Abstract
Florfenicol is a broad-spectrum, primarily bacteriostatic, antibiotic with a range of activity including many gram-negative and gram-positive organisms. This study was carried out to determine the in vivo effect of florfenicol on the paraoxonase (PON), catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPX) activities on Morkaraman normal and lactating sheep. For these studies, three normal and three lactating sheep groups (55–60 kg) were selected for each of intramuscular administration for 24 h of florfenicol (30 mg/kg). Three normal and three lactating sheep groups were included in the study for a control group, which were not subjected to drug administration. For florfenicol, the mean of the hemolysate paraoxonase, glutathione peroxidase, superoxide dismutase, catalase activities and milk paraoxonase, catalase, lactoperoxidase, superoxide dismutase activity was determined and compared to the control group. According to the research results, while PON1 and CAT enzymes were activated, SOD and GPX enzymes were inhibited by florfenicol in both normal and lactating Morkaraman sheep. While florfenicol did not change milk PON1 and SOD activities, it significantly inhibited milk CAT and LPO enzyme activities.
1 Introduction
Florfenicol (2,2-dichloro-N-((1R,2S)-3-fluoro-1-hydroxy-1-(4-(methylsulfonyl)phenyl) propan-2-yl)ethanamide) has been demonstrated to be active in vitro and in vivo against many gram-negative and gram-positive organisms [Citation30]. In the treatment of bovine respiratory disease, florfenicol may be considered as bactericidal agent against some Mannheimia (Pasteurella) hemolytica and Pasteurella multocida when it is administered to achieve minimum inhibitory concentrations (MICs) [Citation7]; the minimum bactericidal concentrations (MBCs) are very close to the MICs.
There are also oxygen and reactive nitrogen species as well as superoxide radical, hydrogen peroxide and hydroxyl radicals in the body. Radicalic and reactive intermediates are chemically very active and they can oxidize nucleic acids, proteins and lipids in the environment leading to the reduction and elimination of them in their biological functions can create negative consequences in the body [Citation17]. Against free radicals produced by the organism itself and the toxic effects of normal oxygen metabolism endogenous antioxidant system consists of antioxidant enzymes, catalase (CAT), paraoxonase (PON), glutathione peroxidase (GPX) and superoxide dismutase (SOD). These antioxidative enzymes prevent resulting/possible oxidative damages by eliminating radicals and reactives. They also take task in detoxification of xenobiotics, some antineoplastic drugs and certain metabolic end-products and enzymatic defense systems [Citation3,Citation13,Citation24,Citation26,Citation28,Citation34].
The enzymatic antioxidant defenses include paraoxonase (PON), superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT). Subsequent studies have shown that PON enzyme (paraoxonase/arylesterase) (PON; aryldialkylphosphatase, EC 3.1.8.1) is an organophosphatase with broad substrate specificity, including aromatic carboxylic acid esters such as phenyl acetate [Citation6,Citation27]. The first functions of paraoxonases are hydrolysis of toxic organophosphates. PON can also be hydrolyzed to the carbonate esters, aromatic lactone, statin type drugs and pharmaceutical substances. In addition, PONs play a role in preventing the oxidation of LDL and the prevention of atherosclerosis, hypercholesterolemia, diabetes, and coronary vascular disease [Citation14,Citation19–Citation21,Citation23,Citation29].
Catalase (CAT, H2O2: H2O2 oxidoreductase; EC 1.11.1.6) is one of the principle antioxidant enzymes. In the presence of molecular oxygen, the primary functions of the CAT are catalyzed of dismutation reaction of a peroxide such as hydrogen peroxide and ROOH is synthesized in some positions of metabolism. It also especially prevents from irreversible damage in membranes [Citation11,Citation22].
Superoxide dismutase (EC 1.15.1.1) is also an antioxidant enzyme that catalyses the dismutation of the highly reactive superoxide anion to O2 and H2O2 [Citation5,Citation17,Citation32]. SOD families include cytosolic Cu, Zn-SOD, mitochondrial Mn-SOD and extracellular Cu, Zn-SOD (ECSOD).
SOD plays a major role in the first line of antioxidant defense and high SOD activities are correlated with high immune competence [Citation31].
GPX (EC 1.11.1.19) catalyses the reaction of hydroperoxides using GSH, protecting mammalian cells against oxidative damage [Citation1].
Reactive oxygen species (ROS) such as the superoxide anion (O2−) and hydrogen peroxide (H2O2), have been implicated in many of the events leading to the development of diseases such as cancer, allergy, atherosclerosis, and Alzheimer's disease [Citation4,Citation12,Citation15]. Peroxidase acts as preventive antioxidants to detoxify damaging from blood and organic substrates [Citation4].
As decreased activity of PON, GPX, LPO, SOD and CAT has been acknowledged as a risk factor for cancer, allergy, coronary vascular disease, organophosphate toxicity, damaging to the structure of the membrane and DNA the factors affecting antioxidative enzymes activities must be well addressed.
While sheep are producing milk, nutrient requirements of sheep are especially high and their blood flows increases during lactation. Although florfenicol is widely used in sheep, their effects on the antioxidant enzymes activities of lactating and non-lactating sheep are not known. It is hypothesized that sheep producing milk eliminates the effects of florfenicol with antioxidative enzymes more than sheep not producing milk during lactation. Although florfenicol are being used commonly in sheep, the exact effect of this drug on paraoxonase, glutathione peroxidase, superoxide dismutase, catalase has been unknown in therapies on lactating and non-lactating sheep. There is also a deep need of understanding of the impact of commonly used this drug on the activity of these antioxidative enzymes. Therefore, the aim of this study was to examine the effects of commonly used florfenicol on normal and lactating sheep PON, GPX, LPO, SOD and CAT activities in vivo on lactating and non-lactating sheep.
2 Experimental
2.1 Chemical and reagent
All chemicals used in this study were obtained from Sigma Chem. Co and Merck (Germany) and they were analytical grade.
2.2 Animals, experimental design and sample collection
In this study, 12 mature clinically healthy Morkaraman sheep (n = 12) were used. The sheep had an average weight of 55–60 kg. Sheep were housed during 15 days in stables of Ataturk University. This animal experiment was approved ethically with protocol in Ethical Committee of Ataturk University.
We allocated 12 sheep (36 weeks old) to four groups of 3 animals: (1) normal control sheep, (2) lactating control sheep, (3) intramuscular administration with florfenicol normal sheep, and (4) intramuscular administration with florfenicol lactating sheep.
It was composed four groups, first group of three Morkaraman normal sheep and second group of three lactating Morkaraman sheep were selected for each of intramuscular administration for 24 h of florfenicol (30 mg/kg). Third and fourth groups of three Morkaraman normal sheep and three Morkaraman lactating sheep were included for a control group, which were not subject to any drug administration. Florfenicol are generally used in dose of 20–40 mg/kg. Therefore, we used a dose rate of 30 mg/kg in this study [Citation8].
Blood and milk samples were taken from normal and lactating Morkaraman sheep during 24 h. While blood samples were taking from normal and lactating sheep, milk samples were taken from lactating sheep. The blood samples were collected from the jugular vein of each sheep at 0.5, 0.75, 1, 2, 4, 6, 8, 12, 18, and 24 h after drug administration in tubes containing heparin. Then, serum was separated by centrifugation to serum and erythrocytes. Serum, erythrocytes and milk samples were kept at −80 °C until analysis. Enzyme activities were not affected by freezing and storage at −80 °C.
2.3 Measurement of PON1 activity
Paraoxonase activity was measured using paraoxon as a substrate by spectrophotometrical method. All rates were determined in triplicate and corrected for the non-enzymatic hydrolysis. Reaction was started by the addition of 50 μL of serum and it was followed for 5 min at 37 °C by monitoring the appearance of p-nitrophenol at 412 nm in a T80 UV/VIS Spectrophotometer (PG Instrument Ltd.) [Citation18].
2.4 Measurement of CAT activity
Catalase activity was measured in the hemolysate. The catalase activity was determined at 25 °C for 3 min with the substrate H2O2 according to the Aebi method [Citation9]. The rate of disappearance of H2O2 per minute in absorbance at 240 nm was determined [Citation9].
2.5 Measurement of SOD activity
The activity of superoxide dismutase was measured by recording the decrease in optical density of nitro-blue tetrazolium (NBT) dye by hemolysate or milk in triplicate. Three milliliters of the reaction mixture contained, 2 μM riboflavine, 13 mM methionine, 75 μM NBT, 0.1 mM EDTA, 50 mM phosphate buffer (pH 7.8), 50 mM sodium carbonate and 25 mL the hemolysate or milk sample. Reaction was started by adding 60 μL from 100 μM riboflavin solution and placing the tubes under two 30 W fluorescent lamps for 15 min, then the reaction was stopped by the switching off the light and putting the tubes into dark and the changes in absorbance was determined at 560 nm. The amount of enzyme required to inhibit the reduction of NBT by 50% under the specified conditions was defined as one unit of SOD activity [Citation34].
2.6 Measurement of GPX activity
Glutathione peroxidase (GPX) catalyzes the oxidation of glutathione using tert-butyl hydroperoxide. Oxidized glutathione was converted to the reduced form in the presence of glutathione reductase and NADPH which was oxidized to NADP. The absorbance of NADPH was measured at 340 nm. The absorbance change per minute and the molar extinction coefficient of NADPH are used as 6.22 × 10−3 mol−1 L cm−1 to calculate GPX activity, expressed as unit per gram of hemoglobin (U/g Hb) [Citation25].
2.7 Measurement of LPO activity
LPO activities were determined by modified the procedure of Shindler and Bardsley [Citation16]. The product was obtained from the oxidation of ABTS as a chromogenic substrate by H2O2 and the charge in absorbance was determined at 412 nm during 3 min for per minute. One unit of enzyme was defined as the amount of enzyme catalyzing the oxidation of 1 μmol of ABTS min−1 at 298 K.
2.8 Statistical analysis
Statistical analysis was performed by using Minitab program for Windows, version 1002 Analysis of variance, ANOVA and mean comparisons were performed by using Duncan's multiple range test. Data were presented as mean-SD. Data were analyzed by using the independent t-test. Statistical significance was considered at p < 0.05.
3 Results and discussion
Florfenicol is useful for the prevention and treatment of bacterial infections due to susceptible pathogens in birds, reptiles, fish, shellfish and mammals. It is a broad-spectrum antibiotic with activity against many gram-negative and gram-positive bacteria. In this study, investigation of effects of florfenicol on normal and lactating sheep's serum PON, erythrocyte CAT, SOD, GPX and milk PON, CAT, SOD, LPO was proposed.
Florfenicol (30 mg/kg) was separately administrated to two groups of three sheep (55–60 kg) for Morkaraman normal and lactating sheep as intramuscularly for 24 h. The results of in vivo effects of the florfenicol as intramuscular administration on Morkaraman normal sheep serum PON, erythrocyte CAT, SOD and GPX are presented in .
Fig. 1 Changes of in activities of serum PON, erythrocyte CAT, SOD and GSH-Px activity of administrated with florfenicol (30 mg kg−1) as intramuscularly on normal Morkaraman sheep.
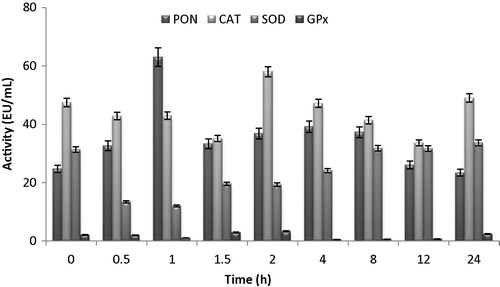
PON enzyme activity was increased in taken serum and milk samples from normal sheep after 1 h. The highest activation was observed in the serum samples taken from normal sheep in 90th minute. Florfenicol was activated to CAT enzyme activity in normal sheep in 4th hour, but it did not change too much. Erythrocyte SOD enzyme was inhibited from 31.35 ± 0.92 EU/mL to 13.4 ± 1.98 EU/mL according to control by Florfenicol in the first half hour. SOD enzyme activity has recovered after 12 h. Florfenicol inhibited to the GPX enzyme activities after 8 h as well. The level of the highest inhibition for GPX enzyme was observed at 8th hour as 0.5 ± 0.03 EU/mL (). The values p < 0.05 were considered significant.
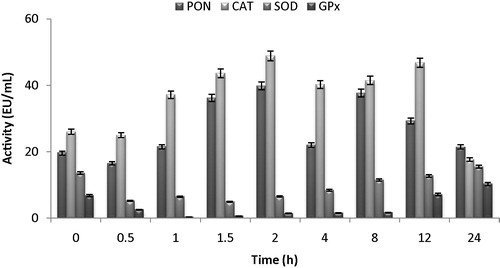
The effects of florfenicol on the activities of PON, CAT, SOD and GPX enzymes in the lactating sheep were presented in . PON and erythrocyte CAT enzymes were activated in taken serum samples from lactating sheep which was applied florfenicol. The highest activation values were determined for PON1 from 24.75 ± 0.78 EU/mL to 33.3 ± 0.28 EU/mL in the 2nd hour and CAT enzyme from 47.5 ± 1.13 EU/mL to 58.05 ± 0.78 EU/mL. Erythrocyte SOD and GPX enzymes were inhibited. While SOD was inhibiting from 31.35 ± 0.92 EU/mL to 19.6 ± 0.00 EU/mL as level of 64% in the 2nd hour, GPX was inhibited from 2.14 ± 0.65 EU/mL to 0.5 ± 0.03 EU/mL as level of 95%. According to the results observed, florfenicol had inhibited to the erythrocyte GPX enzyme from the antioxidant enzyme of lactating Morkaraman sheep at highest level (). The results showed that florfenicol effects on the activities of antioxidant enzymes are similar in both normal and lactating sheep. However, it was determined that in normal sheep, PON and CAT enzymes were activated more in less time.
Fig. 2 Changes of in activities of serum PON, erythrocyte CAT, SOD and GSH-Px activity of administrated with florfenicol (30 mg kg−1) as intramuscularly on lactating Morkaraman sheep.
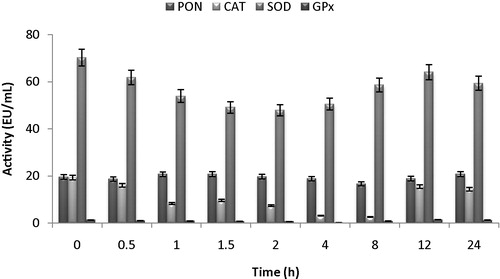
While florfenicol drug did not affect the milk PON and SOD enzyme activities, the drug was inhibited to the activities of CAT and LPO enzymes in the taken samples from lactating sheep. In the 12th hour, milk CAT enzyme activity was inhibited from 19.33 ± 2.42 EU/mL to 2.66 ± 0.31 EU/mL according to control. Milk LPO was also inhibited from 1.34 ± 0.34 EU/mL to 0.264 ± 0.085 EU/mL and it was determined that the activities of both enzymes were gained back their activities after 18 h (). In addition, it was determined from observed data that milk samples had also the highest SOD enzyme activity. The effect of florfenicol on normal serum antioxidant enzymes activity was affected at different rates from lactating serum antioxidant enzymes activity.
Fig. 3 Changes of in activities of milk PON, CAT, SOD and LPO activity of administrated with florfenicol (30 mg kg−1) as intramuscularly on lactating Morkaraman sheep.
There are some researches in literature about the effects of various drugs on PON1 enzyme activity [Citation26,Citation33]. For example, inhibitory effects of glimepiride and acetylsalicylic acid on rat were investigated. It was observed that these drugs significantly inhibited the enzyme activity [Citation26,Citation33]. However any reports could not be found about the effect of florfenicol on the activity of PON 1 of sheep. Thus, we analyzed the alterations on PON1 activity from serum of normal and lactating Morkaraman sheep at dosage of 30 mg/kg for each sheep as in vivo ( and ). We observed that both normal and lactating sheep's serum PON1 activity was increased, but milk PON1 was not effected by florfenicol drug.
Aydin et al. [Citation10] reported the effect of carazolol on catalase and superoxide dismutase in sheep. Both SOD and catalase enzymatic activity failed to exhibit any changes according to control group. There were not enough investigations on sheep CAT and SOD enzymes, so, we aimed to investigate whether they were effected or not by florfenicol. In this research, while erythrocyte CAT was activated, milk CAT enzyme was inhibited in taken samples from normal and lactating sheep (). While SOD was inhibited in normal and lactating sheep, milk SOD enzyme was not affected. CAT and SOD have high affinities and rates of reactions with ROS. For this reason, it may be thought that these enzymes afford more effective protection against acute massive oxidative results, such as inflammation.
The effects of sulfur and molybdenum were investigated on glutathione peroxidase activity at different dietary concentrations in sheep by Abdel-Rahim et al. [Citation2]. They observed that there were probably no major effects of sulfur and selenium on glutathione peroxidase in the sheep. We aimed in the current study to analyze the effect of florfenicol on erythrocyte GPX and milk LPO in normal and lactating sheep. It was also observed from this research that sheep erythrocyte GPX and milk LPO were strongly inhibited in both normal and lactating sheep.
4 Conclusion
PON, CAT, SOD, GPX and LPO activities have a substantial impact on the risk of cancer, organophosphate poisoning, developing cardiovascular disease, damaging cell and DNA. It is thought that more extensive inhibition studies are necessary for a better understanding of the protective role of antioxidative enzymes against the toxic effects of xenobiotics, including environmental heavy metals and oxidative stress by-products. However, there are only few studies regarding effects of drugs on antioxidative enzymes activities in literature. More detailed structure-function analyses are indicated to determine the relationship between its PON, CAT, SOD, GPX and LPO and their antioxidative actions. For this purpose, we analyzed the in vivo influences of commonly used antibacterial, namely florfenicol, on the activity of normal and lactating sheep PON, CAT, SOD, GPX and LPO.
In conclusion, analysis of the data extracted from this study, can obviously present florfenicol as an antibiotic for the safe use in treating bacterial infections. Despite the well known fact that antibiotics are inhibitors or activators, our results have confirmed that the marked diminish or increasing of antioxidative enzyme activities but the activities of all antioxidative enzymes were gained back their activities after 18 h in both normal and lactating sheep.
Notes
Peer review under responsibility of Taibah University.
References
- A.B.SigalovL.J.SternEnzymatic repair of oxidative damage to human apolipoprotein AIFEBS Letters4331998196200
- A.G.Abdel-RahimJ.R.ArthurF.C.MillsSelenium utilization by sheep given diets differing in sulfur and molybdenum contentBiological Trace Element Research821985145155
- A.MazurAn enzyme in animal tissues capable of hydrolysing the phosphorus fluorine bond of alkyl fluorophosphatesJournal of Molecular Biology1641946271289
- B.HalliwellJ.M.C.GutteridgeFree Radicals in Biology and Medicine2nd edn.1998Clarendon PressOxford pp. 134–146, 165–169, 456–457
- B.HalliwellJ.M.C.GutteridgeThe anti-oxidants of human extracellular fluidsArchives of Biochemistry and Biophysics280199018
- B.N.La DuW.KalowPharmacogenetics of Drug Metabolism1992PergamonElmsford, NY5191
- D.J.WilsonP.M.SearsR.N.GonzalezEfficacy of florfenicol for treatment of clinical and subclinical bovine mastitisAmerican Journal of Veterinary Research5741996526528
- F.KocK.UneyM.OzturkY.KadiogluA.AtilaPharmacokinetics and bioavailability of florfenicol in the plasma of Japanese quailsNew Zealand Veterinary Journal572009388391
- H.AebiCatalase in vitro assay methodsMethods Enzymology1051984121126
- H.AydinM.C.GunduzH.D.YardibiEffect of carazolol on plasma molondialdehyde, superoxide dismutase and catalase in sheepsJournal of Animal Veterinary Advances842009771773
- H.N.KirkmanG.F.GaetaniCatalase: a tetrameric enzyme with four tightly bound molecules of NADPHProceedings of the National Academy of Sciences United States of America81198443434347
- H.NadarogluN.DemirIn vivo effects of chlorpyrifos and parathion methyl on some oxidative enzyme activities in chickpea, bean, wheat, nettle and parsley leavesFresenius Environmental Bulletin1852009647652
- I.FridovichSuperoxide radical and superoxide dismutaseAccounts of Chemical Research51972321323
- J.A.BerlinerJ.W.HeineckeThe role of oxidized lipoproteins in atherogenesisFree Radical Biology and Medicine201996707727
- J.P.KehrerFree radicals as mediators of tissue injury and diseaseCritical Reviews in Toxicology2319932148
- J.S.ShindlerW.BardsleyStead-state kinetics of lactoperoxidase with ABST as chromogensBiochemical and Biophysical Research Communications67197513071312
- J.M.McCordHuman disease, free radicals, and the oxidant/anti-oxidant balanceClinical Biochemistry261993351357
- K.N.GanA.SmolenH.W.EckersonB.N.La DuPurification of human serum paraoxonase/arylesteraseDrug Metabolism and Disposition191991100106
- M.C.BlatterR.W.JamesS.MessmerF.BarjaD.PomettaIdentification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45: identity of K-45 with paraoxonaseEuropean Journal of Biochemistry2111993871879
- M.I.MacknessS.ArrolC.AbbottP.N.DurringtonProtection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonaseAtherosclerosis1041993129135
- M.I.MacknessS.ArrolP.N.DurringtonParaoxonase prevents accumulation of lipoperoxides in low-density lipoproteinsFEBS Letters2861991152154
- M.J.MateM.ZamockyL.M.NykyriC.HerzogP.M.AlzariC.BetzelF.KollerI.FitaStructure of catalase A from Saccharomyces cerevisiaeJournal of Molecular Biology2861999135149
- M.NavabS.Y.HamaT.B.NguyenA.M.FogelmanMonocyte adhesion and transmigration in atherosclerosisCoronary Artery Disease51994198204
- M.SalviV.BattagliaA.M.BrunatiN.La RoccaE.TibaldiP.PietrangeliL.MarcocciB.MondoviC.A.RossiA.ToninelloCatalase takes part in rat liver mitochondria oxidative stress defenseJournal of Biological Chemistry2823320072440724415
- N.AvissarJ.R.SlemmonI.S.PalmerH.J.CohenPartial sequence of human plasma glutathione peroxidase and immunologic identification of milk glutathione peroxidase as the plasma enzymeJournal of Nutrition121199112431249
- N.DemirH.NadarogluY.DemirPurification of paraoxonase human serum and effect of acetylsalicylic acid on paraoxonase activity in vitro and rat serum liver, and heart in vivoPharmaceutical Biology4662008393399
- N.DemirH.NadarogluY.DemirPurification of paraoxonase (pon1) from olive (Olea europaea L.) and effect of some chemicals on paraoxonase activity in vitroAsian Journal of Chemistry236201125842588
- N.H.P.CnubbenI.M.C.M.RietjensH.WortelboerJ.Van ZandenP.J.Van BladerenThe interplay of glutathione-related processes in antioxidant defenseEnvironmental Toxicology and Pharmacology102001141152
- P.A.CeruttiOxidant stress and carcinogenesisEuropean Journal of Biochemistry21199115
- R.D.LobellK.J.VarmaJ.C.JohnsonPharmacokinetics of florfenicol following intravenous and intramuscular doses to cattleJournal of Veterinary Pharmacology and Therapeutics171994253258
- T.PrasadM.S.KunduSerum IgG and IgM responses to sheep red blood cells (SRBC) in weaned calves fed milk supplemented with Zn and CuNutrition111995712715
- W.A.PryorOxy-radicals and related species: their formation, lifetimes, and reactionsAnnual Review of Physiology481986657667
- Y.DemirH.NadarogluN.DemirEffect of glimepride on human serum paraoxanase activity in vitro and rat serum, liver and heart in vivoPharmaceutical Biology4452006396399
- Y.SunL.W.OberleyY.LiA simple method for clinical assay of superoxide dismutaseClinical Chemistry341988497500