Abstract
Oxidative stress produced by free radicals has been implicated in the pathogenesis of acute liver injury. The aim of our study was to investigate whether melatonin, a potent antioxidant, could attenuate acute and chronic hepatic injury in rats. Acute liver injury was induced by two consecutive intra peritoneal (i.p.) injections of thioacetamide (TAA; 300 mg/kg, i.p.) at 24 h intervals. Chronic liver injury was induced by (TAA, i.p. injections for 6 weeks twice weekly, 50 mg/kg). Treatment with melatonin (3 mg/kg/daily, i.p.) was initiated 24 h prior to and for 6 weeks post TAA intake. Rats in normal control group received intra-peritoneal injections of normal saline at the same dose and frequency as those in treatment groups. At the end of experiment, animals were sacrificed, liver was removed and weighed, and ratio to body weight was calculated. Serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), reduced glutathione (GSH) and malondialdehyde (MDA) concentration in liver homogenates were assessed. We found that the acute and chronic experiments, liver weight, as well as liver weight/body weight ratio, ALT, AST, GSH and MDA concentration were lower in rats treated with TAA + melatonin compared to acute and chronic TAA groups. Liver histology was significantly improved and the mortality in the melatonin-treated rats was decreased indicating decreased oxidative stress and inflammation. In conclusion both models results suggest that melatonin may be utilized to reduce liver injury associated with oxidative stress.
1 Introduction
Oxidative stress has been accused in the genesis of acute and chronic liver damage in many conditions such as toxin exposures, bile duct obstruction, excess alcohol intake, liver ischemia, and viral infection [Citation1]. Overproduction of reactive oxygen species (ROS) and nitrogen species, together with a significant decrease of antioxidant protection in these pathological conditions, disturbs various cellular functions through the process of lipid peroxidation [Citation2]. Cirrhosis results from induction of oxidative stress, mitochondrial dysfunction and depletion of antioxidant status [Citation3].
Common causes of acute liver failure include acute viral hepatitis, and chemical- or toxin-induced liver injury [Citation4], and can rapidly lead to failure of many organs and death while the liver transplantation is the only effective treatment [Citation5].
Meanwhile, liver cirrhosis is a critical stage of chronic liver diseases that can produce liver failure. It may be due to viral infection, toxic agents, or alcohol. The usual treatment of liver cirrhosis is limited to the treatment of the underlying cause [Citation6]. Cirrhosis is defined as the advanced stage of fibrosis. Hepatic fibrosis can lead to irreversible cirrhosis, and involves multiple cellular and molecular events that ultimately result in hepatic accumulation of collagen and extra cellular matrix protein, this being directly responsible for the decrease of the hepatic function [Citation7,Citation8]. The accumulation of extracellular matrix observed in cirrhosis is due to the activation of fibroblasts, that are produced by the activation of cell precursors, such as hepatic stellate cells [Citation9].
Many studies have been carried out to develop liver failure models. Surgical models include the use of hepatic ischemia and subtotal or total hepatectomy [Citation10], while chemical models are produced by the use of drugs and toxins such as acetaminophen [Citation11], carbon tetrachloride [Citation12], galactosamine [Citation13], and thioacetamide [Citation14].
Thioacetamide [TAA] was originally used as a fungicide [Citation15]. TAA is a weak carcinogen that mainly affects liver and kidney [Citation16]. TAA has been considered to be an inducer of liver fibrosis and cirrhosis [Citation17]. The effects of TAA are not limited to the liver, but also many structural and functional disturbances have been described in the kidney [Citation18], and spleen [Citation19].
Liver cirrhosis induced by thioacetamide is associated with excessive lipid peroxidation [Citation20] and the exhaustion of antioxidant state [Citation21]. In addition, thioacetamide causes hepatocellular necrosis, and has been used to explore the role of reactive oxygen species [Citation14].
In this regard, the decrease of the oxidative stress may be useful to reduce cell injury, cirrhosis and fibrosis in a variety of human diseases and experimental models of liver damage [Citation22,Citation23]. Antioxidants can help to minimize the oxidative stress, in cirrhotic livers [Citation24]. Melatonin (N-acetyl-5-metyoxytryptamine) is a powerful endogenous antioxidant, plays very important roles in regulation of circadian rhythms, sleep, immune system activity, and elimination of oxygen free radicals [Citation25,Citation26], and exerts its antioxidant effects mainly through G protein dependent receptors which lead to the induction of antioxidant enzyme synthesis [Citation27]. In this way, melatonin neutralizes the effects of both oxygen- and nitrogen-based reactive molecules [Citation28].
Moreover, melatonin was demonstrated to interact with many toxic free radicals like peroxyl radicals, singlet oxygen species, and hydrogen peroxide [Citation29,Citation30]. Melatonin may induce up regulation of the activity of antioxidants and antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase and glutathione reductase [Citation31]. Melatonin induces the activity of γ-glutamylcysteinesynthetase, thereby stimulating the production of another intracellular antioxidant, glutathione [Citation32].
Recent studies showed that melatonin exerts its cytoprotective effects in various experimental models of acute liver injury and reduces fibroblast proliferation and collagen synthesis [Citation33], indicating that melatonin may have therapeutic effects on acute and chronic liver injury, through its antioxidant action [Citation34].
In the present study we evaluated the effect of melatonin on oxidative stress damage in thioacetamide-induced acute and chronic hepatic injury in rats.
2 Materials and methods
2.1 Animals
Male Albino rats, weighing between 150 and 250 g at the beginning of the study. The animals were kept in wire-floored cages under standard laboratory conditions of 12 h light–dark cycle, 25 ± 2 °C with free access to food and water. All animals were treated humanely according to the international guidelines for the care of animals.
2.2 Chemicals
Thioacetamide was purchased from Sigma Chemical Co., USA. Melatonin was purchased from Sigma–Aldrich, St Louis, MO, USA.
Experimental induction of liver injury
| - | Acute liver damage was induced by two consecutive injections of thioacetamide (300 mg/kg, i.p.) at 24 h intervals [Citation23]. | ||||
| - | Chronic Liver damage was induced by thioacetamide for 6 weeks (twice weekly 50 mg/kg, i.p. injections) [Citation35]. | ||||
2.3 Experimental protocol
Ten animals were included in each of the following five groups:
| - | Group I [control group]: animals receiving daily i.p. injections of saline. Rats in normal control group received daily i.p. injections of saline at the same dose and frequency as those in treatment groups. | ||||
| - | Group II (acute liver injury): acute liver injury was induced by two consecutive injections of thioacetamide (300 mg/kg, i.p.) at 24 h intervals. | ||||
| - | Group III (melatonin and acute liver injury): animals treated with melatonin (3 mg/kg/daily, i.p. diluted in 0.9% NaCl) was initiated 24 h prior to thioacetamide and 6 weeks post its intake. | ||||
| - | Group IV [chronic liver injury]: chronic liver injury was induced by thioacetamide for 6 weeks (twice weekly 50 mg/kg, i.p. injections). | ||||
| - | Group V (melatonin and chronic liver injury): animals treated with melatonin were initiated 24 h prior to thioacetamide at a dose of 3 mg/kg/daily, i.p. diluted in 0.9% NaCl, and 6 weeks post thioacetamide intake. | ||||
After the last treatment, rats were fasted for 8 h and subjected to sodium thiopental anesthesia (40 mg/kg. i.p.) and sacrificed by cervical dislocation. The blood samples were collected and centrifuged to obtain serum in order to estimate total proteins, ALT, and AST.
The body weights of the animals in each group were determined. The liver from each animal was weighed for determination of the liver weight/body weight ratio. The obtained rat liver homogenate was aliquoted and immediately frozen at −20 °C for biochemical measurement of reduced glutathione (GSH) content, and malondialdehyde (MDA) concentration. Other liver pieces were fixed in buffered 4% formalin and embedded in paraffin. Sections of about 4 μm thickness were stained with hematoxylin and eosin (H&E) for the evaluation of histological changes according to a described procedure [Citation36].
2.4 Statistical analysis
Data were analyzed statistically, using SPSS statistical package Version 13 and expressed as mean (M) ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to compare different groups and to determine overall effect of each treatment, with a confidence level of 95% (p < 0.05) considered significant.
3 Results
3.1 Body weight
In TAA acute models (Groups II and III), the average body weight showed no significant difference compared to the normal control group. Meanwhile there is a significant increase in body weight of chronic models (Groups IV and V) compared to the normal control group (p < 0.001), and these significant changes may be consistent with the age of the animals. The melatonin treated chronic liver damage groups (Group V) showed a significant increase in body weight compared to the acute and chronic TAA-intoxicated animals (Groups II and IV) (p < 0.001; ).
Table 1 Body weight (BW) (g), liver weight (LW) (g) and liver weight (g)/body weight (g) ratio (LW/BW) ratio in different groups (mean ± SD).
3.2 Liver weight
The average liver weights of acute and chronic TAA-intoxicated animals (Groups II and IV) showed significant increase compared to the normal control group (p < 0.001), The melatonin treated acute and chronic liver damage groups (Groups III and V) showed a significant increase in liver weights compared to the acute TAA-intoxicated animals (Groups II; p < 0.001 and ).
3.3 Liver weight/body weight ratio
The mean relative liver weights of acute and chronic TAA-intoxicated animals (Groups II and IV) showed significant increase compared to the control group (p < 0.001). Value of the mean relative liver weights (LW/BW ratio) showed a significant increase in melatonin treated acute liver damage group (Group III) compared to the control group (p < 0.01). Value of the mean relative liver weights (LW/BW ratio) showed a significant increase in melatonin treated chronic liver damage group (Group V) compared to the control group (p < 0.05). The chronic TAA-intoxicated animals (Groups IV) and the melatonin treated acute and chronic liver damage groups (Group III and V) showed a significant increase in LW/BW ratio compared to the acute TAA-intoxicated animals (Group II) (p < 0.001), and the melatonin treated chronic liver damage groups (Group V) showed a significant increase in liver weights compared to the chronic TAA-intoxicated animals (Group IV; p < 0.001) ().
3.4 Serum liver markers
Short term and long-term taken of TAA led to significant increase of biochemical markers ALT, and AST, (p < 0.001, ) were compared to the control group, indicating hepatocytes damage. Treatment of animals with melatonin significantly reduced the level of liver function biomarker (ALT, AST). The toxic effect of TAA was controlled in the rats treated with melatonin and that is approved by restoration of the levels of the liver biomarker. In acute liver injury treated with melatonin, the effect was only marginal but still there was a significant increase in liver enzymes (p < 0.001, ) as compared to control group, whereas in chronic liver injury, melatonin prevented the TAA-induced liver damage effectively.
Table 2 Serum aspartate transaminase (AST, IU/L), alanine transferase (ALT, IU/L), MDA concentration (nmol/mg liver tissue) and GSH content (μg/mg liver protein) in liver tissue of different groups of rats in different groups of rats (mean ± SD).
MDA concentration (nmol/mg liver tissue) and GSH content (μg/mg liver protein) in liver tissue of different groups of rats ()
Lipoperoxidation is a marker of cell membrane damage, and the measure of malondialdehyde, which is a product of this damage. Here we have assessed lipoperoxidation in the liver. We found a significant increase of this marker in both models of the liver injury as compared to the control group (p < 0.01, ) and a decrease in the animals receiving melatonin as compared to thioacetamide-intoxicated animals. In acute liver injury treated with melatonin, the effect was only marginal but still there was a significant increase in this liver tissue marker (p < 0.01, ) as compared to control group, whereas in chronic liver injury, melatonin prevented the TAA-induced liver damage effectively. These data showed that there is oxidative damage to the membrane of these cells, and that melatonin decreases such damage.
GSH could scavenge the lipid peroxide radicals, lipid hydroperoxides and other products which is a toxic metabolite of thioacetamide. Therefore, we measured the content of GSH in liver tissue of rats. From , we can clearly see the vast difference between thioacetamide-intoxicated group and the control group, and the level of GSH was largely decreased (p < 0.001, ). Our results showed that the recovery of the GSH level was near to normal level in chronic models treated with melatonin compared to acute models treated with melatonin.
3.5 Histological examination
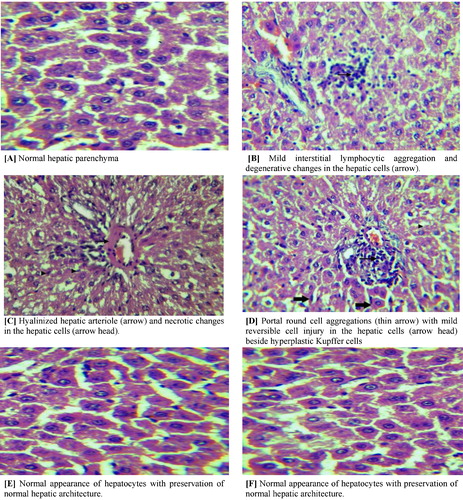
To confirm the protective effect of Melatonin on TAA-induced liver tissue damage, we performed histological examinations (). The normal control rat liver sections showed normal regular cellular architecture of hepatic lobules with distinct hepatic cells, sinusoidal spaces, and a central vein. The hepatic cells displayed prominent nuclei and uniform cytoplasm (A). However, TAA-intoxicated rat liver elicited decrease in the number of hepatocytes, massive hepatocytic necrosis, including bridging necrosis, with influx of inflammatory cells (B and C). The liver sections of the chronic TAA-intoxication revealed extensive damage, characterized by severe necrosis, fatty degeneration, sinusoidal dilatation and congestion, centrilobular necrosis, proliferation of bile duct, presence of collagen bundles surrounding the lobules, which lead to thick fibrotic septae that disrupts the cellular architecture (D). On the other hand, TAA-intoxicated rats administered with Melatonin revealed significant marked regeneration of the hepatocytes with preservation of the normal hepatic architecture, reduced level of necrosis, narrow fibrotic septae, and remarkable increase in bile ductules, fat storing cells, and Kupffer cells (E). Excellent liver recovery was indicated in rats administered with melatonin in chronic model with liver morphology comparable to the control rats. There was minimal disruption of the hepatic cellular structure, very minor fibrotic septae and a low degree of lymphocyte infiltration (F).
4 Discussion
Recently, there has been a growing understanding of the pathophysiology behind fibrosis, which has contributed to the development of agents that could potentially inhibit and even reverse the fibrotic process in the future [Citation37]. Among various hepatotoxins, TAA is known to be the most potent because of its rapid elimination and cumulative injury. The present study showed that TAA administration for 6 weeks induced liver fibrosis with many histopathological alterations. These observations were in agreement with many previous studies, which investigated the induction of liver fibrosis and cirrhosis by TAA in experimental animals [Citation38] and [Citation39].
In the present study, although there were no significant changes in body weights in the different experimental groups except for Groups IV and V (chronic models) and this is consistent with the age, higher liver weights as well as liver body weight ratios had been observed in TAA-treated rats compared to rats in control groups. Measurement of liver body weight ratio is a more accurate approach to determine the changes in liver size compared to the measurement of liver weight alone as the liver weight largely depends on the size of the rat. The enlargement of livers in TAA-treated rats signified hepatic lesions and liver injury associated with the toxic effects of thioacetamide. These significant changes in the liver weights may be attributed to the accumulation of collagen and extra cellular matrix protein in liver tissue [Citation8]. However, the liver enlargement was significantly reduced (p < 0.05) in rats treated with melatonin.
Serum liver biomarkers (ALT, AST) are important criteria for the evaluation of liver toxicity. The amounts of enzymes that leak into the blood stream indicate the severity of hepatic damage [Citation40] and [Citation41]. In the present study, the rats intoxicated with thioacetamide experienced hepatic injury evidenced by significant increase (p < 0.05) in serum liver biomarkers (AST and ALT) when compared to normal control rats. However, melatonin exhibited hepatoprotective effects to attenuate the elevated serum liver parameters, but the effectiveness of melatonin in the attenuation of the elevated liver enzymes was observed more in treated chronic model than treated acute model.
The increased serum levels of AST and ALT are due to the damage to the structural integrity of the liver, since these enzymes are normally located in the cytoplasm and released into the circulation after cellular injury [Citation42]. Also Hajovsky et al. [Citation43] reported that thioacetamide produces free radicals, which affect the cellular permeability of hepatocytes leading to elevated levels of serum biochemical parameters like ALT, and AST.
It was suggested that hepatotoxins including TAA induced liver damage by forming free radicals, which then react with cellular lipids to promote lipid peroxidation [Citation44]. The higher MDA level in TAA control rats observed in the present study also supports this suggestion.
Generally, malondialdehyde (MDA) is used as an index of lipid peroxidation, and lipid peroxidation is postulated as the mechanism of free radical induced tissue injury, hence free radical scavenging is established as the means by which antioxidants inhibit lipid peroxidation. Malondialdehyde has been quantified since the 1960s and is still widely used as a biomarker to detect lipid peroxidation due to the low cost and simplicity of the application [Citation45].
Our results showed that melatonin in both acute and chronic models (3 mg/kg) significantly reduced the hepatic MDA content (). This result is consistent with previous reports that indicated melatonin showed free radical scavenging properties and protective effects on hepatotoxicity induced in rats by thioacetamide [Citation46] and by irradiations [Citation47]. However, the hepatoprotective activity of melatonin in chronic liver injury seemed to be better than to that of melatonin in acute injury. These data show that there is oxidative damage to the membrane of these cells, and that melatonin decreases such damage [Citation48].
Reduced glutathione is an important endogenous antioxidant system that is found in particularly high concentration in liver, and it is known to have key functions in protective processes. The reduced form of GSH becomes readily oxidized to glutathione disulfide (GSSG) on interacting with free radicals. Excessive production of free radicals resulted in the oxidative stress, which leads to damage of macromolecules, e.g. lipids and can induce lipid peroxidation in vivo [Citation49]. In our study, TAA depleted tissue GSH significantly. The post-treatment of animals with melatonin significantly decreased the TAA-induced changes of this oxidative stress marker.
The reductions of endogenous antioxidant enzymes in cirrhotic animals, together with the results of lipoperoxidation, establish a picture of oxidative stress in these animals [Citation50]. Melatonin treatment reduced lipoperoxidation and increased level of reduced glutathione, showing a significant improvement of the oxidative stress in these animals [Citation51–Citation53].
The data lead us to suggest that melatonin decreases the oxidative stress by acting as scavenger of free radicals and providing antioxidant protection of biomolecules. Melatonin significantly reduces the lipoperoxidation levels in liver, and increases the antioxidant substances in the liver tissue.
We suggest that the major reason is that, while the production of the free radicals with various chemical properties in these diseases is widely spread throughout the different tissue and cellular components, the chemical property of an individual antioxidant can only allow it to scavenge the free radicals located in a specific cellular component, e.g., lipid or aqueous phase. Moreover, the efficacy of an antioxidant substance is also dependent on the redox state of the cell. In situations that an imbalanced redox state preexisted, antioxidant treatment will be less, or none, effective [Citation54]. It has been suggested recently that the therapeutic strategy for protecting against oxidative stress will be to target simultaneously the free radicals in both the lipid and the aqueous phases, in extracellular and intracellular spaces [Citation55].
Histologically, in our study, TAA administration produced severe periportal inflammation with pigment deposition and also scattered inflammatory cell infiltration in the hepatic lobule. The post-treatment of animals with melatonin, reversed significantly TAA-intoxicated pathogenic changes in liver. A mild periportal inflammation with no pigmentation or necrosis was observed in the liver sections of animals treated with TAA and melatonin.
As previously mentioned, acute TAA toxicity leads to the prominent changes in the liver tissue architecture including the appearance of necrotic cells and inflammatory cells, mostly macrophages around the central vein. TAA produces centrilobular necrosis with marked accumulation of lipids on acute exposure [Citation56].
5 Conclusion
Based on our results, the concentration of daily 3 mg/kg melatonin is likely to provide significant protection against acute and chronic liver injuries induced by thioacetamide and is a potentially beneficial agent to reduce liver damage by decreasing oxidative stress. Further experiments and clinical trials remain necessary to validate these findings.
Conflict of interest
There is no conflict of interest.
Acknowledgements
We would like to thank all our colleagues for their continuous support to conduct the study. Special thanks to our skillful laboratory technicians and research assistants for their continuous support.
Notes
Peer review under responsibility of Taibah University.
References
- W.E.StehbensOxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zincExperimental and Molecular Pathology7532003265276
- Y.Z.FangS.YangG.WuFree radicals, antioxidants, and nutritionNutrition18102002872879
- T.KitadaS.SekiS.IwaiT.YamadaH.SakaguchiK.WakasaIn situ detection of oxidative DNA damage, 8-hydroxydeoxyguanosine, in chronic human liver diseaseJournal of Hepatology352001613618
- R.Q.GillR.K.SterlingAcute liver failureJournal of Clinical Gastroenterology3332001191198
- W.BernalJ.WendonAcute liver failure: clinical features and managementEuropean Journal of Gastroenterology and Hepatology1191999977984
- T.PoynardJ.MchutchisonM.MannsImpact of Pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis CGastroenterology122200213031313
- S.L.FriedmanSeminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis: mechanisms and treatment strategiesNew England Journal of Medicine328199318281835
- M.PinzaniK.RomboutsLiver fibrosis: from the bench to clinical targetsDigestive and Liver Disease3642004231242
- C.GuyotS.LepreuxC.CombeE.DoudnikoffP.Bioulac-SageC.BalabaudA.DesmouliereHepatic fibrosis and cirrhosis: the (myo) fibroblastic cell subpopulations involvedInternational Journal of Biochemistry and Cell Biology382006135151
- H.MakinoS.TogoT.KubotaD.MoriokaT.MoritaT.KobayashiK.TanakaT.ShimizuK.MatsuoY.NagashimaH.ShimadaA good model of hepatic failure after excessive hepatectomy in miceJournal of Surgical Research1272005171176
- T.M.RahmanA.C.SeldenH.J.HodgsonA novel model of acetaminophen-induced acute hepatic failure in rabbitsJournal of Surgical Research1062002264272
- M.TaniguchiT.TakeuchiR.NakatsukaT.WatanabeK.SatoMolecular process in acute liver injury and regeneration induced by carbon tetrachlorideLife Science75200415391549
- E.CuestaJ.BoadaR.CalafellJ.C.PeralesT.RoigJ.BermudezFructose 1,6-bisphosphate prevented endotoxemia, macrophage activation and liver injury induced by D-galactosamine in ratsCritical Care Medicine342006807814
- V.PallottiniC.MartiniA.M.BassiP.RomanoG.NanniA.TrentalanceRat HMGCoA reductase activation in thioacetamide-induced liver injury is related to an increased reactive oxygen species contentJournal of Hepatology442006368374
- H.V.VadiR.A.NealMicrosomal activation of thioacetamide-S-oxide to a metabolite(s) that covalently binds to calf thymus DNA and other polynucleotidesChemico-Biological Interactions35119812538
- R.D.RekhaA.A.AmaliG.M.Heret alThioacetamide accelerates steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerioToxicology2431-220081122
- A.M.Al-AttarHepatoprotective influence of vitamin C on thioacetamide-induced liver cirrhosis in Wistar male ratsJournal of Pharmacology and Toxicology.632011218233
- M.E.CaballeroJ.BerlangaD.Ramirezet alEpidermal growth factor reduces multiorgan failure induced by thioacetamideGut48120013440
- A.Al-BaderT.C.MathewM.KhoursheedS.AsfarH.Al-SayerH.M.DashtiThioacetamide toxicity and the spleen: histological and biochemical analysisAnatomia, Histologia, Embryologia291200038
- D.MullerM.SommerM.Kretzschmaret alLipid peroxidation in thioacetamide-induced macronodular cirrhosisArchives of Toxicology651991199203
- H.AbulT.C.MathewH.M.DashtiA.Al-BaderLevel of superoxide dismutase, glutathione peroxidase and uric acid in thioacetamide-induced cirrhotic ratsAnatomia, Histologia, Embryologia3120026671
- C.S.LieberM.A.LeoQ.CaoSilymarin retards the progression of alcohol-induced hepatitis in baboonsJournal of Clinical Gastroenterology372003336339
- H.ShapiroM.AshkenaziN.WeizmanM.ShahmurovH.AeedR.BruckCurcumin ameliorates acute thioacetamide-induced hepatotoxicityJournal of Gastroenterology and Hepatology212006358366
- A.PavanatoN.MarroniC.A.MarroniF.LlesuyQuercetin prevents oxidative stress in cirrhotic ratsDigestive Diseases and Sciences52200726162621
- M.AllegraR.J.ReiterD.X.TanC.GentileL.TesoriereM.A.LivreaThe chemistry of melatonin's interaction with reactive speciesJournal of Pineal Research3412003110
- D.X.TanL.C.ManchesterR.J.ReiterB.F.PlummerJ.LimsonS.T.WeintraubW.QiMelatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformationFree Radical Biology and Medicine2911200011771185
- M.H.Abdel-WahabA.R.A.Abd-AllahPossible protective effect of melatonin and/or desferrioxamine against streptozotocin-induced hyperglycaemia in micePharmacological Research412001533537
- E.J.SudnikovichY.Z.MaksimchikS.V.ZabrodskayaV.L.KubyshinE.A.LapshinaM.BryszewskaR.J.ReiterI.B.ZavodnikMelatonin attenuates metabolic disorders due to streptozotocin-induced diabetes in ratsEuropean Journal of Pharmacology5692007180187
- R.J.ReiterD.X.TanM.J.JouA.KorkmazL.C.ManchesterS.D.ParedesBiogenic amines in the reduction of oxidative stress: melatonin and its metabolitesNeuro Endocrinology Letters292008391398
- F.PeyrotC.Houee-LevinC.DucrocqMelatonin nitrosation promoted by NO2: comparison with the peroxynitrite reactionFree Radical Research402006910920
- R.J.ReiterD.X.TanM.D.MaldonadoMelatonin as an antioxidant: physiology versus pharmacologyJournal of Pineal Research392005215216
- K.WiniarskaT.FraczykD.MalinskaJ.DrozakJ.BrylaMelatonin attenuates diabetes-induced oxidative stress in rabbitsJournal of Pineal Research402006168176
- V.TahanR.OzarasB.CanbakanH.UzunS.AydinB.YildirimH.AytekinG.OzbayA.MertH.SenturkMelatonin reduces dimethylnitrosamine-induced liver fibrosis in ratsJournal of Pineal Research3720047884
- R.T.HongJ.M.XuQ.MeiMelatonin ameliorates experimental hepatic fibrosis induced by carbon tetrachloride in ratsWorld Journal of Gastroenterology1512200914521458
- H.OkuyamaY.ShimaharaN.KawadaS.SekiD.B.KristensenK.YoshizatoN.UyamaY.YamaokaRegulation of cell growth by redox-mediated extracellular proteolysis of platelet-derived growth factor receptor betaJournal of Biological Chemistry27620012827428280
- H.M.DashtiT.C.MathewM.M.JadaonE.AshkananiZinc and liver cirrhosis: biochemical and histopathologic assessmentNutrition1331997206212
- V.BhatM.BhatHepatic fibrosis: novel strategies in detection and therapyMcGill Journal of Medicine11120083840
- A.I.MirB.KumarS.A.TasduqD.K.GuptaS.BhardwajR.K.JohriReversal of hepatotoxin-induced pre-fibrogenic events by Emblica officinalis – a histological studyIndian Journal of Experimental Biology4572007626629
- J.F.LiB.C.ChenD.D.Laiet alSoy isoflavone delays the progression of thioacetamide-induced liver fibrosis in ratsScandinavian Journal of Gastroenterology4632011341349
- C.Z.NkosiA.R.OpokuS.E.TerblancheEffect of pumpkin seed (Cucurbitapepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low-protein fed ratsPhytotherapy Research1942005341345
- K.Abul NajmiK.K.PillaiS.N.PalM.AkhtarM.AqilM.SharmaEffect of l-ornithine l-aspartate against thioacetamide-induced hepatic damage in ratsIndian Journal of Pharmacology4262010384387
- J.Y.ShimM.H.KimH.D.KimJ.Y.AhnY.S.YunJ.Y.SongProtective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory responseToxicology and Applied Pharmacology2422010318325
- H.HajovskyG.HuY.KoenD.SarmaW.CuiD.S.MooreJ.L.StaudingerR.P.HanzlikMetabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytesChemical Research in Toxicology259201219551963
- Z.A.FadhelS.AmranEffects of black tea extract on carbon tetrachloride-induced lipid peroxidation in liver, kidneys, and testes of ratsPhytotherapy Research16102002S28S32
- J.LykkesfeldtMalondialdehyde as biomarker of oxidative damage to lipids caused by smokingClinica Chimica Acta3801-220075058
- A.CruzF.J.PadilloE.TorresC.M.NavarreteJ.R.Muñoz-CastañedaF.J.CaballeroJ.BriceñoT.MarchalI.TúnezP.MontillaC.PeraJ.MuntanéMelatonin prevents experimental liver cirrhosis induced by thioacetamide in ratsJournal of Pineal Research3922005143150
- A.ShiraziE.MihandoostG.GhobadiM.MohseniM.Ghazi-KhansariEvaluation of radio-protective effect of melatonin on whole body irradiation induced liver tissue damageCell Journal1442013292297
- G.Pereira-FilhoC.FerreiraA.SchwengberC.MarroniC.ZettlerN.MarroniRole of N-acetylcysteine on fibrosis and oxidative stress in cirrhotic ratsArquivos de Gastroenterologia452008156162
- A.J.SinclairA.H.BarnettJ.LunieFree radical and auto-oxidant systems in health and diseaseJournal of Applied Medicine171991409412
- H.G.OsmanO.M.GabrS.LotfyS.GabrSerum levels of bcl-2 and cellular oxidative stress in patients with viral hepatitisIndian Journal of Medical Microbiology252007323329
- R.KireevS.BitounS.CuestaA.TejerinaC.IbarrolaE.MorenoE.VaraJ.A.TresguerresMelatonin treatment protects liver of Zucker rats after ischemia/reperfusion by diminishing oxidative stress and apoptosisEuropean Journal of Pharmacology7011–32013185193
- P.N.MartinsJ.F.MarkmannAge-related differences in hepatic ischemia/reperfusion: gene activation, liver injury, and protective effect of melatoninJournal of Surgical Research1782012922934
- C.AktasM.KanterM.ErbogaR.MeteM.OranMelatonin attenuates oxidative stress, liver damage and hepatocyte apoptosis after bile-duct ligation in ratsToxicology and Industrial Health2012 (Epub ahead of print)
- S.J.FloraM.PandeA.MehtaBeneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiolchelators in the treatment of chronic lead intoxicationChemico-Biological Interactions14532003267280
- G.VendemialeI.GrattaglianoE.AltomareAn update on the role of free radicals and antioxidant defense in human diseaseInternational Journal of Clinical & Laboratory Research29219994955
- R.A.NealJ.HalpertToxicity of thionosulfur compoundsAnnual Review of Pharmacology and Toxicology221982321329