards acidic or alkaline sides markedly decreased the activity of non-irradiated and irradiated S. fradiae. The moderate alkaline pHs (8.0 and 9.0) was better than the corresponding acidic ones (6.0 and 5.0). Both non-irradiated and irradiated bacteria failed to grow on the extremely acidic pH (pHs 2 and 3), while at the extremist alkaline medium (pH 11), the non-irradiated and radiated bacteria could decompose small amount of cholesterol recording 12.1 and 15.8%, respectively. Yet, the cholesterol decomposing activity of Nd-YAG irradiated S. fradiae was mostly better than the corresponding activity of the non-irradiated one with broader optimum pH peak of activity (pHs 7–8).
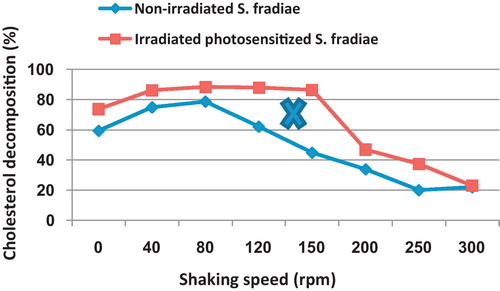
3.2.4 Shaking speed
shows that shaking culture up to 80 rpm induced significant increase in cholesterol decomposition reaching 78.7 and 88.5% at 80 rpm in the case of non-irradiated and radiated species. The maximum peak of the cholesterol decomposing activity of the radiated S. fradiae (86.2–88.6%) was recorded in a range of shaking speed from 40 to 150 rpm. Increasing shaking speed after 150 rpm induced steady decrease in cholesterol degrading activity of irradiated S. fradiae attaining 46.9 and 22.9% at 200 and 300 rpm, respectively. However, the maximum degrading activity of cholesterol by non-irradiated S. fradiae was obtained at 80 rpm reaching 77.5% as compared with 73.8% for the stand culture. There was a gradual decrease in cholesterol degrading activity of the non-irradiated S. fradiae with the increase of shaking speed over 80 rpm.
Fig. 9 Effect of different shaking speeds on the percentage of cholesterol decomposition by non-irradiated and 8 min Nd-YAG laser-irradiated Streptomyces fradiae pre-inocubated with chlorophyllin as a photosensitizer for 5 min. Regression of the line above an X is significant different from that below an X.
4 Discussion
For optimization of cholesterol-degrading activity of the non-irradiated and Nd-YAG laser irradiated S. fradiae, a series of experiments was carried out. Concerning the effect of substrate concentration, the maximum cholesterol degrading activity was evident at cholesterol concentration of 1.0 and 1–1.5 g/l for non-irradiated and irradiated bacterium, respectively. However, the potentiality of degrading cholesterol was higher in the case of irradiated S. fradiae (73.8%) as compared to the non-irradiated one (59.3%). The cholesterol degrading activity by non-irradiated and irradiated bacterium significantly decrease on increasing the concentration of cholesterol up to 4.0 g/l and the test bacterial species failed to grow on media containing 4.5 and 5.0 g/l in the case of non-irradiated and irradiated S. fradiae, respectively. So, it appears that the low concentration of cholesterol (1.5–2.5 g/l) is more suitable than the higher concentration (>3.0 g/l). These results are matching with those reported by Imshenetskii et al. [Citation19] who found that the higher concentrations of cholesterol were decomposed more slowly than low concentration by cholesterol oxidase from Steptomyces lavendulae.
It has been reported that the optimal concentration of cholesterol for the most efficient enzyme production varies with the strain. Arthrobacter simplex [Citation25] Bacillus subtilis SFF34 [Citation23] and R. equi [Citation44] could produce the maximal level of cholesterol oxidase in the presence of 0.3, 0.05, 0.2 and 0.1% cholesterol, respectively. The maximal levels of cholesterol oxidase production have been reported to be 1.50 U ml−1 by A. simplex [Citation26], 3.14 U ml−1 by Bacillus subtilis SFF34 [Citation23] and 0.29 U ml−1 by Rhodococcus sp. [Citation31].
The present results indicate that the cholesterol degrading activity reached the maximum values at 5–6 and 4–6 g/l yeast extract in the case of non-irradiated and irradiated S. fradiae, respectively. This indicates the ability of irradiated bacteria to exhibit cholesterol degrading activity under wider range of yeast extract concentration. Moreover, the cholesterolytic activity was significantly higher in the case of irradiated S. fradiae. This may be due to the more stimulatory effect of yeast extract on the synthesis of cholesterol-decomposing enzyme(s) produced by the irradiated bacterium. Aihara et al. [Citation2] used a medium with 0.5% yeast extract for maximum production of cholesterol oxidase enzyme by three Rhodococcus strains. Voets and Lamot [Citation41] in their study on microbial degradation of cholesterol, reported that addition of small amounts of yeast extract stimulated growth and cholesterol decomposing activity, but addition of higher concentrations of yeast extract decreased cholesterol breakdown by the experimental microorganisms.
The present work also revealed that the irradiated S. fradiae was mostly more active in cholesterol decomposition than non-irradiated one under different sources of nitrogen. Magnesium nitrate and ammonium nitrate was the most suitable nitrogen sources for the non-irradiated and radiated S. fradiae. Although the suitability of magnesium nitrate and ammonium nitrate as nitrogen sources for the production of cholesterol oxidase enzyme by bacteria was proved formerly by Kim et al. [Citation23], however, Zhukova and Kozlova [Citation48] reported earlier that 0.3% asparagines and 0.05–0.07% glutamine were the best nitrogen sources for growth and cholesterol decomposition by Flavobacterium strain and Actinomyces streptomycini, respectively.
It has been reported that the supplementation of glucose to the growth medium enhances the cholesterolytic activity of the bacteria [Citation23]. The results revealed that addition of glucose concentration up to 10 and 10–20 g/l to the growth medium enhanced the cholesterol degrading activity in the case of non-irradiated and irradiated bacteria reaching 65.8% and 85.1% as compared with 59.3 and 73.8% in the case of glucose-free medium for non-irradiated and irradiated bacteria, respectively. The elevation of glucose concentration to more than 25 g/l was inhibitory for cholesterol decomposition of both non-irradiated and irradiated bacterial species. The cholesterol-degrading activity of irradiated S. fradiae showed wider maximal peak over a wider range of glucose concentration as compared with the non-irradiated bacterium. The stimulation of the cholesterol degrading activity of irradiated S. fradiae indicates that cholesterol oxidase production by irradiated bacteria may be regulated by carbon catabolic repression.
The most suitable metallic ions for cholesterol degrading activity of non-irradiated and radiated S. fradiae was MgSO4 followed by CaCl2 (59.3 and 73.8%, respectively for MgSO4 and 53.9 and 61.3%, respectively for CaCl2). NiCl2, BaCl2 and CdSO4 induced low activity for non-treated and treated bacteria. HgCl2 and CuSO4 were toxic for the tested bacterium. Sabry [Citation36] found that MgSO4 followed by ZnSO4 were the most stimulatory ions for enzymes recovered from Pseudonocardia compacta. He stated that the maximum activity was attained at 100 μg MgSO4/ml, while relative maximum activity was induced by ZnSO4 at 50 μg/ml. Ammar et al. [Citation3] reported that Mg++ is the best inducer for exo-enzyme at 250 μg/ml and for endo-enzyme at 100 μg/ml, while Cd++, Co++, Ba++ and Ca++ were considered as enzyme inhibitors.
The incubation period is an important factor that must be considered. Since the production of cholesterol-decomposing enzyme is related to internal biosynthetic processes inside the organism, theses processes are quite complicated, so certain enzymes could be subjected to alteration with the incubation period. In this research, the maximum decomposition of cholesterol by non-irradiated and Nd-YAG irradiated bacteria was detected at the end of the sixth days. The percentage of cholesterol decomposition, on the sixth day, amounted 82.8 in the case irradiated S. fradiae as compared to 66.9% in the case of non-irradiated one. Generally, the decomposition of cholesterol, at any incubation time, by the irradiated S. fradiae significantly exceeded that recorded by non-irradiated bacterium.
Several investigators indicated different incubation periods for different microorganisms. Abo-El-Khair [Citation1] reported that the cholesterol decomposing activity of Mycobacterium fortuitum increased rapidly during the first three days of incubation followed by insignificant variations with extension of incubation up to 10 days. It can be mentioned here that the process of cholesterol decomposition by microorganisms occurs during 6 days for Streptomyces spp. [Citation32] and 43 hr. for the most active strains of Actinomycetes [Citation47]. Watanabe et al. [Citation43] reported that most Rhodococcus strains complete the cholesterol degradation during 3–7 days incubation period.
The present results indicated that the maximum cholesterol decomposition by irradiated bacterium was achieved at wider range of temperature (30–35 °C) reaching 73.8–71.7% as compared to 59.3% in the case of non-irradiated bacterium incubated at 30 °C. The non-irradiated S. fradiae was more susceptible to higher temperature and failed to grow at 50 °C as compared to 55 °C, in the case of irradiated bacteria. It is also obvious that, at any incubation temperature, the cholesterol decomposing activity of irradiated S. fradiae exceeds the corresponding activity of the non-irradiated one.
The optimum incubation temperature for cholesterol decomposition varies in different microorganisms. Johnson and Somkuti [Citation20] found that egg yolk cholesterol degradation by R. equi 33706 had an optimum temperature of 40 °C. Chang et al. [Citation10] found that degradation of pure cholesterol or egg cholesterol by R. equi 12859, isolated from butter, was best at 45 °C.
The maximum cholesterol decomposing activity by irradiated S. fradiae was attained at pH 7–8 as compared to pH 7 for non-irradiated bacterium. The shifting of pH away from the optimum steadily decrease the cholesterol decomposing activity of non-irradiated and irradiated S. fradiae. It is noticed that, at any pH, the cholesterol decomposing activity of Nd-YAG irradiated S. fradiae was mostly better than the corresponding activity of the non-irradiated one. Buckland et al. [Citation8] found that pH 6.7 ± 0.2 is the optimum pH for the same enzyme produced by Nocardia sp. Chang et al. [Citation10] found that degradation of pure cholesterol or egg cholesterol by R. equi 12859 was best at pH 7.5.
The reached results in this research indicated that shaking culture from 40 to 150 rpm kept the maximum peak of the cholesterol decomposing activity of the irradiated S. fradiae as compared to 40–120 rpm for the non-irradiated bacterium. However, the maximum cholesterol decomposing activity of the non-irradiated S. fradiae was achieved at 80 rpm. Increasing shaking speed, after 80 and 150 rpm, induced steady decrease in cholesterol degrading activity of non-irradiated and irradiated S. fradiae, respectively. The increase in production of cholesterol decomposing enzyme on shaking culture as compared with stationary one, might be due to the increase in aeration rate and consequently increase in the dissolved oxygen. Based on this assumption, it is concluded that the irradiated S. fradiae could induce maximum cholesterol decomposing activity in presence of a limited oxygen concentration as compared to the non-irradiated one. In this connection, Kapat et al. [Citation22] determined the dissolved oxygen concentration and the volumetric oxygen transfer coefficient at corresponding combinations of impeller speed and aeration rate. They found that the maximal specific extracellular glucose oxidase production (3.17 U mg−1 dry cell mass) was achieved when the initial dissolved oxygen concentration was 6.83 mg−1 at the impeller speed of 420 rev min−1 and the rate of aeration of 0.25 vvm.
Acknowledgement
Authors are very grateful to the deanship of Graduate Studies and Research, Taibah University, Saudi Arabia for funding and supporting this research project (project no. 258/429).
Notes
Peer review under responsibility of Taibah University.
References
- I.A. Abo-El-Khair, Studies on the metabolism of cholesterol by some microorganisms, Ph.D. Thesis, Fac. Sci., Zagazig Univ., Egypt, 1989.
- H.AiharaK.WatanabeR.NakamuraCharacterization of production of cholesterol oxidase in three Rhodococcus strainsJournal of Applied Bacteriology611986269
- M.S.AmmarI.F.LashinH.E.AbdullahCholesterol decomposing microorganisms isolated from some human skin microfloraEgyptian Journal of Microbiology2519909
- K.ArimaM.NagasawaM.Bac.G.TamuraMicrobial transformation of steroids. Part I: Decomposition of cholesterol by microorganismsAgricultural and Biological Chemistry33196916361643
- P.ArmitageStatistical Methods in Medical Research1971Blackwell Scientific PublicationLondon
- M.BoultonJ.MarchallLasers in the Life Sciences11986125134
- A.BrzostekB.DziadekA.Rumijowska-GalewiczJ.Pawel-czykJ.DziadekCholesterol oxidase is required for virulence of Mycobacterium tuberculosisFEMS Microbiology Letters2752007106112
- B.C.BucklandM.D.LillyP.DunnillThe kinetics of cholesterol oxidase synthesis by Nocardia rhodocrousBiotechnology and Bioengineering181976601
- M.E.CardonaE.NorinT.MidtvedtBiochemical functions of Bacillus licheniformis in gnotobiotic miceMicrobial Ecology in Health and Disease1520034042
- H.S.ChangJ.I.WangP.P.ChangStudies on the isolation and identification of Rhodococcus equi NCHUl and it’s egg yolk cholesterol degradationTaiwanese Journal of Agricultural Chemistry and Food Science382000555564
- Y.W.ChungC.Y.RochY.H.ShunDecomposing cholesterol in egg yolk by enzymes of Rhodococcus equi. No. 23Food Science, Taiwan2219956976
- O.DrzyzgaL.Fernández de las HerasV.MoralesJ.M.Navarro LlorensJ.PereraCholesterol degradation by Gordonia cholesterolivoransApplied and Environmental Microbiology77201148024810
- L.Fernández de las HerasE.García FernándezJ.M.Navarro LlorensJ.PereraO.DrzyzgaMorphological, physiological, and molecular characterization of a newly isolated steroid-degrading actinomycete, identified as Rhodococcus ruber strain Chol-4Current Microbiology592009548553
- N.GeweelyS.A.OufEnhancement of fungal degradation of starch based plastic polymer by laser-induced plasmaAfrican Journal of Microbiology Research520201132733281
- N.S.GeweelyS.A.OufM.A.EldesokyA.A.EladlyStimulation of alkalothermophilic Aspergillus terreus xylanase by low-intensity laser radiationArchives of Microbiology186200619
- H.M.GhalebN.M.HanafyA.A.El-GhandourSome trials to produce yoghurt of low cholesterol content. 2: Bacterial cholesterol assimilationProceedings of the 7th Egyptian Conference for Dairy Science and TechnologyCairo, Egypt, 7–9 November1998251260
- G.GomoriPreparation of buffer for use in enzyme studiesS.P.ColwickN.O.KaplanMethods in Enzymolvol. I1955Academic Press. Inc. PublishersNew York
- A.HosonoT.Tono-OkaBinding of cholesterol with lactic acid bacterial cellsMilchwissenschaft: Milk Science International501995556560
- A.A.ImshenetskiiT.S.NazarovaL.E.NikitinDecomposition of cholesterol by enzyme preparation from Streptomyces lavendulaeMicrobiologia451976298301
- T.L.JohnsonG.A.SomkutiCholesterol removal from egg yolk and milk cream by sonicated extracts of Rhodococcus equiJournal of Dairy Science721989118
- H.K.JongK.O.MinH.R.YongC.C.KiK.L.YongY.S.SeungSelection and physico-chemical characteristics of lactic acid bacteria which had cholesterol lowering activitiesHanguk Nongwhahak Hoechi4219998390
- A.KapatJ.K.JungY.H.ParkEnhancement of glucose oxidase production in batch cultivation of recombinant Saccharomyces cervisiae: optimization of oxygen transfer conditionJournal of Applied Microbiology902001216222
- K.P.KimC.H.RheeH.D.ParkDegradation of cholesterol by Bacillus subtilis SFF34 isolated from Korean traditional fermented flatfishLetters in Applied Microbiology352002468472
- M.T.LiongN.P.ShahOptimization of cholesterol removal by probiotics in the presence of prebiotics by using a response surface methodApplied and Environmental Microbiology71200517451753
- W.LiuJ.HsuW.WangProduction of cholesterol oxidase by antibiotic resistant mutant and a constitutive mutant Arthrobacter simplex B-7Proc. Natl. Sci. Council, vol. 7Republic of China1983225260
- N.LiuT.ZhangY.J.WangJ.H.HuangP.OuA.ShenA heat inducible tyrosinase with distinct properties from Bacillus thuringiensisLetters in Applied Microbiology32004407412
- S.MichaelZ.JamesP.DonaldG.DenyseGrowth and cholesterol oxidation by Mycobacterium species in tween 80 mediumApplied and Environmental Microbiology59199314251429
- W.W.MohnR.van der GeizeG.R.StewartS.OkamotoJ.LiuL.Dijkhuizenet alThe actinobacterial mce4 locus encodes a steroid transporterJournal of Biological Chemistry28320083536835374
- S.A.OufA.Q.AlsarraniA.A.Al-AdlyM.K.IbrahimEvaluation of low-intensity laser radiation on stimulating the cholesterol degrading activity. Part I: Microorganisms isolated from cholesterol-rich materialsSaudi Journal of Biological Sciences192012185193
- S.A.OufA.Q.AlsarraniA.A.Al-AdlyM.K.IbrahimM.H.IsmailEvaluation of low-intensity laser radiation on stimulating cholesterol degrading activity of Streptomyces fradiae. Part III: Cholesterol oxidase purification and applicationAfrican Journal of Microbiology Research67201215091519
- T.H.ParkH.U.KimStudies on cholesterol-degrading microorganisms in animal food productsKorean Journal of Animal Science361994682692
- G.E.PetersonJ.R.DavisCholesterol utilization by Streptomyces spp.Journal of Steroid41964677688
- A.I.RaafatE.ArabyS.LotfyEnhancement of fibrinolytic enzyme production from Bacillus subtilis via immobilization process onto radiation synthesized starch/dimethylaminoethyl methacrylate hydrogelCarbohydrate Polymers87201213691374
- C.R.RatilffF.HallLaboratory Manual of Clinical Bio-chemistry1973Scott and White Memorial Hospital Publications OfficeTemple, TX
- C.H.RheeK.P.KimH.D.ParkTwo novel extracellular oxidases of Bacillus sp. isolated from fermented flatfishBiotechnology Letters24200213851389
- A. Sabry, Decomposition of cholesterol by some organisms isolated from certain Egyptian soils, M.Sc. Thesis, Botany and Microbiology Dept., Fac. Sci., Al-Azhar Univ., Cairo, Egypt, 1994.
- Y.TamaiN.YoshimitsuY.WatanabeY.KuwabaraS.NagaiEffects of milk fermented by culturing with various lactic acid bacteria and a yeast on serum cholesterol level in ratsJournal of Fermentation and Bioengineering811996181182
- N.W.TietzFundamentals of Clinical Chemistry1987W.B. Saunders CompanyPhiladelphia p. 563
- H.VarleyPractical Clinical Biochemistry4th ed.1969Gulab Vazirani for Arnold Heinemann PublishersIndia p. 316
- T.H.VesaCan lactic acid bacteria reduce blood serum cholesterol? Twelfth Forum for applied Biotechnology, Provincial Court, Brugge, Belgium, 24–25 September, 1998. Part II: Mededelingen-Faculteit Landbouwkundige en Toegepaste-Biologishe WetenschappenUniversiteit Gent634b199815051510
- J.P.VoetsE.LamotMicrobial degradating of cholesterolZeitschrift fur Allgemeine Mikrobiologie: Morphologie, Physiologie und Okologie der Mikroorganismen14197477
- C.T.WangX.Q.BaiL.Q.ZhuT.Y.LiuInfluence of 60Co-gamma ray irradiation on the growth of Armillaria mellea mycelia and on enzyme activity in themJournal of Sourthwest Agricultural University2019981923
- K.WatanabeH.ShimizuH.AiharaR.NakamuraK.SuzukiK.KomagataIsolation and identification of cholesterol degrading Rhodococcus strains from food of animal origin and their cholesterol oxidase activitiesJournal of General and Applied Microbiology3219861317
- K.WatanabeH.AiharaY.NakagawaR.NakamuraT.SasakiProperties of the purified extracellular cholesterol oxidase from Rhodococus equiJournal of Agricultural and Food Chemistry37198911781182
- X.YangN.M.NesbittE.DubnauI.SmithN.S.SampsonCholesterol metabolism increases the metabolic pool of propionate in Mycobacterium tuberculosisBio-chemistry48200938193821
- M.T.YazdiZ.T.YazdiA.GhasemianG.ZarriniN.H.OlyaeeZ.SepehrizadehPurification and characterization of extra-cellular cholesterol oxidase from Rhodococcus sp. PTCC1 633Biotechnology72008751756
- V.A.ZaninDecomposition of cholesterol by actinomycetesMikrobiologyia3719681024
- A.I.ZhukovaV.Kh.KozlovaCholesterol oxidation capacity of Actinomyces streptomycini Strain 2105Mikrobiologyia371968223227