?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A convenient synthesis of a new series of nano steroidal pyrazolones is reported. They were characterized by X-ray diffraction, scanning electron microscopy, UV–vis light, Fourier transform-infrared spectroscopy, 1H and 13C nuclear magnetic resonance, mass spectroscopy and analytical data. The X-ray diffraction patterns at room temperature showed that two nano steroids, 7 and 8, are formed in single phase with hexagonal crystal symmetry, while nano steroid 9 is formed with orthorhombic crystal symmetry. Compounds 1–3 are formed with hexagonal, monoclinic and tetragonal crystal symmetry, respectively. Scanning electron microscopy showed that the crystals of nano steroids 7–9 are brick-shaped agglomerates with less sharp edges and a rough surface with a few microcrystals, while the homogeneous crystal of nano steroid 3 has an approximate spherical morphology of nano particles conjoined to the chains. UV–vis absorption analysis showed that the band gap energy of nano steroids 7 and 8 was 3.70 eV and 4.61 eV, while that of 2 and 3 was 4.38 eV and 4.27 eV, respectively. Nano steroids 2, 3 and 7–9 were screened for antimicrobial activity against various strains; nano compound 7 showed potential antimicrobial activity.
1 Introduction
Self-assembly of organic compounds with cholesteryl groups has proved to be an attractive field in nanotechnology research. Some steroid derivatives are known to form ordered structures, which indicate thermotropic and lyotropic liquid crystalline, monolayers, multilayers and micelles [Citation1]. As steroids are important constituents of most eukaroytic cell membranes, a great deal is known about certain aspects of their function. In model systems, cholesterol is distributed evenly on both sides of the bilayer, with its polar hydroxyl group held in the vicinity of the phosphate groups of phospholipids [Citation2,Citation3]. The molecule undergoes rapid uncatalysed transmembrane and inter-membraneous transfer [Citation4,Citation5]. In addition, the bulk effects of cholesterol on membrane phase transitions and, as a consequence, on membrane fluidity and permeability have been well documented [Citation6,Citation7].
Table 2 The XRD parameters of the nano compounds 7–9 prepared in this study.
Cholesterol derivatives are broadly categorized as hydrophobic or amphiphilic on the basis of the chemical subunits that are present in the molecule. The ordered arrays of cholesterol molecules or mesogens result in the formation of liquid crystalline mesophases, during which orientation order arises from parallel arrangement of cholesterol, while positional order is obtained from attractive forces that hold the assembly together [Citation8–Citation11].
X-ray diffraction (XRD) is the most widely used technique for determining the phase, crystal structure and lattice parameter of crystalline solids and is an appropriate technique for all types of sample, i.e. powder and bulk as well as thin film. This technique can be used to obtain information on the crystalline nature of a compound, the nature of the phase present, the lattice parameter and grain size [Citation12]. We attempted to synthesize new steroidal derivatives and study their physical properties and report the synthesis of new steroidal pyrazolones and their physical properties. We also report the in vitro antimicrobial activity of some known steroid compounds, 3β-acetoxycholest-5-ene and 3β-acetoxy-6-nitrocholest-5-ene.
2 Materials and methods
Cholesterol (3β-hydroxycholest-5-ene) was purchased from Sigma. All other reagents and solvents were obtained from best-known commercial sources and were purified, dried or freshly distilled as required according to the specified literature procedure [Citation13]. Melting-points (mp) were recorded on a Kofler apparatus. Infrared (IR) spectra were recorded on KBr pellets with a Perkin Elmer RXI Spectrometer (without microscope) and given in cm−1. 1H and 13C nuclear magnetic resonance (NMR) spectra were run in CDCl3 on a JEOL Eclipse (400 MHz) instrument with tetramethylsilane as the internal standard; values are given in ppm (δ). Mass spectra were recorded on a JEOL SX 102/DA-6000 mass spectrometer. Thin-layer chromatography plates were coated with silica gel G and exposed to iodine vapours to check the homogeneity and the progress of reaction. Sodium sulphate (anhydrous) was used as the drying agent.
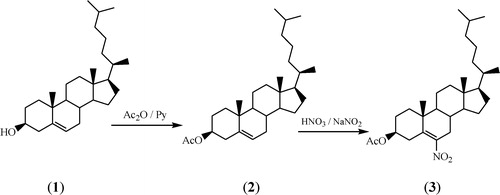
2.1 Synthesis of 3β-acetoxycholest-5-ene (2)
A mixture of cholesterol 1 (12.5 mg), pyridine (16.25 ml) and acetic anhydride (12.5 ml) was heated in a water bath for 2 h under anhydrous conditions. After completion of the reaction, the mixture was poured into ice-cold water, and a solid mass was obtained. It was filtered under suction, washed thoroughly with water and air-dried. Recrystallization from acetone provided pure product 2, mp 114 °C, reported mp, 115–116 °C [Citation14].
2.2 Synthesis of 3β-acetoxy-6-nitrocholest-5-ene (3)
To a cooled mixture of 3β-acetoxycholest-5-ene (8 g) and nitric acid (100 ml, d 1.41), sodium nitrite (8 g) was added gradually with constant stirring over about 45 min. After complete addition, stirring was continued for an additional 2 h. Cooled water (150 ml) was added to the reaction mixture, and the yellow solid thus separated out was placed in diethyl ether, washed with water and NaHCO3 (5%) until the washing was pink and finally dried in anhydrous sodium sulphate. Removal of solvents gave an oil, which was recrystallized from methanol to give the pure product 3, mp, 104 °C, reported mp 103–104 °C [Citation15].
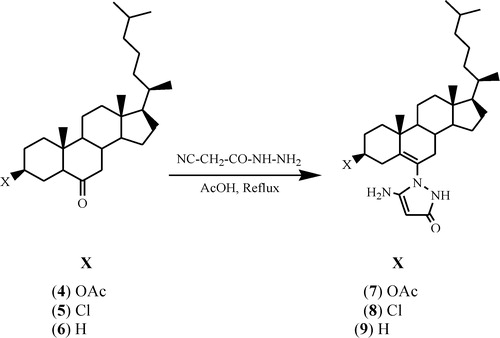
2.3 General synthesis of steroidal pyrazolones (7–9)
Cyanoacetohydrazide was added in an equimolar ratio to a solution of steroidal ketones 4–6 (1 mmol) in acetic acid (15 ml) in the same solvent. The reaction mixture was stirred under refluxing for 7 h. The progress of the reaction was monitored by thin-layer chromatography. After completion of the reaction, the excess solvent was removed to three fourths of the original volume under reduced pressure. The reaction mixture was washed with water (30 ml), neutralized with saturated aqueous NaHCO3 and again washed with water and taken in ether. The ethereal layer was further washed with water and dried over anhydrous sodium sulphate. Removal of the solvent gave the crude product, which was recrystallized from methanol to furnish the corresponding steroidal pyrazolones 7–9.
2.3.1 3β-Acetoxy-cholest-6[5′-amino-1′,2′-dihydropyrazol-3-one-1′-yl]5-ene (7)
White solid, yield (80%); mp, 145 °C; calculated analysis of C32H51N3O3: C, 73.04, H, 9.65, N, 7.95; found: C, 73.14, H, 9.71, N, 8.0; IR (KBr, υ cm−1): 3345, 3280 (NH, NH2), 1735 (OCOCH3), 1680 (CONH), 1625, 1617 (C=C), 1210 (C–O), 1455 (C–N), 1480 (N–N); 1H NMR (CDCl3, 400 MHz): δ 2.2 (s, 2H, NH2, exchangeable with D2O), 9.0 (s, 1H, NH, exchangeable with D2O), 5.2 (s, 1H, CH), 4.7 (m, 1H, C3 α-H, W ½ = 15 Hz), 2.01 (s, 3H, OCOCH3), 1.12 (s, 3H, C10–CH3), 0.71 (s, 3H, C13–CH3), 0.92 and 0.85 (other methyl protons); 13C NMR (CDCl3, 100 MHz): δ 171.1 (CO), 165.4 (C3′), 159 (C5′), 135 (C6),119 (C5), 83.2 (C4′), 70.2 (C3); MS: m/z 525 [M+•].
2.3.2 3β-Chloro-cholest-6[5′-amino-1′,2′-dihydropyrazol-3-one-1′-yl]5-ene (8)
White solid, yield (75%); mp, 131 °C; calculated analysis of C30H48ClN3O: C, 72.32, H, 9.58, N, 8.32. found: C, 72.14, H, 9.61, N, 8.41; IR (KBr, υ cm−1): 3375, 3291 (NH, NH2), 1685 (CONH), 1621, 1618 (C=C), 1448 (C–N), 1467 (N–N), 741 (C–Cl); 1H NMR (CDCl3, 400 MHz): δ 2.3 (s, 2H, NH2, exchangeable with D2O), 8.5 (s, 1H, NH, exchangeable with D2O), 5.4 (s, 1H, CH), 3.9 (1H, m, C3 α-H, W ½ = 17 Hz), 1.12 (s, 3H, C10–CH3), 0.71 (s, 3H, C13–CH3), 0.92 and 0.85 (other methyl protons); 13C NMR (CDCl3, 100 MHz): δ 162.3 (C3′), 154.7 (C5′), 136 (C6), 117 (C5), 84.4 (C4′), 50.6 (C3); MS: m/z 499/501 [M+•].
2.3.3 Cholest-6[5′-amino-1′,2′-dihydropyrazol-3-one-1′-yl]5-ene (9)
White solid, yield (70%); mp, 119 °C; calculated analysis of C30H49N3O: C, 77.01, H, 10.41, N, 8.63. found: C, 77.08, H, 10.49, N, 8.99; IR (KBr, υ cm−1): 3470, 3296 (NH, NH2), 1680 (CONH), 1620, 1617 (C=C), 1441 (C–N), 1461 (N–N); 1H NMR (CDCl3, 400 MHz): δ 2.1 (s, 2H, NH2, exchangeable with D2O), 8.8 (s, 1H, NH, exchangeable with D2O), 5.6 (s, 1H, CH), 1.12 (s, 3H, C10–CH3), 0.71 (s, 3H, C13–CH3), 0.92 and 0.85 (other methyl protons); 13C NMR (CDCl3, 100 MHz): δ 161 (C3′), 151 (C5′), 137 (C6),117 (C5), 85.7 (C4′), 25.6 (C3); MS: m/z 467 [M+.].
2.4 Characterization
2.4.1 X-ray diffraction
The phase transition characteristics, i.e. the crystallanity, structure and particle size, of nano steroids 1–3 and 7–9 were studied by XRD. The XRD pattern of freeze-dried compounds was measured with a Rigaku diffractometer (2400) using graded d-space elliptical side-by-side multilayer optics, monochromatic Cu Kα radiation (40 kV, 30 mA) and an imaging plate (R-axis IV). The typical exposure time was 10 min, with a 150-mm camera length. The freeze-dried compounds 1–3 and 7–9 were vacuum-dried to constant weight and placed in capillary tubes, without being powdered [Citation16].
2.4.2 Scanning electron microscopy
Scanning electron microscopy (SEM) was used for morphological analysis with a JEOL JSM-6700F field-emission scanning electron microscope. After drying, compounds 2, 3 and 7–9 were mounted on circular metallic sample holders so that they did not fall off during handling. As only conducting samples can be mounted for SEM analysis, the compounds were coated with a thin layer of gold to prevent charging of the samples [Citation17].
2.4.3 UV–vis absorption and emission spectrophotometry
Optical absorbance spectra of compounds 1, 2, 3, 7 and 8 were obtained on an UV–vis light (UV–vis) spectrophotometer, in which the samples were scanned in dilute solutions of 1 mg of compound in a suitable solvent made up to 100 ml. A little of this solution was taken into a silica cell to form a thickness of 1 cm. Pure solvent was taken in an exactly similar cell (reference cell). The cells were exposed to a monochromatic beam of equal intensity in the spectrophotometer. After the beams had passed through both cells, the intensities were compared over whole wavelength range of the instrument [Citation18].
2.4.4 Infrared spectroscopy
Compositional analysis was derived from IR spectra recorded on KBr pellets with a Perkin Elmer RXI spectrometer (without microscope). The compounds were ground with KBr in a pestle and mortar and formed into discs after drying and then pressing under elevated temperature and high pressure. To avoid scattering, the particle size of the ground mixture was limited to 2 μm [Citation18].
2.5 In vitro antibacterial activity
Compounds 2, 3 and 7–9 were screened against cultures of Streptococcus pyogenes (ATCC-29213), Staphylococcus aureus (ATCC-25923), Pseudomonas aeruginosa (ATCC-27853) and Escherichia coli (ATCC-25922) by the disc diffusion method [Citation19,Citation20]. Standard inocula of 1 × 107 to 2 × 107 colony-forming units ml−1 (0.5 McFarland standards) were introduced onto the surface of sterile agar plates and spread with a sterile glass spreader for even distribution. Discs measuring 6 mm in diameter were prepared from Whatman No. 1 filter paper and sterilized by dry heat at 140 °C for 1 h. Sterile disks previously soaked in a known concentration of the test compounds were placed in nutrient agar medium. Solvent and growth controls were also kept. Chloramphenicol was used as a positive control and a disc with DMSO as a negative control. The plates were inverted and incubated at 37 °C for 24 h. The susceptibility of the bacterial strains was assessed from the diameter of the zone of inhibition in comparison with the standard drug. The bacterial zones of inhibition values for compounds 2 and 3 are shown in .
Table 3 The zones of inhibition of nanocompounds 2 and 3 with respect to positive and negative control in antibacterial activity.
Nutrient broth containing logarithmic serially twofold diluted amounts of the test compounds and control was inoculated with approximately 5 × 105 colony-forming units ml−1 of actively dividing bacterial cells. The cultures were incubated at 37 °C for 24 h, and growth was monitored visually and spectrophotometrically.
2.6 In vitro antifungal activity
Candida albicans (ATCC-10231), Aspergillus fumigates (ATCC-1022), Trichophyton mentagrophytes (ATCC-9533) and Penicillium marneffei (ATCC-18224) were re-cultured in DMSO by the agar diffusion method [Citation21,Citation22]. Sabouraud agar media was prepared by dissolving peptone (1 g), d-glucose (4 g) and agar (2 g) in distilled water (100 ml) and adjusting the pH to 5.7. Normal saline was used to make a suspension of spores of each strain for lawning. A loopful of each strain was transferred to 3 ml saline to obtain a suspension of the corresponding species, and 20 ml agar media were poured into each Petri dish. Excess suspension was decanted, and the plates were dried by placing them in an incubator at 37 °C for 1 h. Wells were made with an agar punch, and each well was labelled. A control was prepared in triplicate and maintained at 37 °C for 3–4 days.
The inhibition zones of compounds 2, 3 and 7–9 were compared with that of nystatin used as the standard drug (). Nutrient broth containing logarithmic serially twofold diluted amounts of the test compounds and control was inoculated with approximately 1.6 × 104 to 6 × 104 colony-forming units ml−1 of actively dividing fungal cells. The cultures were incubated at 35 °C for 48 h, and growth was monitored.
Table 4 The zones of inhibition of nanocompounds 2 and 3 with respect to positive and negative control in antifungal activity.
3 Results and discussion
3.1 Chemistry
The synthesis of 3β-acetoxycholest-5-ene 2 involves acetylation of 3β-hydroxycholest-5-ene 1 in the presence of pyridine [Citation14], while 3β-acetoxy-6-nitrocholest-5-ene 3 was obtained by nitration of 3β-acetoxycholest-5-ene 2 in the presence of sodium nitrite at room temperature [Citation15]. During both processes, compounds 2 and 3 were obtained in good yields (80%). A schematic representation of the formation of nano steroids 2 and 3 is shown in .
3β-Acetoxycholestan-6-one 4, 3β-chlorocholestan-6-one 5 and cholestan-6-one 6 were used as starting compounds and were prepared by known methods [Citation23–Citation25]. All three new compounds () were synthesized by the reaction of steroidal ketones 4–6 with cyanoacetohydrazide under refluxing conditions of 7 h in acetic acid. The yield of steroidal pyrazolone derivatives 7–9 was 70–80%.
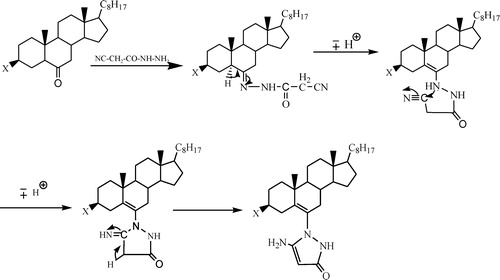
shows that the formation of cyanoacetohydrazone takes place first, involving a nucleophilic attack of nitrogen on the terminal carbon (C≡N) of cyanoacetohydrazone to convert it to (C=NH), which later changes into (C–NH2). Hence, cyclization of the heterocycle occurs [Citation26].
3.2 X-ray diffraction analysis
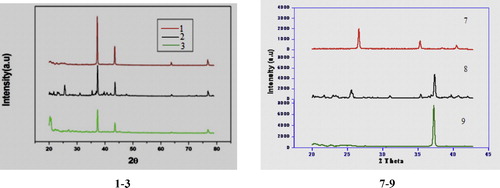
A typical powder XRD pattern of nano steroids 1–3 and 7–9 at room temperature is shown in . The analysis showed that nano steroids 1–3 are formed in a single phase with hexagonal, monoclinic and tetragonal crystal symmetry, respectively. Analysis of the powder XRD patterns of nano compounds 7–9 at room temperature showed that 7 and 8 are formed in single phase with hexagonal crystal symmetry, while 9 is formed with orthorhombic crystal symmetry. All the peaks matched well with the crystal structures, and no indication of a secondary phase was found. The lattice parameters calculated from the XRD pattern are given in and ; they are close to the values reported in the literature [Citation27].
The maximum deviation between the observed and calculated values of interplanar spacing (d) remained below 0.0011 Å. In order to calculate the particle size (D) of sample 3, Scherer's formula was used [Citation28].(1)
(1) where λ is the X-ray wavelength (1.5418 Å), B is the full width at the half-maximum of the most intense peak, and θ is the diffraction angle.
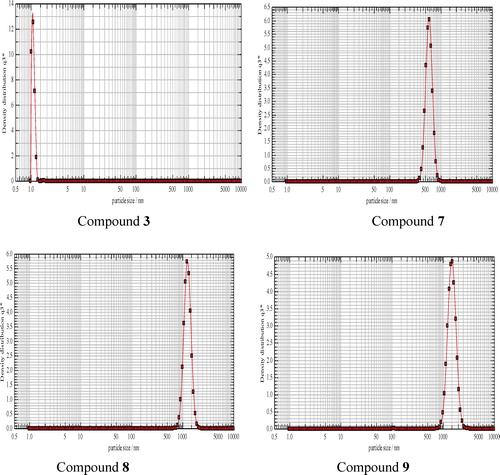
The particle size of 3β-acetoxy-6-nitrocholest-5-ene 3 was found to be ∼19 nm, estimated from the line width of the (1 0 1) XRD peak. Well-crystallized diffraction peaks were observed, from which the calculated d values are in good agreement with those given in the standard data (JCPDS, 36-1451) for 2 and 3 (). This suggests that compounds 2 and 3 crystallized in monoclinic and tetragonal symmetry, respectively. Particle size analysis of nano compounds 7–9 revealed average particle sizes of 476.30, 1003.67 and 1157.88 nm, respectively. The particle size graphs of nano compounds 3 and 7–9 are shown in . Well-crystallized diffraction peaks were observed for all the compounds for which cell parameters, lattice type and d values were calculated ( and ), which were also in good agreement with those given in the standard data (JCPDS, 36-1451).
Table 1 The XRD parameters of the nano compounds 2 and 3 prepared in this study.
The uniform values for the particle sizes of compounds 7–9 is due to the preparation mode. The uniform, smaller particle size of nano steroid 3 is illustrated by the sharp, strong peak shown in .
3.3 Scanning electron microscopy
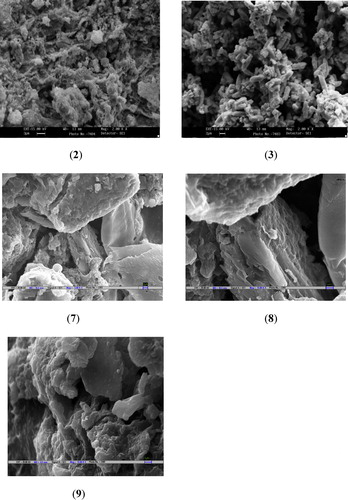
The surface morphology of nano steroids 2, 3 and 7–9 was investigated by SEM. The images for nano steroids 2 and 3 have a width of 13 mm and a magnification of 2.00 KX, while those for nano steroids 7–9 have a width of 10.5 mm and a magnification of 15.00 KX (). The signals from SEM provide information about the external morphology (texture), crystalline structure, homogeneity, thickness and orientation of the materials making up the nano compounds. SEM showed that the homogeneous crystals of nano steroid 3 are approximately spherical and particle agglomerates are formed by spherical nanoparticles conjoined to the chains. The crystals of nano steroids 7–9 are brick-shaped agglomerates with less sharp edges and a rough surface with a few microcrystals.
3.4 Optical properties
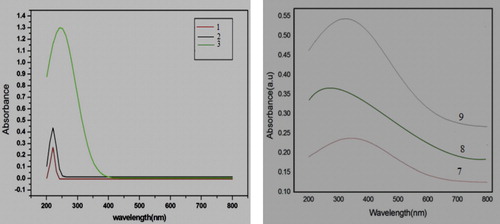
In order to determine the photo-absorption behaviour of nano compounds 1–3 and 7–9, portions of these compounds were uniformly dispersed in ethanol, and the UV–vis absorption and emission spectra were recorded at room temperature. The optical absorption spectra of nano compounds 1–3 were in the range 200–800 nm (). These nano compounds show extremely strong absorption at wavelengths of 200–250 nm. Nano compound 3 also showed relatively strong absorption at ∼400 nm, due to the presence of NO2 in this compound, which causes conjugation that leads to a decrease in the energy gap between the highest and lowest unoccupied molecular orbitals. Thus, energy of maximum wavelength is required to cause absorption, and red shift is observed. The optical absorption spectra of nano compounds 7–9 were also in the range 200–800 nm (). Strong absorption at wavelengths 200–400 nm is shown by these compounds 7–9, with strong absorption at 340 nm for compound 7, 260 nm for compound 8 and 320 nm for compound 9. The reason for strong absorption at higher wavelengths for these compounds is the presence of conjugation in the pyrazolone moiety, but the reason for the strong absorption at a higher wavelength for compound 7 is the presence of an acetate substituent at the 3β-axial position, which creates additional conjugation through the lone pair of electron of oxygen with the carbonyl group, leading to the decrease in the energy gap between the highest and lowest unoccupied molecular orbitals. Thus, the energy of the maximum wavelength is required to cause absorption, and red shift is observed in compound 7.
The fluorescence spectra of nano steroids 2 and 3, measured on a Shimadzu spectrofluorimeter-5000 (Japan), were in the range 350–600 nm. shows that the maximum fluorescence intensity is ∼475 nm for both nano compounds. Nano steroid 3 gave the maximum fluorescence intensity because of a smaller energy gap between the ground state and the excited state, which makes electrons easily excited; after deactivation, maximum energy is emitted. This fact is also seen from the red shift () and the band gap energy values shown in .
The band gap energy of nano steroids 2 and 3 () was 4.38 eV and 4.27 eV, respectively, indicating that nano steroid 3 is red-shifted as compared to nano steroid 2 ( and ). The lower band gap energy of nano steroid 3 is due to the presence of a nitro (NO2) group, which acts as a chromophore and results in conjugation. The presence of a chromophore decreases the energy gap between the ground state and the excited state, resulting in easy excitation of electrons.
The band gap energy of nano steroids 7 and 8 () was 3.70 eV and 4.61 eV, respectively, indicating that nano steroid 7 is red-shifted as compared with nano steroid 8. This is also obvious in the UV–vis spectra of compounds 7–9.
3.5 Infrared spectroscopy
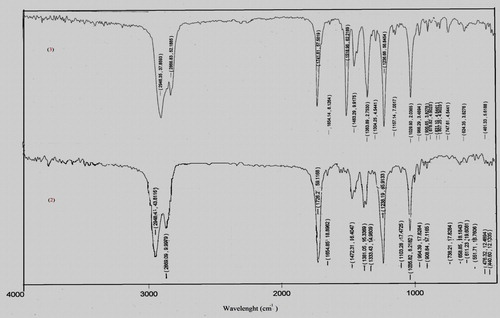
The characteristic absorption peaks of nano steroids 2 and 3 were obtained from their IR spectra (). Compound 2 showed absorption peaks at ν 1726 cm−1 (OCOCH3) and a weak absorption peak at 1654 cm−1 (C=C), while compound 3 showed strong absorption at ν 1741 cm−1 (OCOCH3), 1365–1518 (NO2), 1325 (C–N) and a weak absorption peak at ν 1654 cm−1(C=C). The IR spectra () indicate that 3β-acetoxycholest-5-ene 2 and 3β-acetoxy-6-nitrocholest-5-ene 3 are readily formed from 3β-hydroxycholest-5-ene 1.
3.6 Antimicrobial activity
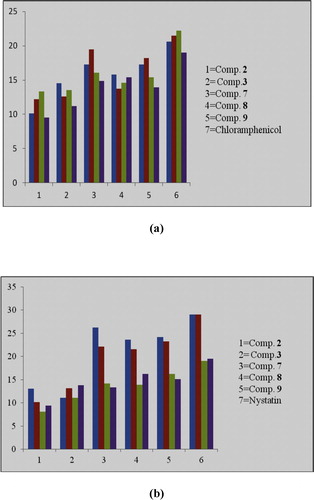
and indicate that nano steroids 2 and 3 have varying inhibitory effects on the growth of bacterial and fungal strains, with moderate to good antimicrobial behaviour. Nano compound 3 had a large zone of inhibition (14.5 mm) in comparison with chloramphenicol against S. aureus and also showed large zone of inhibition (13.8 mm) in comparison with nystatin against A. fumigatus.
shows that compound 3 had large zones of inhibition in comparison with chloramphenicol against P. aeruginosa (a) and S. aureus (b) and a large zone of inhibition in comparison with nystatin against A. fumigates (c).
Fig. 8 Antimicrobial activity of 3 against P. Aeruginosa (a), S. aureus (b) and A. fumigatus (c) Antimicrobial activity of 7 against S. Pyogenes (d) and C. Albicans (e).
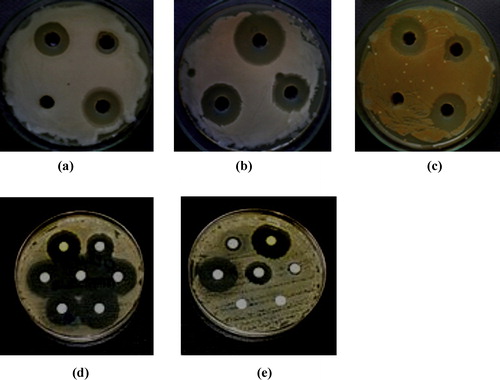
Compound 3 is biologically active because it contains a nitro (NO2) group at the 6-position of the steroid molecule. It has been reported [Citation29] that the biological characteristics of the nitrogen-containing heterocyclics depends on the metabolic reactions that lead to the reduction of the NO2 group and the formation of a highly reactive intermediate, which is responsible not only for antimicrobial activity but also for cytotoxic and mutagenic properties.
Nano steroids 7–9 also had varying inhibitory effects on the growth of bacterial and fungal strains, with moderate to good antimicrobial behaviour. Compound 7 had a large zone of inhibition (19.5 mm) in comparison with chloramphenicol (21.5 mm) against S. pyogenes and a large zone of inhibition (26.2 mm) in comparison with nystatin (29.0 mm) against C. albicans.
Lakshmia et al. [Citation30] reported enhanced penetration of nano steroids into lipid membranes, as the hydrocarbon skeleton functions as a lipophilic group to drive the compounds through the semipermeable membrane of the cell, blocking the binding sites of the enzymes of the micro-organism. A graphical representation of the antimicrobial activity of nano compounds 2, 3 and 7–9 and of the positive and negative controls is shown in .
4 Conclusion
Our convenient, operationally simple strategy for better synthesis of new steroidal pyrazolones involves the reaction of steroidal ketones with cyanoacetohydrazide in acetic acid. This is a straightforward, efficient method for obtaining steroidal pyrazolones. XRD at room temperature showed single-phase formation of the nano compounds, with monoclinic crystal symmetry for 2, tetragonal crystal symmetry for 3, hexagonal crystal symmetry for 7 and 8 and orthorhombic crystal symmetry for 9. SEM showed that the homogeneous crystals of nano steroid 3 have a spherical morphology and that particle agglomerates are formed by conjunction to the chains. SEM of nano steroids 7–9 showed that they were brick-shaped agglomerates with less sharp edges and a rough surface with a few microcrystals. The band gap energy of nano compounds 2, 3, 7 and 8, calculated from UV–vis spectra, indicated that nano compound 3 is red-shifted as compared to 2, and nano compound 7 is red-shifted as compared to 8. In biological screening, compound 7 showed potential antimicrobial behaviour against S. pyogenes and C. albicans, and nano compound 3 showed potential antimicrobial behaviour against S. aureus, P. aeruginosa, T. mentagrophytes and A. fumigates. However, further modification and derivatization of these nano compounds may increase their antimicrobial activity.
Acknowledgements
The authors are thankful to the Chairman, Department of Chemistry, for providing research facilities, the All India Institute of Medical Sciences, New Delhi, for providing SEM micrographs, Zakir Hussain College of Engineering and Technology, Aligarh Muslim University, for X-ray diffraction analysis, UV–vis spectrometry and particle size analysis. The financial assistance provided by the (UGC) India and SAP (DRS-1) is also acknowledged.
Notes
Peer review under responsibility of Taibah University
References
- S.I.YusaSelf-assembly of cholesterol-containing water-soluble polymersInt. J. Polym. Sci.2011110
- N.P.FranksStructural analysis of hydrated egg lecithin and cholesterol bilayers. I. X-ray diffractionJ. Mol. Biol.1001976345358
- D.L.WorsterN.P.FranksStructural analysis of hydrated egg lecithin and cholesterol bilayers. II. Neutrol diffractionJ. Mol. Biol.1001976359378
- J.M.BackerE.A.DawidowiczTransmemberane movement of cholesterol in small unilamellar vesicles detected by cholesterol oxidaseJ. Biol. Chem.2561981586588
- J.M.BackerE.A.DawidowiczMechanism of cholesterol exchange between phospolipid vesiclesBiochemistry20198138053810
- E.J.ShimshickH.M.McConnellLateral phase separations in binary mixtures of cholesterol and phospholipidsBiochem. Biophys. Res. Commun.531973446451
- T.N.EstepD.B.MountcastleR.L.BiltonenT.E.ThompsonStudies on the anomalous thermotropic behaviour of aqueous dispersions of dipalmitoylphosphatidylcholine–cholesterol mixturesBiochemistry17197819841989
- C.J.DrummondC.FongSurfactant self-assembly objects as novel drug delivery vehiclesCurr. Opin. Colloid Interface Sci.41999449456
- D.A.MannockR.N.McElhaneyThermotropic and lyotropic phase properties of glycolipid diastereomers: role of head group and interfacial interactions in determining phase behaviourCurr. Opin. Colloid Interface Sci.82004426447
- T.ThiemannV.VillHomologous series of liquid crystalline steroidal lipidsJ. Phys. Chem. Ref. Data261997291333
- J.Q.HaoH.LiX.X.ZhuRecent advances in functional polymers based on steroid cholic acidChinese J. Org. Chem.24200411291132
- D.LiuQ.WangH.L.M.ChangH.ChenVariant structure in metal-organic-chemical-vapor-deposition-derived SnO2 thin films on sapphire (0 0 0 1)J. Mater. Res.10199515161522
- J.A.W.BarnardJ.E.LydonA crystallographic examination of 14 straight chain alkyl esters of cholesterolMol. Cryst. Liq. Cryst.261974285296
- L.F.FieserM.FieserSteroid1959Reinhold Publishing Corp.New Yorkp28
- C.E.AnagostopoulosL.F.FieserNitration of unsaturated steroidsJ. Am. Chem. Soc.761954532536
- S.J.LeeE.KimM.L.SeoY.DoY.-A.LeeS.S.LeeJ.H.JungM.KogisoT.ShimizuSelf-assembled helical ribbon and tubes of alanine-based amphiphiles induced by two different formation mechanismsTetrahedron64200813011308
- Y.WenW.HuangB.WangJ.FanZ.GaoL.YinSynthesis of Cu nanoparticles for large-scale preparationMater. Sci. Eng. B1772012619624
- P.S.KalsiSpectroscopy of Organic Compounds6th ed.2002New Age InternationalNew Delhi
- A.H.CollinsMicrobiological Methods4th ed.1976ButterworthLondon
- R.CruickshankJ.P.DuguidR.H.A.SwainB.P.MarmionMedicinal Microbiology12th ed.1975Churchill LivingstoneLondon196202 Vol. II
- Z.K.KhanIn vitro and vivo screening techniques for bioactivity screening and evaluationProceeding International workshop UNIDO-CDRI1997210211
- R.S.VermaI.K.KhanA.P.SinghAntifungal Agents: Past, Present, Future Prospects1998National Academy of Chemistry and BiologyLucknow55128
- R.K.CallowV.H.T.JamesBy-ways of synthesis of cortisone from hecogenin. Part II. A route to 11-oxygenated compounds through 3b: 20b-diacetoxy-11-bromoallopregnan-12-oneJ. Chem. Soc.195647444749
- A.H.MillurnE.V.TruterThe components of wool wax. Part III. 7-Oxocholesterol and the alleged presence of cholestanolJ. Chem. Soc.341195617361739
- G.W.DaubenK.H.TakemuraA study of the mechanism of conversion of acetate to cholesterol via squaleneJ. Am. Chem. Soc.75195363026304
- S.BondockA.E.TarhoniA.A.FaddaUtility of cyanoacetic acid hydrazide in heterocyclic synthesisARKIVOC92006113156
- D.C.LookJ.W.HemskyJ.R.SizeloveResidual native shallow donor in ZnOPhys. Rev. Lett.82199925522555
- B.D.CullityElements of X-ray Diffraction2nd ed.1978Addison-WesleyReading102p
- M.CastelliM.MalagoliL.LupoS.RoffiaF.PaolucciC.CermelliA.ZancaG.BaggioCytotoxicity and probable mechanism of action of sulphimidazoleJ. Antimicrob. Chemother.462000541550
- P.V.A.LakshmiaP.S.ReddyV.J.RajuSynthesis, characterization and antimicrobial activity of 3d transition metal complexes of a biambidentate ligand containing quinoxaline moietySpectrochim. Acta A7420095257