Abstract
A simple, rapid, sensitive reversed-phase high-performance liquid chromatography method was developed and validated for simultaneous measurement of albendazole and praziquantel with an internal standard, simvastatin, at single wavelength of 225 nm. Chromatographic separation was performed on an Enable C18 column (250 mm × 4.6 mm, 5 μm: Spinco Biotech Pvt Ltd) and a mobile phase consisting of acetonitrile:water (60:40, v/v) with 10% orthophosphoric acid to adjust the pH to 3.2, at a flow rate of 1.0 ml/min. The calibration curve was linear (r2 ≥ 0.999) over the concentration range 0.05–8.0 μg/ml. The concentrations of simvastatin was 1.0 μg/ml. The limit of quantification was 0.05 μg/ml for both albendazole and praziquantel. No interference was found by the excipients in the synthetic mixture. The proposed methods were validated as per International Conference on Harmonisation guidelines for linearity, accuracy, precision and robustness for estimation of albendazole and praziquantel in bulk and in a synthetic mixture, and the results were found to be satisfactory.
1 Introduction
Neurocysticercosis is the commonest helminthic disease of the nervous system and is considered a serious public health problem in developing countries of Latin America, Asia, and Africa [Citation1–Citation3]. Although the treatment of neurocysticercosis is restricted to palliative measures, it has advanced over the past 20 years with the use of praziquantel and albendazole, which are effective against the cystic larvae [Citation2,Citation4]. Albendazole is more effective than praziquantel, but cysts persist in some patients even after repeated use of albendazole [Citation2]. For these cases, alternative treatment schedules such as simultaneous use of praziquantel and albendazole have been evaluated [Citation4,Citation5]. The combination of praziquantel with albendazole has also been extensively used in human hydatid disease [Citation6–Citation9]. Albendazole is extensively metabolized to its active metabolite albendazole sulfoxide, which is further metabolized to the inactive albendazole sulfone [Citation10]. Because of this extensive metabolism, plasma concentrations of albendazole are usually low, and pharmacokinetics are studied by measuring the sulfoxide and sulfone concentrations [Citation11–Citation15]. Praziquantel is metabolized to several hydroxylated metabolites [Citation16–Citation18], mainly trans-4-hydroxypraziquantel, an active metabolite [Citation19].
Table 1 Linear regression equations generated from validation of albendazole and praziquantel: slope, intercept and coefficient of determination.
Table 3a Intra-day (n = 3) precision.
Table 3b Inter-day (n = 3) precision.
Table 3c Repeatability.
Table 6 Determination of robustness for ABZ.
Table 7 Determination of robustness for PRQ.
In order to evaluate the kinetics of albendazole and praziquantel, selective, sensitive, reproducible analytical methods are required for their quantification in plasma samples as well as for their metabolites. High-performance liquid chromatography (HPLC) [Citation20–Citation29] and capillary electrophoresis [Citation30,Citation31] have been used, and the coupling of mass spectrometry to liquid chromatography (LC–MS and LC–MS–MS) has brought new insight into quantitative bioanalysis. Use of these techniques for the analysis of albendazole metabolites, praziquantel and trans-4-hydroxypraziquantel has been described only for isolated drugs. Bonato et al. [Citation32] and Chen et al. [Citation33] reported the use of LC–MS–MS for two methods, with quantification limits for albendazole sulfoxide of 5.0 and 4.0 μg/ml, respectively, and a quantification limit of 0.5 μg/ml for albendazole sulfone. LC–MS–MS was used only for qualitative analysis of praziquantel metabolites [Citation16,Citation34].
The aim of this work was to develop an HPLC method for simultaneous estimation of albendazole and praziquantel in bulk and in the synthetic mixture. The method was validated according to the International Conference on Harmonisation guidelines.
2 Materials and methods
2.1 Chemicals and reagents
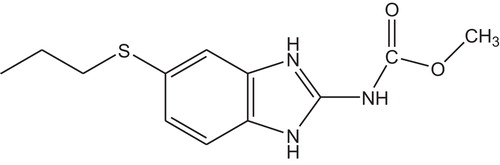
Albendazole () was obtained from Mercury Pharmaceutical Ltd, Vadodara, Gujarat, India, praziquantel () from Micro Labs Ltd, Goa, India, and simvastatin (internal standard) () from Dr Reddy's Lab, Hyderabad, India. Acetonitrile, methanol and water of HPLC grade were used. All the other reagents (including 10% ortho-phosphoric acid) were of analytical grade.
2.2 Chromatographic conditions
The high-performance liquid chromatograph (Shimadzu, Kyoto, Japan) was composed of an LC-20AT Prominence solvent delivery module, a manual rheodyne injector with a 20-μl fixed loop and a SPD-20A Prominence ultraviolet–visible detector. Separation was performed on an Enable C18 G column (particle size 5 μm; 250 mm × 4.6 mm) preceded by an ODS guard column (10 μm, 10 mm × 5 mm) at ambient temperature. Data were acquired on a Spinchrom Chromatographic Station® CFR Version 2.4.0.195 (Spinchrom Pvt. Ltd, Chennai, India). The mobile phase consisted of acetonitrile:water in a ratio of 60:40, the pH was adjusted to 3.2 with ortho-phosphoric acid, and the flow rate was 1.0 ml/min. Mo was vacuum filtered and degassed through 0.2 μm pore polymeric PTFE filters.
2.3 Method
2.3.1 Preparation of standard stock solutions
A sample of 25 mg of each drug is weighed and transferred to a 25-ml volumetric flask; 15 ml of methanol are added, and the solution is sonicated for 15 min. The volume is made up to the mark with methanol to obtain a stock solution of 1000 μg/ml. Simvastatin is also prepared in the diluent to obtain a working standard solution of 1000 μg/ml.
2.3.2 Preparation of working standard solutions
From the standard stock solutions, 2.5 ml are withdrawn and transferred to 25-ml volumetric flasks, and the volume is made up to the mark with diluent to obtain working standard solutions of 100 μg/ml. The working standard solution of simvastatin is diluted to a final solution of 10 μg/ml.
2.4 Validation
The method was validated by evaluating recovery, linearity, precision, accuracy, quantification limit and stability. Coefficients of variation and relative errors <2% were considered acceptable [Citation35,Citation36].
2.4.1 Linearity
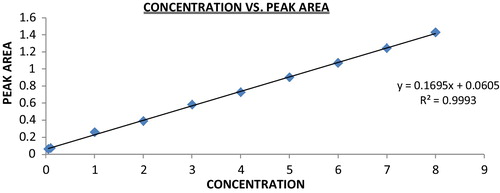
Linearity was tested at concentrations of 8, 7, 6, 5, 4, 3, 2, 1, 0.5 and 0.05 μg/ml for albendazole and praziquantel with a fixed concentration of 1 μg/ml diluted from 10 μg/ml of the working standard solution. The calibration curve was constructed and its coefficient of determination (r2) determined. The calibration plot (peak area ratio of albendazole and praziquantel to internal standard versus albendazole and praziquantel concentration) was generated by replicate analysis (n = 10) at all concentrations, and the linear relation was evaluated by the least-squares method in Microsoft Excel®. For minimum error, 10 concentrations were tested, giving a wide range of linearity.
2.4.2 Accuracy
The accuracy of the method was determined by replicate analyses with two standard addition methods at six concentrations, the first at 80%, 100% and 120% and the second at 50%, 100% and 150%. Comparison of the difference between the spiked value (theoretical value) and that found gave the accuracy.
2.4.3 Precision
The precision of the method, measured as within-day repeatability, was determined by replicate analyses of three sets of samples spiked with three concentrations of albendazole and praziquantel (0.05, 1 and 8 μg/ml) and a fixed concentration of internal standard (1 μg/ml). The reproducibility (day-to-day variation) of the method was validated with the same concentration range, but with only a single determination of each concentration on three days. The relative standard deviation was calculated from the ratio of the standard deviation to the mean and expressed as a percentage.
2.4.4 Specificity
Specificity was measured by analysing the standard solutions in the presence of an excipient (talc) to determine any interference in the percentage recovery. Standard solutions containing 10 mg of each drug were spiked with 50% (5 mg), 100% (10 mg) or 150% (15 mg) of talc and analyzed for albendazole and praziquantel recovery by HPLC with a fixed concentration of internal standard (1 μg/ml). The acceptable level of interference was <0.5%.
2.4.5 Robustness
The robustness of the method for determining albendazole and praziquantel was evaluated by varying the flow rate, pH and mobile phase ratio. The percentage recovery and relative standard deviation were recorded for both drugs.
3 Results and discussion
3.1 Calibration curve
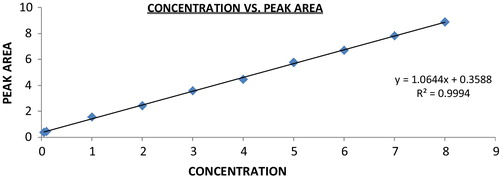
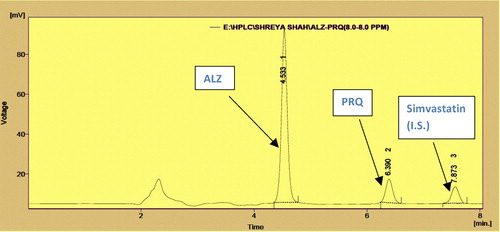
The coefficient correlation r, slope and intercept were 0.999, 1.064 and 0.358 for albendazole and 0.999, 0.169 and 0.060 for praziquantel with ultraviolet detection () with absorbance maxima set at 225 nm. The retention times were 4.533 for albendazole, 6.39 for praziquantel and 7.8 for the internal standard (). Linear regression of data from the calibration curve indicated a linear response over the concentration range of both drugs. The curve can therefore be used for determination of albendazole and praziquantel in synthetic mixtures.
3.2 Validation
3.2.1 Linearity
The coefficient of determination (r2) for both albendazole and praziquantel was 0.999 ( and and and ).
3.2.2 Limit of detection and limit of quantification
The limit of detection and the limit of quantification were determined from the calibration curve, according to the formulae 3.3 × SD/slope and 10 × SD/slope, respectively. The results are shown in .
Table 2 Spectral and statistical data for determination of albendazole and praziquantel by proposed RP-HPLC method.
3.2.3 Precision
The relative standard deviation was found to be <2.0% for both albendazole and praziquantel, indicating satisfactory precision (). The intermediate precision of the expected results is expressed as a percentage.
3.2.4 Accuracy
The mean recovery of praziquantel and albendazole was found to be in the range 100.81–100.92% and 99.71–100.86%, respectively, within the acceptable limits at 80%, 100% and 120% () and 98.33–100.66% and 98.46–100.73% within the acceptable limits at 50%, 100% and 150% ().
Table 4a Accuracy.
Table 4b Accuracy.
3.2.5 Specificity
Interference was found to be 100.81–100.92% for albendazole and 100.39–100.89% for praziquantel, which is within the acceptable limit. Hence the excipients do not interfere with estimates of drug concentrations ().
Table 5 Specificity.
3.2.6 Robustness
The retention times of the analytes did not change significantly when the flow rate, mobile phase ratio and pH were changed. The percentage recovery and relative standard deviation were within the limits for both albendazole and praziquantel ( and ).
3.2.7 Sample stock solutions for assay
Ten tablets powdered equivalent were mixed in a ratio of 300 mg albendazole: 25 mg praziquantel. A quantity of this synthetic mixture powder equivalent to 65 mg was taken up in a 100-ml volumetric flask, and diluent was added up to the mark. The solution was sonicated for 5 min. This solution was further diluted to obtain a concentration of 12 μg/ml albendazole and 5 μg/ml praziquantel. The results are summarized in , and the chromatogram is shown in .
Fig. 8 Chromatogram of Albendazole and Praziquantel assay containing 6 μgm/ml of ABZ & 0.5 μg/ml of PRQ with simvastatin as an Internal Standard (IS) (7.873).
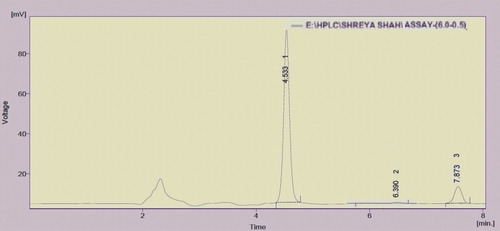
Table 8 Determination of % assay for ABZ & PRQ.
3.2.8 System suitability
The system suitability test is an integral part of chromatographic analysis. It is used to verify that the resolution and reproducibility of the system are adequate for the analysis. A system suitability test according to the United States Pharmacopeial Convention was performed on chromatograms obtained for standard and test solutions to check differences in the above-mentioned parameters. The results obtained with six replicate injections of the standard solution are summarized in .
Table 9 System suitability parameters for ABZ & PRQ.
4 Conclusions
An HPLC method for albendazole and praziquantel has been developed, which is simple, precise and selective for simultaneous determination of the two drugs in bulk and in synthetic mixtures. It can be used for process control of bulk drug and formulated products in ordinary laboratories and in the pharmaceutical industry.
Acknowledgements
The authors are grateful to the Principal Dr U.M. Upadhyay and to assistant professors in the Quality Assurance Department of the Sigma Institute of Pharmacy, Vadodara, Gujarat. We also thank the management team for support and for providing chemicals and equipment for this work. We also thank Mercury Pharmaceutical Ltd, Vadodara, Gujarat, for providing albendazole and Micro Labs Ltd, Goa, for providing praziquantel, both free of charge.
Notes
Peer review under responsibility of Taibah University.
References
- A.FlisserE.SartiM.LightowlersP.SchantzNeurocysticercosis: regional status, epidemiology, impact and control measures in the AmericasActa Trop.8720034351
- O.M.TakayanaguiTherapy for neurocysticercosisExpert Rev. Neurother.42004129139
- O.H.Del BruttoSeminars on neurologyNeurocysticercosis252005243251
- F.PolomaresG.PalenciaJ.R.AmbrosioA.OrtizH.Jung-CookEvaluation of the efficacy of albendazole sulphoxide and praziquantel in combination on Taenia crassiceps cysts: in vitro studiesJ. Antimicrob. Chemother.572006482488
- D.M.GuoS.P.XieJ.P.JiaTherapeutic efficacy of praziquantel, albendazole and a combination of the two drugs in cysticercosisChin. J. Parasitol. Parasit. Dis.212003187188
- M.I.YasawyM.A.A.L.KarawiA.R.E.MohamedCombination of praziquantel and albendazole in the treatment of hydatid diseaseTrop. Med. Parasitol.441993192194
- F.CoboC.YarnozB.SesmaP.FraileM.AizcorbeR.TrujilloA.Diazde- LiañoM.A.CigaAlbendazole plus praziquantel versus albendazole alone as a pre-operative treatment in intra-abdominal hydatidosis caused by Echinococcus granulosusTrop. Med. Int. Health31998462466
- A.E.MohamedM.I.YasawyM.A.Al KarawiCombined albendazole and praziquantel versus albendazole alone in the treatment of hydatid diseaseHepatogastroenterology45199816901694
- H.M.AylesE.L.CorbettI.TaylorA.G.A.CowieJ.BlighK.WalmsleyA.D.M.BrycesonA combined medical and surgical approach to hydatid disease: 12 years’ experience at the Hospital for Tropical DiseasesAnn. R. Coll. Surg. England842002100105
- C.VillaverdeA.I.AlvarezP.RedondoJ.VocesJ.L.Del EstalJ.G.PrietoSmall intestinal sulphoxidation of albendazoleXenobiotica251995433441
- E.A.FormentiniO.N.MestorinoE.L.MariñoJ.O.ErrecaldePharmacokinetics of ricobendazole in calvesJ. Vet. Pharmacol. Ther.242001199202
- O.M.TakayanaguiP.S.BonatoS.A.C.DreossiV.L.LanchoteEnantioselective distribution of albendazole metabolites in cerebrospinal fluid of patients with neurocysticercosisBr. J. Clin. Pharmacol.542002125130
- A.GoudahAspects of the pharmacokinetics of albendazole sulphoxide in sheepVet. Res. Commun.272003555566
- V.L.LanchoteO.M.TakayanaguiF.H.MateusEnantioselective renal excretion of albendazole metabolites in patients with neurocysticercosisChirality162004520525
- K.PengsaaK.Na-BangchangK.LimkittikulK.KabkaewK.LapphraC.SirivichayakulP.WisetsingC.Pojjaroen-AnantP.ChanthavanichA.SbchareonPharmacokinetic investigation of albendazole and praziquantel in Thai children infected with Giardia intestinalisAnn. Trop. Med. Parasitol.982004349357
- C.LerchG.BlaschkeInvestigation of the stereoselective metabolism of praziquantel after incubation with rat liver microsomes by capillary electrophoresis and liquid chromatography–mass spectrometryJ. Chromatogr. B7081998267275
- H.MeierG.BlaschkerInvestigation of praziquantel metabolism in isolated rat hepatocytesJ. Pharmaceut. Biomed. Anal.262001409415
- A.Godawska-MatysikE.PekalaK.Kiec-KononowiczThe research on biotransformation of praziquantelActa Polon. Pharmaceut.6120047578
- U.StaudtG.SchmahlG.BlaschkeH.MehlhornLight and scanning electron microscopy studies on the effects of the enantiomers of praziquantel and its main metabolite on Schistosoma mansoni in vitroParasitol. Res.781992392397
- F.WesthoffG.BlaschkeHigh-performance liquid chromatographic determination of the stereoselective biotransformation of the chiral drug praziquantelJ. Chromatogr.5781992265271
- M.E.C.ValoisO.M.TakayanaguiP.S.BonatoV.L.LanchoteD.CarvalhoDetermination of albendazole metabolites in plasma by HPLCJ. Anal. Toxicol.1819948690
- S.R.PoloJ.TorradoF.BolasS.TorradoA selective and simple RP-HPLC assay to quantify albendazole metabolites in plasmaJ. Liq. Chromatogr. Relat. Technol.21199823272340
- J.J.GarciaF.Bolas-FernandezJ.J.TorradoQuantitative determination of albendazole and its main metabolites in plasmaJ. Chromatogr. B7231999265271
- A.MirfazaelianS.DadashzadehM.R.RouiniA high performance liquid chromatography method for simultaneous determination of albendazole metabolites in human serumPharm. Pharmacol. Commun.62000563566
- D.KitzmanK.J.ChengL.FleckensteinHPLC assay for albendazole and metabolites in human plasma for clinical pharmacokinetic studiesJ. Pharmaceut. Biomed. Anal.302002801813
- W.RidtitidM.WongnawaW.MahatthanatrakulJ.PunyoM.SunbhanichLC determination of praziquantel in human plasmaJ. Pharmaceut. Biomed. Anal.282002181186
- A.MirfazaelianS.DadashzadehM.R.RouiniA high performance liquid chromatography method for simultaneous determination of albendazole metabolites in human serumJ. Pharmaceut. Biomed. Anal.30200212491254
- R.SarinA.P.DashV.K.DuaAlbendazole sulphoxide concentrations in plasma of endemic normals from a lymphatic filariasis endemic region using liquid chromatographyJ. Chromatogr. B7992004233238
- Z.WuN.J.MedlicottM.RazzakI.G.TuckerDevelopment and optimization of a rapid HPLC method for analysis of ricobendazole and albendazole sulfone in sheep plasmaJ. Pharmaceut. Biomed. Anal.392005225232
- A.ProcházkovaM.ChoukiR.TheurillatW.ThormannTherapeutic drug monitoring of albendazole: determination of albendazole, albendazole sulfoxide, and albendazole sulfone in human plasma using nonaqueous capillary electrophoresisElectrophoresis212000729736
- H.MeierG.BlaschkeInvestigation of praziquantel metabolism in isolated rat hepatocytesJ. Chromatogr. B7482000221231
- P.S.BonatoV.L.LanchoteO.M.TakayanaguiSimultaneous liquid chromatography-tandem mass spectrometric determination of albendazole sulfoxide and albendazole sulfone in plasmaJ. Chromatogr. B7832003237245
- X.ChenL.ZhaoH.XuD.ZhongSimultaneous determination of albendazole and its major active metabolite in human plasma using a sensitive and specific liquid chromatographic-tandem mass spectrometric methodJ. Pharmaceut. Biomed. Anal.352004829836
- A.J.B.MeloY.IamamotoA.P.J.MaestrinJ.R.L.SmithM.D.SantosN.P.LopesP.S.BonatoBiomimetic oxidation of praziquantel catalysed by metalloporphyrinsJ. Mol. Catal. A Chem.22620052331
- ICH Guidelines: Validation of Analytical Procedures: Q2B, 1996.
- ICH Guidelines: Validation of Analytical Procedures: Q2A, 1994.