Abstract
Growth factors (GFs) are naturally occurring proteins or steroid hormones which act as signaling molecules between cells that play a key role in the processes of proliferation, cell differentiation and maturation of a wide variety of cells and tissues. A recently purified synthetic basic b-FGF was assessed using a routine tissue culture assay via application of a wide range of doses ranged between 0.1 and 300 ng/mL of the basic fibroblast growth factor (b-FGF) in phosphate buffer saline (PBS) and 10% fetal calf serum (FCS) on the growth rate of Rama-27 mammary cell line. Applying SPSS “Student T-test” biostatistics the result showed significant increase (p ≤ 0.05), almost 7 folds in tissue proliferation at a low dose of 0.3 ng/mL FGF in comparison with control tissue (PBS) only. It is concluded that 0.3 ng/mL dose represents the lower optimal dose suggesting its possibility of an in vivo technique to test its potency in curing skin wounds in rats.
1 Introduction
All living cells are characterized by their ability to replicate or go through cell-division, during which the cell duplicate its DNA and the mitosis phase follows by the cell splitting itself into two distinct cells, often called “daughter cells” [Citation8,Citation9,Citation37]. Cell division can be detected by exposing the cells to radioactively labeled nucleotide precursors which helps to observe and measure these “landmark” events providing the basis for defining the kinetic and regulatory properties of the cell cycle. A longitudinal observation of a population of cells grown outside the normal habitat of the body e.g. in culture, can most easily be studied within the laboratories under relatively controlled conditions [Citation13]. Cells depending upon the environmental stimuli established within laboratories using tissue culture techniques can retain their ability to divide further in a process called “quiescence” which is a reversible phenomenon [Citation2,Citation6,Citation26,Citation30]. It also can reveal basic information about cells and to investigate fundamental processes of growth and development in both normal and abnormal tissues.
Continuous works in tissue culture field have approved that a mixture of nutrient to ‘add back’ ingredient until an optimal mixture for cell proliferation is obtained is a step to environment of cells within the body. This approach converges on the identification of a core set of molecules required for the survival and most importantly proliferation of mammalian cells in culture, known as growth factors [Citation3,Citation4]. The mammary cell line of rats (Rama-27) isolated and grown under excellent critical environmental conditions was used to assess the potential of the basic fibroblast growth factor (b-FGF) and to optimize the right dose of it as in vitro for future use as in vivo for wound healing.
Growth factors (GFs) are proteins or steroid hormones [Citation39] which are important for regulating a variety of cellular processes and they typically act as signaling molecules between cells. In normal tissue, b-FGF is present in basement membranes and in the subendothelial extracellular matrix of blood vessels. It stays membrane-bound as long as there is no signal peptide [Citation16,Citation25]. A single GF could be used in assays to assess its potency in promoting growth of certain cell line as in vitro. Application of synthesized GFs is one of the ways being utilized as a way to maintain skin's youth. They increase the rate at which cells in the body grow, play roles in cell division, new cell and blood vessel growth, collagen and elastin production and distribution [Citation34]. GFs exert their effect on cells through cell-surface receptors, and may bind to one or several receptors. In normal tissue, the b-FGF is present in basement membranes and in the sub-endothelial extracellular matrix of blood vessels while it stays membrane-bound as long as there is no signal peptide.
The FGFs are a family of GFs involved in angiogenesis, wound healing, and embryonic development. They are heparin-binding proteins and interactions with cell-surface associated heparan sulfate proteoglycans have been shown to be essential for FGF signal transduction and as key players in the processes of proliferation and differentiation of wide variety of cells and tissues. A novel chemical-defined medium with b-FGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells [Citation20]; and interacts with free ribosomal protein S19 [Citation31] while intracellular association of b-FGF is found with the ribosomal protein L6/TAXREB107 [Citation28]. It can bind to protein kinase CK2 correlates with mitogenicity [Citation29].
Synthetic GFs are polypeptide that can mimic the role of the natural GFs in vitro experiments. They are a specific subgroup of cytokines whose main activity is the induction of mitosis. They are secreted by a wide range of cells including macrophages, fibroblasts, endothelial cells, and platelets [Citation22,Citation27] and may bind to one or several receptors. They contribute to blood vessel growth, collagen and elastin production and distribution [Citation4,Citation22–Citation27 Citation28 Citation29 ,Citation36]. GFs also influence and regulate the degradation and formation of collagen [Citation7,Citation10,Citation11,Citation14,Citation17,Citation18,Citation20,Citation21,Citation24,Citation26,Citation32,Citation38] also exert a mitogenic effect on mesodermal cells, manifested as stimulated angiogenesis and granulation tissue formation [Citation35].
The aim of the present work was designed to optimize the dose of b-FGF that produces the highest cell growth in vitro by using mammary gland cells Rama-27 cell line. It has been assumed that its role in interacting with certain cell membrane receptors to provide nutrients to the germinating cells; act as catalyst to accelerate the cell growth.
The b-FGF has been synthesized, its chemical structures and purity being determined at Biochemistry Department, Liverpool University, UK by professor David Fernig who reserves the right for disclosing its chemical components. Rama-27 from passages 31st of a rat mammary cell line used in the present study has been derived from the fast sticking fraction of cells. They have been isolated from a normal rat mammary gland and defined as fibroblast on the basis of its ability to differentiate to the adipocyte phenotype [Citation26].
2 Materials and methods
2.1 Dose preparations
An amount of basic stock of 20 μL b-FGF (0.33 mg/mL b-FGF dissolved in 1000 μL phosphate buffer saline [PBS], 2 M NaCl, 20 mM phosphate buffer [PB], 1 mM DTT, pH 6.8) was provided as a stock solution to prepare working doses. Four sub-stocks (i.e. 5 ng/mL; 100 ng/mL; 1000 ng/mL and 10,000 ng/mL) were prepared from the basic stock. The final doses (0.01; 0.03; 0.1; 0.3; 1.0; 3.0; 10.0; 100.0 and 300.0 ng/mL) were then diluted from the above sub-stocks. The equation of (N1 × V1 = N2 × V2) has applied for dilution and preparation of the doses. All doses were prepared of 500 μL as working volume.
2.2 The Rama-27 cell line
The Rama-27 cell line has been cultured in Dulbecco's Modification of Eagle Medium (DMEM) [Gibco] supplemented with 5% fetal calf serum (FCS), 50 ng/mL hydrocortisone [Sigma]. These cell lines were collected from the Tissue bank of the department kept at −140 °C for long-term storage. Rama-27 cells were kept sparse during the culture because high-density cells do not synchronize readily. The pH was always kept checked because they could easily change when adding GFs and [3H]-thymidine as DNA synthesis is somewhat dependent on bicarbonate. Eight plates were used a time to pool an appropriate amount of confluent cells to use in these plates. Each sample was tested in triplicate and have included a negative and a positive control (SDM and 5% FCS respectively) on each plate. Equal volume of 50 μL was used in all experiments. Medium is aspirated from the cells, which were then washed twice with PBS. 1 mL trypsin–EDTA solution was added and incubated for 5–6 min.
2.3 Procedure details of the experiments
In day 1, two plastic Petri dish plates of 80% confluent cells were trypsinized, cells were suspend in 10 mL RM, counted using Coulter counter and the suspension was diluted with RM to give between 30,000 and 40,000 cells per mL. 500 μL of cell suspension was pipetted into each well of the assay plate (to make between 15,000 and 20,000 cells/well) followed by incubation for 24 h at 37 °C in a moist oven in 5% CO2. In day 2, the wells were washed twice with 500 μL of PBS, replaced with 500 μL SDM and incubated for 24 h at 37 °C in a moist oven in 5% CO2. In day 3, 50 μL of cell suspension was added to the wells and incubated for 18 h at 37 °C in a moist oven in 5% CO2.
In day 4, 20 μL of 40 μCi/mL [3H]-thymidine was added to each well and incubated for 1 h at 37 °C inside oven followed by washing twice with PBS. An amount of 500 μL of ice-cold TCA was added and left (15–30 min) in cold room. Replaced with another 500 μL TCA and removed immediately then washed twice with 500 μL of ice-cold ethanol. The plates were left to dry on top of hot oven for 15 min. 500 μL of 0.2 M NaOH was added to each well and incubated for 1 h at 37 °C in a moist oven in 5% CO2. Only 300 μL of cell suspension was added to 1 mL of Optiphase scintillation fluid in a scintillation vial and counted for 10 min in scintillation counter. Always both positive and negative controls were used in all experiments and growth activity was recorded as the mean count/minute (CPM) with standard deviation (±sd). The results were expressed as a fold activity of the negative control or as a percentage of the positive control.
3 Results
3.1 Growth activity records
Results in and refer to three highest records of growth density of Rama-27 cell line as mean cell per count (CPM) recorded at 3 different doses of b-FGF (0.3, 0.1 and 3.0 ng/mL). Significant differences (p ≤ 0.05) in the growth at different doses were detected in comparison with both negative (step-down media SDM/bovine serum albumin) and positive control (10% fetal calf serum {FCS}). The rate of growth indicates a regular ascending pattern with the increased dose in vitro up to 0.3 ng/mL. The highest record of Rama-27 cell line growth activity was at 0.3 ng/mL that represents almost 7 folds of the negative control and around 3–4 folds of the positive control. The growth rate, however, revealed a descending pattern beyond 1.0 ng/mL indicating the threshold ranges of the b-FGF doses.
Table 1 The arithmetic mean cell per minute (CPM) and standard deviation (±sd) of Rama-27 cell line counted at different doses of b-FGF (0.01–300 ng/mL). Note the maximum growth is achieved at 3 different doses ranged between 0.3 and 3.0 ng/mL. (NS) Insignificant (p > 0.05); significant (p ≤ 0.05); triplicate readings were done for six experiments (total 18 readings). Biostatistics analysis was done using SPSS software.
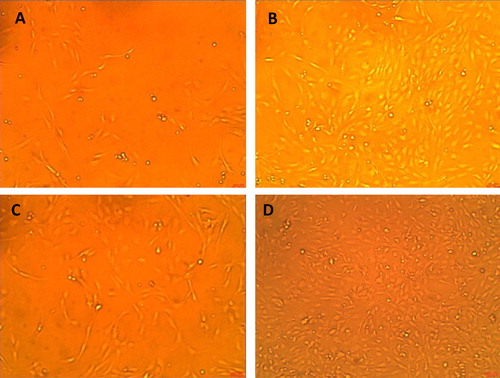
Fig. 1 Effects of growth promoting activity of b-FGF is notable on proliferation of Rama-27 cell line by over confluent treated with b-FGF in comparison with positive control (10% FCS) at both 9 and 18 h. (A) 10% confluent after 9 h with 10% FCS only; (B) 90% confluent after 9 h treatment with 0.3 ng/mL FGF; (C) 50% confluent after 18 h with 10% FCS only and (D) 180% confluent after 18 h treatment with 0.3 ng/mL FGF (100×).
4 Discussion
Many natural GFs e.g. insulin-like growth factor (IGF) and epidermal growth factor (EGF) do exist in various mammalian fresh milk i.e. human, mouse, rat and swine which all have growth promoting activity regarding growth of cells both in vitro and in vivo [Citation13,Citation19]. Any milk, once boiled, the growth factors will be disabled due to the de-naturalization of amino acid components of the GFs. Bovine milk contains a major growth-promoting activity for rat mammary fibroblast cell line (Rama-27) which is structurally different than other GFs [Citation3,Citation4,Citation13]. Due to the fear of some unexpected germs i.e. virus being carried on and transmitted to human being via naturally produced products which have growth promoting activity, attention has been given to produce synthetic peptides on industrial scales to mimic the action of these growth hormones [Citation3,Citation6]. The synthetic GFs alone had a little or no significant effects on growth of Rama-27 cell line unless with Natural Standard Product (NSP) [Citation3]. No additives of the NSP were added to the b-FGF in these experiments as the target was to assess solely its activity. It therefore is likely that the mixture of the present synthesized b-FGF with NSP could produce better outcome; however, this was out of scope of this study. It is therefore recommended to carry out further experiments to check the activity of the current b-FGF together with the NSP in future. The results showed significantly higher activity of b-FGF on its own than our previous works [Citation3,Citation4]. They may therefore indicate a relative improvement into the synthesis, preparation procedure and purity of GFs in comparison with the previous works.
The mechanism of action of this b-FGF molecules has not been the scope of this study to investigate but it is likely that it exerted its effect on these cells through the cell-surface receptors via binding to one or more receptors to induce cell proliferation [Citation1,Citation5,Citation12,Citation15,Citation28,Citation31,Citation33]. Further investigation using molecular biology techniques is recommended which is out of scope of the present in vitro study.
Naturally, every cell does contain a trace of GF while with the addition of 0.3 ng/mL of b-FGF it triggered a rapid proliferation in a shorter time. The dose 0.3 ng/mL had produced the highest cell proliferation among other doses. It is not wrong to assume that 0.3 ng/mL dose is an optimal dose which provided enough nutrients needed to the cells and acted as catalyst to speed up cell division. In other words, it is not wrong to conclude that such a low dose might solely be specific to b-FGF whereas other GF might vary in their optimal doses. That would also depend upon other factors i.e. the structure and components of the GF itself, mechanism of action, temperature, pHs, etc. [Citation23]. The b-FGF has proved its compatibility to work on Rama-27 cells to accelerate their division in vitro. The 0.3 ng/mL b-FGF has provided the least workable dose to produce the highest number of cell and indicated as a threshold dose. Therefore it represents the optimal dose for rat tissues.
Our next intension would be to assess the potency of the above optimal dose of the synthetic b-FGF in curing skin healing wounds by topical application on injured skin of rats.
5 Conclusion
The new synthesized b-FGF used as in vitro in this work, without NSP, has proved to be potential in activating the cell division in Rama-27 cell cultures at a dose (0.3 ng/mL in PB). The latter might represent an ideal dose candidate in pharmaceutical uses for medical purposes i.e. “in vivo” applications with other medications at surgical operations to speed up the process of skin healing and perhaps as a juvenile hormone (skin care) as an anti-wrinkle agent.
Acknowledgements
The authors do acknowledge Professor David Fernig for his generous gift of the synthesized b-FGF being used in this project. FOB does thank Drs. Thamir Ismael and Nicola Wells for their technical assistance regarding preparation of the doses.
Notes
Peer review under responsibility of Taibah University.
References
- J.AndraeR.GalliniC.BetscholtzRole of platelet-derived growth factors in physiology and medicineGenes & Development22200812761312
- R.BarracloughK.DawsonP.S.RudlandElongated Cells Derived from Rat Mammary Cuboidal Epithelial Cell Lines Resemble Cultured Mesenchymal Cells in Their Pattern of Protein Synthesis1984 Also available at:http://www.sciencedirect.com/science
- A.A. Bazzaz, Assessment of growth promoting activity of different synthetic peptides using Rama-27 Cell-Line, M.Sc. Thesis, University of Liverpool, UK, 2002.
- A.A. Bazzaz, N.A. Chelebi, J.A. Smith, S.A. Bazzaz, Assessment of growth potency of six synthetic peptide using Rama-27 cell line, Journal of Environmental Sciences and Engineering, submitted.
- H.-D.BeerM.BittnerG.NiklausC.MundingN.MaxA.GoppeltS.WernerThe fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repairOncogene24200552695277
- B.BoillyA.S.Vercoutter-EdouartH.HondermarckV.NurcombeX.Le- BourhisFGF signals for cell proliferation and migration through different pathwaysCytokine & Growth Factor Reviews112000295302
- A.C.ConnerD.L.HayS.G.HowittK.KilkU.LangelM.WheatleyD.M.SmithD.R.PoynerInteraction of calcitonin-gene-related peptide with its receptorsBiochemical Society Transactions3042002451455
- G.M.CooperChapter 14: The eukaryotic cell cycleThe Cell: A Molecular Approach2nd ed.2000ASM PressWashington, DC
- C.P.De SouzaS.A.OsmaniMitosis, not just open or closedEukaryotic Cell69200715211527
- M.DouglasA.L.MillerNutritional support for wound healingAlternative Medicine Review842003359
- L.Ferrero-MilianiO.H.NielsenP.S.AndersenS.E.GirardinChronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generationClinical and Experimental Immunology147February (2)2007227235
- L.GoretzkiM.A.BurgK.A.GrakoW.B.StallcupHigh-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycanThe Journal of Biological Chemistry2742419991683116837 Also available at: http://www.genecards.org/cgi-bin/carddisp.pl?gene=FGF2
- J.K.HeathGrowth FactorsChapter in “Focus Growth factors”, Oxford, IRL Press, UK1993
- A.KohlM.G.GrutterFire and death: the pyrin domain joins the death-domain superfamilyComptes Rendus Biologies327200410771086
- E.V.KooninL.AravindThe NACHT family – a new group of predicted NTPases implicated in apoptosis and MHC transcription activationTrends in Biochemical Sciences252000223234
- M.C.KühnH.S.WillenbergM.SchottC.PapewalisU.StumpfS.FlohéW.A.ScherbaumS.SchinnerAdipocyte-secreted factors increase osteoblast proliferation and the OPG/RANKL ratio to influence osteoclast formationMolecular and Cellular Endocrinology34922012180188
- S.LalaY.OguraC.Osborneet alCrohn's disease and the NOD2 gene: a role for paneth cellsGastroenterology12520034757
- M.LamkanfiW.DeclercqB.T.VandenP.VandenabeeleCaspases leave the beaten track: caspase-mediated activation of NF-kappaBThe Journal of Cell Biology1732006165171
- Q.-M. Liu, A novel growth promoting activities in bovine milk, Ph.D. Thesis, University of Liverpool, UK, 2002.
- Y.LiuZ.SongY.ZhaoH.QinJ.CaiH.ZhangT.YuS.JiangG.WangM.DingH.DengA novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cellsBiochemical and Biophysical Research Communications34612006131139
- J.MasumotoS.TaniguchiJ.SagaraPyrin N-terminal homology domain- and caspase recruitment domain-dependent oligomerization of ASCBiochemical and Biophysical Research Communications2802001652655
- Mescher A.L., Skin, in: Junqueira's Basic Histology; Text & Atlas, 12th ed. McGraw Hill Companies, Bloomington, Indiana, USA; 2010.
- R.NakanishiN.NagayaT.YoshimatsuT.HanagiriK.YasumotoOptimal dose of basic fibroblast growth factor for long-segment orthotopic tracheal autograftsThe Journal of Thoracic and Cardiovascular Surgery113199726036
- Y.OguraN.InoharaA.BenitoF.F.ChenS.YamaokaG.NunezNod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaBThe Journal of Biological Chemistry276200148124818
- D.RibattiA.VaccaM.RusnatiM.PrestaThe discovery of basic fibroblast growth factor/fibroblast growth factor-2 and its role in haematological malignanciesCytokine & Growth Factor Reviews183–42007327334
- P.S.RudlandW.SeifertD.GospodarowiczGrowth control in cultured mouse fibroblast: induction of the pleiotypic and mitogenic responses by purified growth factorsProceedings of the National Academy of Sciences of the United States of America717197426002604
- A.ShaiH.I.MaibachWound Healing and Ulcer of the Skin, Diagnosis and Therapy. The Practical Approach2005Springer-VerlagBerlin/Heidelberg, Germany
- B.ShenM.AreseA.GualandrisD.B.RifkinIntracellular association of FGF-2 with the ribosomal protein L6/TAXREB107Biochemical and Biophysical Research Communications (United States)252November (2)1998524528
- C.S.SkjerpenT.NilsenJ.WescheS.OlsnesBinding of FGF-1 variants to protein kinase CK2 correlates with mitogenicityThe EMBO Journal (England)21August (15)200240584069
- J.A.SmithL.MartinDo cells cycle?Proceedings of the National Academy of Sciences of the United States of America704197312631267
- F.SouletT.Al-SaatiS.RogaF.AmalricG.BoucheFibroblast growth factor-2 interacts with free ribosomal protein S19Biochemical and Biophysical Research Communications (United States)289November (2)2001591596
- C.StehlikJ.C.ReedThe PYRIN connection: novel players in innate immunity and inflammationThe Journal of Experimental Medicine2002004551558
- A.Tassi EAl-AttarA.AignerM.R.SwiftK.McDonnellA.KaravanovA.WellsteinEnhancement of fibroblast growth factor (FGF) activity by an FGF-binding proteinThe Journal of Biological Chemistry264320014024740253
- M.TaylorSkin careRx2011 Also available at: http://skincarerx.com/growth-factors.html.imovic
- A.ThompsonM.PrescottN.ChelebiJ.SmithT.BrownG.SchmidtElectrospray ionisation-cleavable tandem nucleic acid mass tag-peptide nucleic acid conjugates: synthesis and applications to quantitative genomic analysis using electrospray ionisation-MS/MSNucleic Acids Research102007113
- A.TingstromC.-H.HeldinC.RubinRegulation of fibroblast-mediated collagen gel contraction by platelet derived growth factor, interleukin-1 α and transforming growth factor β1Journal of Cell Science1021992315322
- G.TooleS.TooleCellular organizationUnderstanding Biology for Advanced Level3rd ed.1995Stanley Thomas Publisher Ltd.UK
- A.M.VerhagenE.J.CoulsonD.L.VauxInhibitor of apoptosis proteins and their relatives: IAPs and other BIRPsGenome Biology220013009
- T.YorioA.F.ClarkM.B.WaxOcular Therapeutics: “Eye on New Discoveries”2007Academic Press p. 88. Also available at: http://books.google.com/books?id=7jVNuIt8vm4C&pg=PA88