Abstract
The classical Hantzsch reaction is one of the simplest and most economical methods for the synthesis of biologically important and pharmacologically useful 1,4-dihydropyridine derivatives. Melamine trisulfonic acid (MTSA) under thermal condition is proven to act as a very efficient catalyst for a one-pot synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives in excellent yields from aldehydes, dimedone, malononitrile and ammonium acetate under solvent-free conditions. The present environmentally benign procedure for the synthesis of 1,4-dihydropyridines is suitable for library synthesis and it will find application in the synthesis of potent biologically active molecules. The process presented here is operationally simple, environmentally benign and has excellent yield. Furthermore, the catalyst can be recovered conveniently and reused efficiently.
1 Introduction
Multi component reactions allow the creation of several bonds in a single operation and are attracting increasing attention as one of the most powerful emerging synthetic tools for the creation of molecular diversity and complexity. They also have considerable advantages in terms of user and environmental friendliness because of the step reduction and atom economy associated to their use [Citation1]. Therefore, the design of novel MCRs has attracted great attention from research groups working in medicinal chemistry and drug discovery.
One-pot, four-component synthesis of symmetrically substituted 1,4-dihydropyridines were first reported by Arthur Hantzsch in 1882 [Citation2]. 1,4-Dihydropyrdines have been reported as anticancer [Citation3], neurotropic [Citation4], glycoprotein inhibitors [Citation5], bronchodilating [Citation6] and antidiabetic [Citation7] agents. Tuberculosis (TB) is a common and often deadly infectious disease caused by various strains of mycobacterium, usually Mycobacterium tuberculosis. Tuberculosis has been considered to be a disease of poverty for many years with quite rare occurrence in the developed countries. Recently, studies showed that 3,5-dicarbamoyl derivatives of 1,4-dihydropyridine (DHP) with lipophilic groups have considerable antitubercular activity against M. tuberculosis H37Rv [Citation8,Citation9].
In order to model and understand these biological properties and to develop new chemotherapeutic agents based upon the 1,4-DHP motif, considerable effort has been devoted to establish efficient and rapid methods for their synthesis. 1,4-DHPs are generally synthesized using the Hantzsch method, which involves cyclocondensation of an aldehyde, a β-ketoester and ammonia either in acetic acid or under reflux in alcohols for long reaction times. Recently, a number of modified methods using different catalysts like K7[PW11CoO40] [Citation10], p-TSA [Citation11], grinding [Citation12], FeF3 [Citation13], Yb(OTf)3 [Citation14], HClO4-SiO2 [Citation15], cellulose sulfuric acid [Citation16], triphenylphosphine [Citation17] and L-Proline [Citation18] have been developed. Many of these reported methods involve the use of expensive reagents, hazardous solvents, long reaction times and tedious workup procedures. Thus, the search for new reagents and methods is still of growing importance.
Recently, the use of melamine trisulfonic acid as a catalyst in organic synthesis has increased considerably. Melamine trisulfonic acid can be easily prepared. It is effectively used as a catalyst in organic reactions, such as regioselevtive nitration of aromatic compounds [Citation19], N-formylation of amines [Citation20], aryldithienylmethanes [Citation21], spiro[pyrazolo[3,4-b]pyridine-4,3′-indoline] derivatives [Citation22].
In continuation of our ongoing work on multi-component reactions (MCRs) such as 3,4-dihydropyrimidin-2(1H)-ones/-thiones/imines [Citation23], β-amino ketone compounds [Citation24], amidoalkyl naphthols [Citation25], 2-amino-4,6-diphenylpyridine-3-carbonitrile derivatives [Citation26] and α-amino nitriles [Citation27], we now wish to report a facile and rapid one-pot three component reaction for preparation of DHP derivatives 4a–4l from aldehydes, dimedone, malononitrile and ammonium acetate using MTSA as reusable catalyst.
2 Experimental
2.1 Apparatus and analysis
Chemicals were purchased from Merck, Fluka and Aldrich Chemical Companies. Melamine and chlorosulfonic acid were used for the preparation of MTSA. The benzaldehydes used were with substituents H, p-OCH3, p-CH3, p-Cl, p-NO2, p-Br, p-F, p-OH, m-Cl, m-NO2, m-Br and m-OH. The solid aldehydes were used as such and the liquid aldehydes were used after vacuum distillation. Dimedone and ammonium acetate were also used for the synthesis. The solvents like methanol, 1,4-dioxane, ethanol, cyclohexane, acetonitrile and toluene were used to study the optimization of solvent for the synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline. All yields refer to isolated products unless otherwise stated. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were obtained using Bruker DRX-500 Avance at ambient temperature, using TMS as internal standard. FT-IR spectra were obtained as KBr discs on Shimadzu spectrometer. Mass spectra were determined on a Varion-Saturn 2000 GC/MS instrument. Elemental analysis was measured by means of Perkin Elmer 2400 CHN elemental analyzer flowchart.
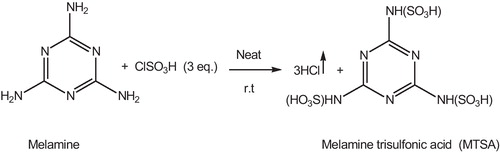
2.2 Preparation of melamine trisulfonic acid (MTSA)
Melamine trisulfonic acid was prepared according to the previously reported method by Shirini et al. [Citation28]. A 250 ml suction flask charged with chlorosulfonic acid (5 ml, 75.2 mmol) was equipped with a gas inlet tube for conducting HCl gas over an adsorbing solution i.e. water. Melamine (3.16 g, 25.07 mmol) was added in small portions over a period of 30 min at room temperature. HCl gas evolved from the reaction vessel immediately (). After completion of the addition of melamine, the mixture was shaken for 30 min; meanwhile, the residual HCl was exhausted by suction. The mixture was triturated with n-hexane (10 ml) and then filtered. The solid residue was washed with n-hexane (10 ml) and dried under vacuum. Melamine trisulfonic acid (7.6 g, 84%) was obtained as a white solid.
2.3 General experimental procedure for the synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (4a–4l)
A mixture of aldehyde (2 mmol), dimedone (2 mmol), malononitrile (2 mmol) and ammonium acetate (3 mmol) was stirred at 60 °C in the presence of MTSA (5 mol%) for the appropriate time. After completion of the reaction (TLC monitoring), the reaction mixture was cooled to ambient temperature, CH2Cl2 was added, and the MTSA was filtered off. The filtrate was concentrated to dryness, and the crude solid product was crystallized from EtOH to afford the pure 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline. All the products obtained were fully characterized by spectroscopic methods such as IR, 1H NMR, 13C NMR, mass spectroscopy and elemental analysis and have been identified by the comparison of the spectral data with those reported.
2.4 The spectroscopic and analytical data for the synthesized compounds are presented below
2.4.1 2-Amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (4a)
IR (KBr, cm−1): 3433, 3322, 3213, 2199, 1697, 1623, 1495. 1H NMR (500 MHz, CDCl3): δ = 1.01 (s, 3H, CH3), 1.08 (s, 3H, CH3), 2.00–2.36 (m, 4H, 2×CH2), 4.36 (s, 1H, CH), 5.36 (s, 2H, NH2), 7.05–7.29 (m, 5H, ArH), 8.92 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 27.0, 29.6, 32.4, 36.5, 39.5, 50.6, 59.5, 113.2, 119.9, 126.3, 127.4, 128.2, 143.8, 155.7, 166.0, 197.0 ppm; MS (ESI): m/z 294 (M+H)+. Anal. Calcd for C18H19N3O: C, 73.72; H, 6.48; N, 14.33. Found: C, 73.66; H, 6.57; N, 14.32.
2.4.2 2-Amino-4-(4-bromophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (4b)
IR (KBr, cm−1): 3438, 3332, 3221, 2192, 1694, 1601, 1500. 1H NMR (500 MHz, CDCl3): δ = 0.95 (s, 3H, CH3), 1.06 (s, 3H, CH3), 2.04–2.32 (m, 4H, 2×CH2), 4.33 (s, 1H, CH), 5.41 (s, 2H, NH2), 7.02–7.28 (m, 4H, ArH), 8.81 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 27.3, 29.4, 32.6, 36.6, 39.4, 50.8, 59.5, 112.8, 119.8, 126.3, 127.3, 128.5, 144.0, 155.2, 167.0, 195.6 ppm; MS (ESI): m/z 372.9 (M+H)+. Anal. Calcd for C18H18BrN3O: C, 58.08; H, 4.84; N, 11.29. Found: C, 58.02; H, 4.80; N, 11.25.
2.4.3 2-Amino-4-(4-nitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (4c)
IR (KBr, cm−1): 3444, 3329, 3215, 2205, 1688, 1600, 1502. 1H NMR (500 MHz, CDCl3): δ = 1.05 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.07–2.47 (m, 4H, 2×CH2), 4.26 (s, 1H, CH), 5.50 (s, 2H, NH2), 7.22–7.33 (m, 4H, ArH), 8.88 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 26.7, 29.7, 32.8, 36.8, 39.6, 51.0, 59.5, 113.4, 120.6, 126.5, 127.0, 128.3, 144.2, 154.7, 167.3, 195.5 ppm; MS (ESI): m/z 339 (M+H)+. Anal. Calcd for C18H18N4O3: C, 63.90; H, 5.32; N, 16.56. Found: C, 63.78; H, 5.31; N, 16.49.
2.4.4 2-Amino-4-(4-methylphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(4d)
IR (KBr, cm−1): 3436, 3318, 3219, 2208, 1698, 1598, 1494. 1H NMR (500 MHz, CDCl3): δ = 0.99 (s, 3H, CH3), 1.01 (s, 3H, CH3), 2.06 (s, 3H, CH3), 1.99–2.30 (m, 4H, 2×CH2), 4.42 (s, 1H, CH), 5.48 (s, 2H, NH2), 7.02–7.16 (m, 4H, ArH), 8.78 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 26.9, 29.9, 32.7, 36.6, 39.6, 50.5, 59.7, 113.3, 121.4, 126.5, 127.2, 128.3, 144.3, 156.2, 168.3, 195.8 ppm; MS (ESI): m/z 308 (M+H)+. Anal. Calcd for C19H21N3O: C, 74.27; H, 6.84; N, 13.68. Found: C, 74.18; H, 6.82; N, 13.6.
2.4.5 2-Amino-4-(4-methoxylphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(4e)
IR (KBr, cm−1): 3442, 3322, 3219, 2206, 1690, 1603, 1498. 1H NMR (500 MHz, CDCl3): δ = 0.94 (s, 3H, CH3), 1.00 (s, 3H, CH3), 3.66 (s, 3H, OCH3), 2.10–2.44 (m, 4H, 2×CH2), 4.29 (s, 1H, CH), 5.55 (s, 2H, NH2), 7.17–7.37 (m, 4H, ArH), 8.80 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 26.5, 29.6, 32.5, 37.0, 39.4, 51.0, 59.3, 114.0, 120.9, 126.8, 127.3, 128.6, 144.6, 156.6, 168.0, 195.9 ppm; MS (ESI): m/z 324 (M+H)+. Anal. Calcd for C19H21N3O2: C, 70.58; H, 6.50; N, 13.00. Found: C, 70.47; H, 6.48; N, 13.00.
2.4.6 2-Amino-4-(3-bromophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (4f)
IR (KBr, cm−1): 3429, 3327, 3212, 2192, 1694, 1618, 1510. 1H NMR (500 MHz, CDCl3): δ = 0.95 (s, 3H, CH3), 0.99 (s, 3H, CH3), 2.14–2.40 (m, 4H, 2×CH2), 4.35 (s, 1H, CH), 5.38 (s, 2H, NH2), 7.09–7.19 (m, 4H, ArH), 8.89 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 27.3, 29.4, 32.5, 36.8, 39.3, 50.8, 59.5, 113.3, 120.2, 126.2, 127.2, 128.4, 144.0, 155.3, 166.9, 196.5 ppm; MS (ESI): m/z 372.9 (M+H)+. Anal. Calcd for C18H18BrN3O: C, 58.08; H, 4.84; N, 11.29. Found: C, 58.02; H, 4.82; N, 11.30.
2.4.7 2-Amino-4-(3-nitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (4g)
IR (KBr, cm−1): 3426, 3320, 3217, 2195, 1688, 1608, 1508. 1H NMR (500 MHz, CDCl3): δ = 1.06 (s, 3H, CH3), 1.14 (s, 3H, CH3), 2.20–2.55 (m, 4H, 2×CH2), 4.55 (s, 1H, CH), 4.78 (s, 2H, NH2), 7.48–7.70 (m, 4H, ArH), 8.92 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 27.2, 30.3, 32.7, 36.8, 39.8, 51.0, 59.5, 112.9, 120.5, 126.7, 127.0, 128.4, 144.4, 155.1, 168.0, 195.0 ppm; MS (ESI): m/z 339 (M+H)+. Anal. Calcd for C18H18N4O3: C, 63.90; H, 5.32; N, 16.56. Found: C, 63.80; H, 5.31; N, 16.53.
2.4.8 2-Amino-4-(4-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(4h)
IR (KBr, cm−1): 3438, 3329, 3216, 2189, 1679, 1604, 1501. 1H NMR (500 MHz, CDCl3): δ = 0.96 (s, 3H, CH3), 1.03 (s, 3H, CH3), 2.02–2.33 (m, 4H, 2×CH2), 4.12 (s, 1H, CH), 5.47 (s, 2H, NH2), 7.08–7.18 (m, 4H, ArH), 8.98 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 26.7, 29.6, 32.4, 36.8, 39.6, 51.3, 59.4, 113.3, 120.5, 126.3, 127.3, 128.2, 145.0, 155.3, 166.5, 194.5 ppm; MS (ESI): m/z 328.45 (M+H)+. Anal. Calcd for C18H18ClN3O: C, 65.96; H, 5.50; N, 12.83. Found: C, 65.88; H, 5.48; N, 12.81.
2.4.9 2-Amino-4-(4-hydroxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(4i)
IR (KBr, cm−1): 3438, 3382, 3325, 3205, 2197, 1689, 1602, 1492. 1H NMR (500 MHz, CDCl3): δ = 1.03 (s, 3H, CH3), 1.02 (s, 3H, CH3), 2.11–2.44 (m, 4H, 2×CH2), 4.33 (s, 1H, CH), 5.88 (s, 2H, NH2), 7.07–7.27 (m, 4H, ArH), 8.77 (s, 1H, NH), 9.74 (s, 1H, OH) ppm; 13C NMR (125 MHz, CDCl3): δ = 26.9, 29.9, 32.5, 36.6, 39.4, 50.8, 59.6, 113.1, 120.9, 126.6, 127.2, 128.0, 144.1, 154.9, 166.8, 194.8 ppm; MS (ESI): m/z 310 (M+H)+. Anal. Calcd for C18H19N3O2: C, 69.90; H, 6.15; N, 13.59. Found: C, 69.84; H, 6.12; N, 13.57.
2.4.10 2-Amino-4-(3-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(4j)
IR (KBr, cm−1): 3427, 3330, 3225, 2199, 1692, 1605, 1508. 1H NMR (500 MHz, CDCl3): δ = 1.04 (s, 3H, CH3), 1.01 (s, 3H, CH3), 1.97–2.36 (m, 4H, 2×CH2), 4.32 (s, 1H, CH), 5.38 (s, 2H, NH2), 7.21–7.38 (m, 4H, ArH), 8.93 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 27.3, 29.6, 32.4, 36.4, 39.5, 51.2, 59.3, 113.3, 120.6, 126.7, 127.1, 128.2, 143.5, 155.5, 167.3, 193.9 ppm; MS (ESI): m/z 328.45 (M+H)+. Anal. Calcd for C18H18ClN3O: C, 65.96; H, 5.50; N, 12.83. Found: C, 65.94; H, 5.49; N, 12.82.
2.4.11 2-Amino-4-(3-hydroxyphenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline(4k)
IR (KBr, cm−1): 3428, 3382, 3333, 3222, 2200, 1688, 1601, 1505. 1H NMR (500 MHz, CDCl3): δ = 0.94 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.99–2.29 (m, 4H, 2×CH2), 4.28 (s, 1H, CH), 5.44 (s, 2H, NH2), 7.18–7.26 (m, 4H, ArH), 8.86 (s, 1H, NH), 9.79 (s, 1H, OH) ppm; 13C NMR (125 MHz, CDCl3): δ = 26.8, 29.7, 32.3, 36.4, 39.3, 50.6, 59.4, 113.6, 121.3, 126.5, 127.4, 128.3, 143.9, 155.0, 166.0, 195.9 ppm; MS (ESI): m/z 310 (M+H)+. Anal. Calcd for C18H19N3O2: C, 69.90; H, 6.15; N, 13.59. Found: C, 69.78; H, 6.13; N, 13.56.
2.4.12 2-Amino-4-(4-fluorophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline (4l)
IR (KBr, cm−1): 3440, 3331, 3211, 2185, 1698, 1605, 1502. 1H NMR (500 MHz, CDCl3): δ = 1.00 (s, 3H, CH3), 1.07 (s, 3H, CH3), 2.11–2.47 (m, 4H, 2×CH2), 4.20 (s, 1H, CH), 5.50 (s, 2H, NH2), 7.16–7.29 (m, 4H, ArH), 8.82 (s, 1H, NH) ppm; 13C NMR (125 MHz, CDCl3): δ = 27.3, 30.0, 32.3, 36.6, 39.6, 50.9, 59.7, 113.3, 121.3, 127.2, 127.5, 128.2, 144.4, 155.3, 167.7, 196.0 ppm; MS (ESI): m/z 312 (M+H)+. Anal. Calcd for C18H18FN3O: C, 69.45; H, 5.78; N, 13.50. Found: C, 69.30; H, 5.79; N, 13.52.
3 Results and discussion
We have developed an efficient synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives from the reaction of aldehydes, dimedone, malononitrile and ammonium acetate promoted by a catalytic amount of MTSA (5 mol%) under solvent free conditions at 60 °C. Herein, we wish to report our results.
To choose optimum conditions, first, the effect of solvent on the rate of the reaction was studied for the preparation of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline by four-component condensation reaction of benzaldehyde, dimedone, malononitrile and ammonium acetate in presence of 5 mol% melamine trisulfonic acid under various solvents like methanol, 1,4-dioxane, ethanol, cyclohexane, acetonitrile and toluene at 60 °C (, entries 1–6). It was found that the best results were obtained with 5 mol% MTSA under solvent-free condition (, entry 7). The reaction was completed within 3 h and the expected product was obtained in a 94% yield.
Table 1 Optimization of solvent for the synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline.Table Footnotea
Next, the study set out to determine the optimal amount of MTSA. The reaction was carried out by varying amount of the catalyst (). Maximum yield was obtained with 5 mol% of the catalyst. Further increase in amount of MTSA in the mentioned reaction did not have any significant effect on product yield.
Table 2 Optimization of catalyst for the synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline.Table Footnotea
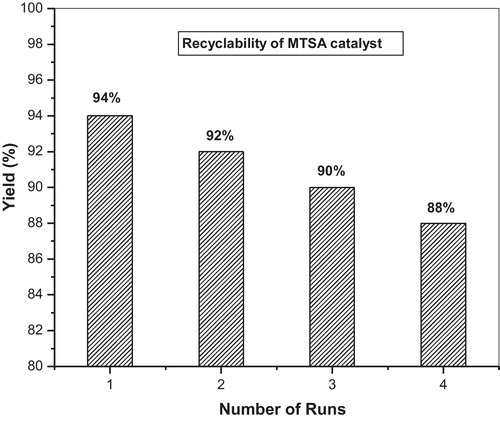
The reusability of the catalyst was tested in the synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline. The catalyst was recovered after each run, washed with CH2Cl2, dried in an oven at 100 °C for 30 min prior to use, and tested for its activity in the subsequent run with no fresh catalyst added. The catalyst was tested for four runs. It was seen that the catalyst displayed very good reusability ( and ).
Table 3 The effect of recyclability of MTSA catalyst for the synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline.Table Footnotea
Fig. 1 Recyclability of MTSA catalyst for the synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline. The catalyst MTSA reused four times.
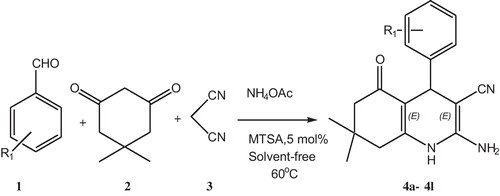
Under the optimized set of reaction conditions a number of aromatic aldehydes 1 were allowed to undergo MCR with dimedone 2, malononitrile 3 and ammonium acetate in the presence of MTSA (5 mol%) under solvent-free condition at 60 °C (). The results were given in . All the electron-rich and electron-deficient aldehydes worked well leading to excellent yields of the products. The progress of the reaction was monitored by TLC.
Table 4 Synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives in the presence of MTSA (5 mol%).Table Footnotea
Scheme 2 Synthesis of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives in the presence of MTSA (5 mol%) as catalyst at 60 °C under solvent free conditions.
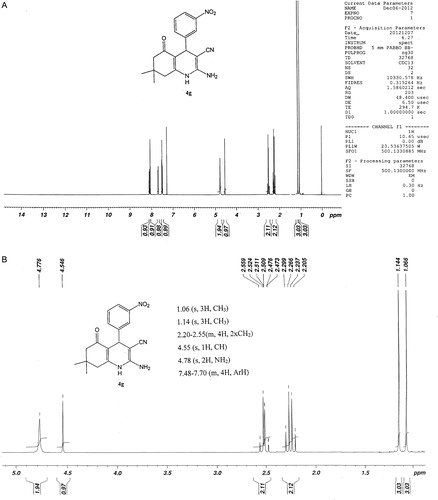
The structures of isolated products 4a–4l were deducted by physical and spectroscopic data such as: IR, 1H NMR and 13C NMR spectroscopy, mass and elemental analysis. In IR spectra, symmetrical and unsymmetrical stretching frequency of NH2 is formed in region between ν = 3444–3318 cm−1. The stretching vibration of C–N in nitrile group was appeared in the region between ν = 2200–2185 cm−1. 1H NMR spectra for compound 4g is given in A and B. In the 1H NMR spectra the two singlet signals around δ = 4.77 and δ = 4.54 ppm corresponding to NH2 group at C-2 and CH at C-4 in 2-amino-4-(3-nitrophenyl)-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline. Presence of these two signals confirmed the formation of desired products in the reaction. The other two singlet signals around δ = 1.06 and δ = 1.14 ppm corresponding to two methyl groups at C-7. The signal around δ = 2.20–2.55 ppm is due to the presence of two CH2 at C-6 and C-8.
4 Conclusion
In conclusion, we have developed an easy and efficient method to prepare a variety of 2-amino-4-phenyl-3-cyano-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives from the reaction of different aryl aldehydes, dimedone, malononitrile and ammonium acetate in the presence of catalytic amount of MTSA at 60 °C under solvent-free conditions and produced the corresponding products in good to excellent yields. Also the catalyst could be successfully recovered and recycled at least for four runs without significant loss in activity. The one-pot nature and the use of reusable and an eco-friendly catalyst make it an interesting alternative to multi-step approaches.
Acknowledgements
The authors gratefully acknowledge University Grants Commission, Government of India, New Delhi for financial support (Major Research Project: F. No. 40-44/2011(SR)). The authors also acknowledge C. Abdul Hakeem College Management, Dr. W. Abdul Hameed, Principal and Dr. M. S. Dastageer, Head of the Research Department of Chemistry for the facilities and support.
Notes
Peer review under responsibility of Taibah University.
References
- A.DomlingI.UgiMulticomponent reactions with isocyanidesAngewandte Chemie International Edition39200031683210
- A.HantzschUeber die synthese pyridinartiger verbindungen aus acetessigather und aldehydammoniakJustus Liebigs Annalen der Chemie2151882182
- T.TsuruoH.IidaM.NojiriS.TsukagoshiY.SakuraiCircumvention of vincristine and adriamycin resistance in vitro and in vivo by calcium influx blockersCancer Research43198329052910
- A.KrauzeS.GermaneO.EberlinsI.SturmsV.KlusaG.DubursDerivatives of 3-cyano-6-phenyl-4-(3′-pyridyl)pyridine-2(1H)-thione and their neurotropic activityEuropean Journal of Medicinal Chemistry341999301310
- X.ZhouL.ZhangE.TsengE.Scott-RamsayJ.J.SchentagR.A.CoburnM.E.MorrisNew 4-aryl-1,4-dihydropyridines and 4-arylpyridines as P-glycoprotein inhibitorsDrug Metabolism and Disposition332005321328
- R.W.ChapmanG.DankoM.I.SiegelsEffect of extra- and intracellular calcium blockers on histamine and antigen-induced bronchospasm in guinea pigs and ratsPharmacology291984282291
- W.J.MalaiseP.C.F.MathiasStimulation of insulin release by organic calcium-agonistDiabetologia281985153156
- A.R.TrivediD.K.DodiyaB.H.DholariyaV.B.KatariaV.R.BhuvaV.H.ShahSynthesis and biological evaluation of some novel N-aryl-1,4-dihydropyridines as potential antitubercular agentsBioorganic & Medicinal Chemistry Letters21201151815183
- M.KhoshneviszadehN.EdrakiK.JavidniaA.AlborziB.PourabbasJ.MardanehR.MirSynthesis and biological evaluation of some new 1,4-dihydropyridines containing different ester substitute and diethyl carbamoyl group as anti-tubercular agentsBioorganic & Medicinal Chemistry17200915791586
- M.M.HeraviK.BakhtiriN.M.JavadiF.F.BamoharramM.SaeediH.A.OskooiK7[PW11CoO40]-catalyzed one-pot synthesis of polyhydroquinoline derivatives via the Hantzsch three component condensationJournal of Molecular Catalysis A: Chemical26420075052
- S.R.CherkupallyR.Mekalanp-TSA catalyzed facile and efficient synthesis of polyhydroquinoline derivatives through Hantzsch multi-component condensationChemical and Pharmaceutical Bulletin56200810021004
- S.KumarP.SharmaK.K.KapoorM.S.HundalAn efficient, catalyst- and solvent-free, four-component, and one-pot synthesis of polyhydroquinolines on grindingTetrahedron642008536542
- R.SurasaniD.KalitaA.V.D.RaoK.YarbagiK.B.ChandrasekharFeF3 as a novel catalyst for the synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reactionJournal of Fluorine Chemistry13520129196
- L.M.WangJ.ShengL.ZhangJ.W.HanZ.FanH.TianC.T.QianFacile Yb(OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reactionTetrahedron61200515391543
- M.MaheswaraV.SiddaiahG.L.V.DamuC.V.RaoAn efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using a heterogeneous catalyst under solvent-free conditionsARKIVOC22006201206
- J.SafariS.H.BanitabaS.D.KhaliliCellulose sulfuric acid catalyzed multicomponent reaction for efficient synthesis of 1,4-dihydropyridines via unsymmetrical Hantzsch reaction in aqueous mediaJournal of Molecular Catalysis A: Chemical33520114650
- A.DebacheW.GhalemR.BoulcinaA.BelfaitahS.RhouatiB.CarboniAn efficient one-step synthesis of 1,4-dihydropyridines via a triphenylphosphine-catalyzed three-component Hantzsch reaction under mild conditionsTetrahedron Letters50200952485250
- N.N.KaradeV.H.BudhewarS.V.ShindeW.N.JadhavL-Proline as an efficient organo catalyst for the synthesis of polyhydroquinoline via multicomponent Hantzsch reactionLetters in Organic Chemistry420071619
- J.AlbadiF.ShiriniB.GhabeziT.SeiadatnasabMelamine trisulfonic acid catalyzed regioselevtive nitration of aromatic compounds with sodium nitrate under solvent-free conditionsArabian Journal of Chemistry201210.1016/j.arabjc.2012.10.011
- X.J.YangY.S.ZhangMelamine trisulfonic acid-catalyzed N-formylation of amines under solvent-free conditionsResearch on Chemical Intermediates201210.1007/s11164-012-0803-7
- L.WuY.YanF.YanMelamine trisulfonic acid: a new efficient catalyst for the synthesis of aryldithienylmethanesPhosphorus, Sulfur, and Silicon and the Related Elements18722012149154
- L.YangP.SunL.WuOne-pot three-component synthesis of spiro[pyrazolo[3,4-b]pyridine-4,3′-indoline] derivatives catalyzed by melamine trisulfonic acidJournal of the Chinese Chemical Society201210.1002/jccs.201200046
- S.S.MansoorS.S.ShafiS.Z.AhmedAn efficient one-pot multicomponent synthesis of 3,4-dihydropyrimidine-2-(1H)-ones/thiones/imines via a Lewis base catalyzed Biginelli-type reaction under solvent-free conditionsArabian Journal of Chemistry201110.1016/j.arabjc.2011.09.018
- S.S.MansoorK.AswinK.LogaiyaS.P.N.SudhanAn efficient synthesis of β-amino ketone compounds through one-pot three-component Mannich-type reactions using bismuth nitrate as catalystJournal of Saudi Chemical Society201210.1016/j.jscs.2012.04.008
- S.S.MansoorK.AswinK.LogaiyaS.P.N.SudhanZrOCl2.8H2O: an efficient and recyclable catalyst for the three-component synthesis of amidoalkyl naphthols under solvent-free conditionsJournal of Saudi Chemical Society201210.1016/j.jscs.2012.06.003
- S.S.MansoorK.AswinK.LogaiyaS.P.N.Sudhan[Bmim]BF4 ionic liquid: an efficient reaction medium for the one-pot multi-component synthesis of 2-amino-4,6-diphenylpyridine-3-carbonitrile derivativesJournal of Saudi Chemical Society201210.1016/j.jscs.2012.07.011
- S.S.MansoorK.AswinK.LogaiyaS.P.N.SudhanAn efficient one-pot three-component synthesis of α-amino nitriles via Strecker reaction catalysed by bismuth(III) nitrateJournal of Saudi Chemical Society201210.1016/j.jscs.2012.10.009
- F.ShiriniM.A.ZolfigolJ.AlbadiMelamine trisulfonic acid as a new, efficient and reusable catalyst for the solvent free synthesis of coumarinsJournal of the Iranian Chemical Society742010895899