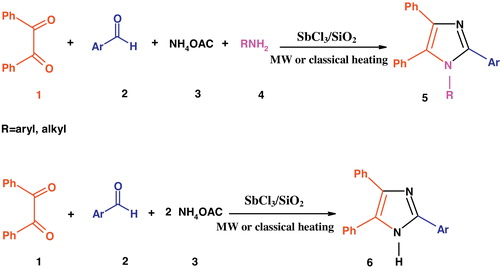
Abstract
One-pot cyclo-condensation of benzil, aldehydes, ammonium acetate and primary amines were used to synthesize 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted-1H-imidazole derivatives under microwave irradiation with silica-supported SbCl3 (SbCl3/SiO2) as a heterogeneous catalyst. The operational simplicity, practicability and applicability of this protocol to various substrates make it an interesting alternative to previous procedures. From the environmental stand-point, this eco-friendly, green catalyst is stable, highly active, easy to prepare and handle.
1 Introduction
Microwave-assisted organic synthesis results in spectacular acceleration of many chemical reactions as a consequence of three-dimensional heating of the reaction mass, which cannot be reproduced by classical heating methods. High yields, simple work-up, improved selectivity and clean reaction pathways are additional advantages of this synthetic technique [Citation1]. Moreover, even reactions that do not occur with conventional heating can be performed with microwave irradiation. In most cases, microwave irradiation coupled with solvent-free techniques represents a powerful, eco-friendly, green alternative to conventional synthesis. Theoretical calculations also suggest that reactions with high activation energies can be performed under microwave conditions without the use of harsh organic solvents [Citation2].
Numerous important heterocycle compounds have been synthesized under solvent-free conditions accelerated by microwave technology. Nitrogen-containing heterocyclics are widespread in nature, and their applications in agrochemicals, pharmaceuticals and functional materials are gaining importance [Citation3,Citation4]. A wide variety of N-containing heterocyclic imidazole ring systems have received considerable attention because of their pharmacological properties and roles in biochemical processes [Citation4,Citation5].
Multi-substituted imidazoles are known to possess NO synthase inhibition and antifungal, antibacterial, antiulcerative, antibiotic, antitumour, antimycotic, and CB1 receptor antagonistic activities [Citation6,Citation7]. Various substituted imidazoles act as inhibitors of p38 MAP kinase [Citation8] and B-Raf kinase [Citation9], glucagon receptors [Citation10], plant growth regulators [Citation11] and pesticides [Citation12]. Accordingly, a number of synthetic methods have been reported for the construction of substituted imidazoles.
In 1882, Radziszewski and Japp [Citation13,Citation14] reported the first synthesis of a highly substituted imidazole from a 1,2-dicarbonyl compound, aldehydes and ammonia. A number of synthetic processes have also been developed for the synthesis of 1,2,4,5-tetrasubstituted imidazole and 2,4,5-trisubstituted imidazole derivatives. Syntheses of 1,2,4,5-tetrasubstituted imidazoles are carried out by four-component cyclo-condensation of a 1,2-diketone/α-hydroxyketone with various aldehydes, primary amine and ammonium acetate with molecular HBF4-SiO2 [Citation15], FePO4 [Citation16], BF3·SiO2 [Citation17], sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl] ester [Citation18], cyclic phosphoric acid [Citation19], silica-supported Wells–Dawson acid [Citation20], heteropolyacid [Citation21,Citation22], ionic liquids [Citation23,Citation24], SBA-15/2,2,2-trifluoroethanol [Citation25] and clay-supported titanium [Citation26]. 1,2,4,5-Tetrasubstituted imidazoles can also be obtained by condensation of a 1,2-diketone, aryl nitrile and primary amine under microwave conditions [Citation27], by N-alkylation of trisubstituted imidazoles and by hetero-cope rearrangement [Citation5]. 2,4,5-Trisubstituted imidazole derivatives are generally synthesized by three-component condensation of a 1,2-diketone/α-hydroxyketone with various aldehydes and ammonium acetate, including ionic liquids [Citation28], refluxing in acetic acid [Citation29], silica-immobilized sulfuric acid [Citation30], InCl3·3H2O [Citation31], ceric ammonium nitrate [Citation32], NiCl2·6H2O/Al2O3 [Citation33] and microwave irradiation [Citation34].
Although these protocols are suitable for certain synthetic conditions, many have one or more disadvantages, such as long reaction times, expensive reagents and catalysts, low selectivity, tedious work-up procedure and large amounts of catalysts, which eventually results in the generation of large amounts of toxic waste.
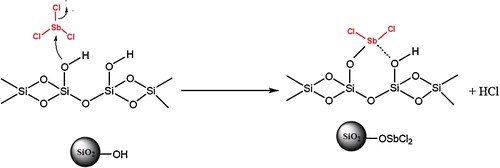
Use of Lewis acids supported on “inert” carriers as catalysts has received considerable interest for organic synthesis [Citation35]. The features of supported Lewis acids, such as ease of handling, enhanced reaction rates, greater selectivity, simple workup and recoverability of catalysts make them attractive alternatives to conventional reagents [Citation36]. Although there is a large variety of supported Lewis acids, silica-supported antimony (III) chloride is a recoverable, reusable heterogeneous catalyst in organic synthesis [Citation36–Citation39], making the processes truly eco-friendly, green methods [Citation35]. SbCl3 has been studied for its catalytic activity in only one study [Citation35], while this compound is not only commercially available and inexpensive, but is also easier to handle than other metal halides. Reports on the safety of SbCl3, however, show that it is highly toxic. Therefore, immobilized antimony (III) chloride has recently received considerable importance. This catalyst can be easily separated from the reaction products by simple filtration and recovered quantitatively in the active form. Supported SbCl3 can be recycled, making the preparation of sophisticated fine chemicals less expensive and, at the same time, avoiding contamination of the products by trace amounts of catalyst [Citation40].
In this context and in continuation of our studies on the applications of new catalysts for the synthesis of heterocyclic compounds [Citation41–Citation43] and to extend them to cleaner processes, we herein report a facile, efficient synthetic strategy for obtaining multi-substituted imidazoles in excellent yield with SbCl3/SiO2 under microwave irradiation and solvent-free conditions. Although the use of various solid heterogeneous catalysts has been reported in literature, silica-supported heterogeneous Lewis acid catalysts like SbCl3/SiO2, have not yet been explored for the preparation of imidazoles under solvent-free conditions and microwave irradiation ().
2 Experimental
2.1 Chemicals and apparatus
Chemical reagents of high purity were purchased from Merck, and all materials were of commercial reagent grade. Melting-points were determined in open capillaries on an Electrothermal Mk3 apparatus and are uncorrected. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded on a Bruker DRX-400 spectrometer at 400 and 100 MHz, respectively, and are reported as parts per million (ppm) downfield from tetramethylsilane as the internal standard. Fourier transform (FT)–infrared (IR) spectra were obtained with potassium bromide pellets in the range 400–4000 cm−1 on a Perkin-Elmer 550 spectrometer. A domestic microwave oven (Bajaj, ET–B at 2450 MHz, 100% power, 1300 W) was used in all experiments.
2.2 Preparation of SbCl3/SiO2 catalyst
The catalyst was prepared as reported elsewhere [Citation35]. Thus, 20 g of silica gel (80–200 mesh) were activated by refluxing with 150 mL of 6 mol/L hydrochloric acid under stirring for 24 h. The activated silica gel was filtered and washed with double-distilled water to neutral and dried overnight at 70 °C under vacuum to give preconditioned silica gel. Antimony trichloride (1.99 g) was added to a suspension of activated silica gel (1.54 g) in ethanol (50.0 mL), and the mixture was stirred at room temperature for 1 h. The solvent was removed, and the residue was washed three times with absolute ethanol and heated at 100 °C under vacuum for 5 h to furnish silica-supported antimony (III) chloride (2.83 g) as a white, free-flowing powder ().
2.3 General synthesis for the preparation of 1,2,4,5-tetrasubstituted imidazoles
SbCl3/SiO2 (0.1 g), an efficient catalyst, was added to the mixture of benzil (1 mmol), benzaldehyde (1 mmol), NH4OAc (4 mmol) and primary aliphatic and aromatic amine (4 mmol). The resulting mixture was heated in a microwave oven at 850 W or in an oil bath (at 120 °C) for the appropriate time until the reaction was complete. After thin-layer chromatography (eluent, petroleum ether:ethyl acetate: 9:1) indicated disappearance of the starting materials, the reaction was cooled to room temperature. The resulting solid was dissolved in acetone and filtered. The solid product obtained was washed with water and recrystallized from acetone:water 9:1 (v/v) to provide pure 1,2,4,5-tetrasubstituted imidazole derivatives as colourless crystals.
2.4 General synthesis for the preparation of 2,4,5-trisubstituted imidazoles
In a 50-mL round-bottom flask, a mixture of benzil (1 mmol), benzaldehyde (1 mmol), ammonium acetate (4 mmol) and SbCl3/SiO2 (0.1 g) was stirred under microwave irradiation at 850 W or in an oil bath (at 120 °C) in solvent-free conditions. Pure 2,4,5-trisubstituted imidazole derivatives were obtained by a method similar to that for 1,2,4,5-tetrasubstituted imidazoles.
3 Results and discussion
Synthesis of tri- and tetra-substituted imidazoles by cyclo-condensation of aldehyde, benzil, ammonium acetate and amine in refluxing acetic acid for a few hours is a well-established protocol [Citation44]. The drawbacks of this procedure include low yields, long reaction time, tedious workup and drastic reaction conditions. An improved method for the synthesis of multi-substituted imidazoles, using silica gel, zeolite and acidic alumina under microwave irradiation, has been described [Citation45,Citation46]; however, this technique does not meet the definition of ‘no solvent’, as it requires the use of an appreciable amount of solvent at the reaction stages. We modified the supported Lewis acid technique to make it an environmentally friendly green synthesis, in which the reaction is carried out in the absence of solvent and solid support by a microwave technique.
In an initial study, we considered cyclo-condensation of benzaldehyde with aniline, benzil and ammonium acetate under microwave irradiation and solvent-free conditions a model reaction. We screened various common SbCl3/SiO2 for their ability to catalyze the four-component cyclo-condensation at 30 W under microwave irradiation. The results are summarized in . Use of 0.1 g SbCl3/SiO2 afforded a 95% yield of the desired product. A physical mixture of silica and SbCl3 showed lower activity. Thus, SbCl3/SiO2 presumably acts in a synergistic fashion to catalyze this reaction. The experimental results show that the reaction times are shorter and the yields of the products higher under microwave conditions. In the absence of catalyst, the yield of product was low (30%), showing that the catalyst is necessary for synthesis of 1,2,4,5-tetrasubstituted imidazoles.
Table 1 Optimization of catalyst amount in synthesis of 1,2,4,5-tetrasubstituted imidazole derivatives under microwave irradiation.
1,2,4,5-Tetrasubstituted imidazole was synthesized by reacting benzil, aliphatic and aromatic amines and ammonium acetate with various aromatic aldehydes and various functional groups under microwave conditions and classical heating. The aromatic aldehydes and aliphatic and aromatic amines were converted to the corresponding products in good yields and in short time (). The reactions were also performed with conventional heating, with similar reaction conditions. Direct heating of the reactants without solvent took longer to complete the reactions and gave high yields. The yields obtained were good to excellent with no formation of side products such as 2,4,5-trisubstituted imidazoles, oxidized products of anilines or aldehydes, which are usually observed under the influence of strong acids.
Table 2 Synthesis of 1,2,4,5-tetrasubstituted imidazole derivatives catalyzed by SbCl3/SiO2.
In order to explore the applicability of this method, the same reaction conditions were used for synthesis of 2,4,5-trisubstituted imidazoles via a one-pot, cyclo-condensation of 1,2-diketone (1 mmol), aldehyde (1 mmol) and ammonium acetate (4 mmol). Good to excellent yields and a short reaction time were found with SbCl3/SiO2. Therefore, application of SbCl3 dispersed on dry silica gel not only affords excellent yield but also avoids the problems of catalyst cost, handling, safety and pollution. This ecofriendly, green catalyst is a heterogeneous acid that can be easily separated from the reaction mixture simply by filtration ().
Table 3 Synthesis of 2,4,5-trisubstituted imidazole derivatives catalyzed by SbCl3/SiO2.
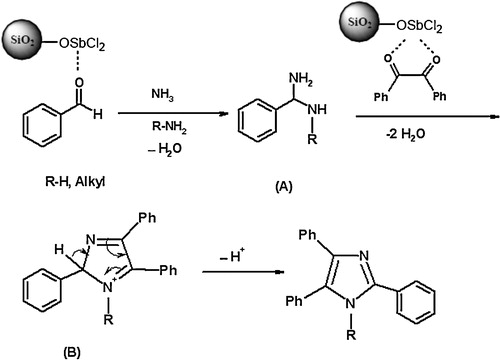
The probable mechanism for the synthesis of multi-substituted imidazoles in the presence of SbCl3/SiO2 is outlined in . The reaction proceeds via the diamine intermediate [A], which is formed by activation of the aldehyde carbonyl group by Lewis acids on the surface of the silica gel. Condensation of diamine with 1,2-diketone, followed by dehydration and then rearrangement through the imino intermediate [B], yielded the desired product. The Lewis acid site on silica-supported antimony (III) chloride acts as a highly efficient catalyst, even for low-activity substrates, in the preparation of imidazole derivatives. The higher catalytic activity of SbCl3/SiO2 is due to good dispersion of SbCl3 over the large surface area of the silica support. The results indicated that dispersed SbCl3 coordinates with surface hydroxyl groups, leading to formation of O–Sb–Cl as stable Lewis acid sites under the reaction condition [Citation35].
Scheme 3 Proposed mechanism for the formation of substituted imidazoles in the presence of SbCl3/SiO2.
We also examined the possibility of recycling the catalyst in the model reaction. When the reaction was complete, ethyl acetate was added to the reaction mixture, the mixture was stirred, and the SbCl3/SiO2 was filtered off, washed with ethyl acetate and activated by heating for 2 h at 110 °C. The recovered catalyst was used in another reaction with the same substrates with no significant loss of activity after five cycles (from 95% in the first run to 90% in the fifth run).
In order to show the advantages of this catalyst over other reported catalysts, we compared the reactions of SbCl3/SiO2 with ionic liquid [EMIM] OAc, Zr(acac)4, zinc (II) [tetra(4-methylphenyl)] porphyrin and BO3H3 for the preparation of imidazole (6a in ). SbCl3/SiO2 was a better catalyst with respect to reaction times and yields of products. Furthermore, SbCl3/SiO2 provides products of high purity in a short reaction time and is also cheap, speedy, facile and eco-friendly throughout the course of the reaction.
Table 4 Comparison of SbCl3/SiO2 with other catalysts reported in the literature for the synthesis of imidazoles.
4 Conclusion
In conclusion, SbCl3/SiO2 can catalyze the one-pot synthesis of a large number of multi-substituted imidazoles at moderate temperature under microwave conditions very efficiently. Microwave-promoted solvent-free solid acid reactions are environmentally benign methods, usually with improved selectively, enhanced reaction rates, cleaner products and manipulative simplicity. SbCl3/SiO2 is also an excellent catalyst for microwave-assisted organic synthesis. We expect that this method will find extensive applications in the fields of combinatorial chemistry, diversity-oriented synthesis, heterogeneous catalytic systems and drug development.
Acknowledgement
This work was financially supported by a grant (No. 256722/19) from the research foundation of the University of Kashan.
Notes
Peer review under responsibility of Taibah University
References
- V.SinghP.KumarR.SanghiUse of microwave irradiation in the grafting modification of the polysaccharides – a reviewProg. Polym. Sci.372012340364
- K.BougrinA.LoupyM.SoufiaouiMicrowave-assisted solvent-free heterocyclic synthesisJ. Photochem. Photobiol. C: Photochem. Rev.62005139167
- M.V.ReddyG.C.S.ReddyY.T.JeongMicrowave-assisted, montmorillonite K-10 catalyzed three-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones under solvent-free conditionsTetrahedron Lett.68201268206828
- S.MaityS.PathakA.PramanikMicrowave assisted synthesis of 2,3-diaryl-6,7-dihydro-5H-pyrrolo[1,2-a]imidazoles through direct condensation of aryl 1,2-diketones and l-proline under solvent-free conditionTetrahedron Lett.54201325282532
- K.SivakumarA.KathirvelA.LalithaSimple and efficient method for the synthesis of highly substituted imidazoles using zeolite-supported reagentsTetrahedron Lett.51201030183021
- M.AntoliniA.BozzoliC.GhironG.KennedyT.RossiA.UrsiniAnalogues of 4,5-bis(3,5-dichlorophenyl)-2-trifluoromethyl-1H-imidazole as potential antibacterial agentsBioorg. Med. Chem. Lett.9199910231028
- L.WangK.W.WoodsQ.LiK.J.BarrR.W.McCroskeyS.M.HannickL.GherkeR.B.CredoY.H.HuiK.MarshR.WarnerJ.Y.LeeN.Z.MozngD.FrostS.H.RosenbergH.L.ShamPotent, orally active heterocycle-based combretastatin A-4 analogues: synthesis, structure–activity relationship, pharmacokinetics, and in vivo antitumor activity evaluationJ. Med. Chem.45200216971711
- J.C.LeeJ.T.LaydonP.C.McDonnellT.F.GallagherS.KumarD.GreenD.McNultyM.J.BlumenthalJ.R.KeysS.W.L.VatterJ.E.StricklerM.M.McLaughlinI.R.SiemensS.M.FisherG.P.LiviJ.R.WhiteJ.L.AdamsP.R.YoungA protein kinase involved in the regulation of inflammatory cytokine biosynthesisNature3721994739746
- A.K.TakleM.J.B.BrownS.DaviesD.K.DeanG.FrancisA.GaibaA.W.HirdF.D.KingP.J.LovellA.NaylorA.D.ReithJ.G.SteadmanD.M.WilsonThe identification of potent and selective imidazole-based inhibitors of B-Raf kinaseBioorg. Med. Chem. Lett.162006378381
- J.HeeresL.J.J.BackxJ.H.MostmansJ.Van CustemAntimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agentJ. Med. Chem.22197910031005
- R. Schmierer, H. Mildenberger, H. Buerstell, German Patent 361464, 1987, Chem. Abstr. 108 (1988) 37838.
- T. Maier, R. Schmierer, K. Bauer, H. Bieringer, H. Buerstell, B. Sachse, U.S. Patent 4,820,335, 1989, Chem. Abstr. 111 (1989) 19494w.
- B.RadziszewskiUeber die Constitution des Lophins und verwandter VerbindungenChem. Ber.15188214931496
- F.R.JappH.H.RobinsonConstitution des Lophins und des AmarinsChem. Ber.15188212681270
- D.KumarD.N.KommiA.R.PatelA.K.ChakrabortiCatalytic procedures for multicomponent synthesis of imidazoles: selectivity control during the competitive formation of tri- and tetrasubstituted imidazolesGreen Chem.14201220382049
- F.K.BehbehaniT.YektanezhadA greener route for the one-pot synthesis of 1,2,4,5-tetraarylated imidazolesMonatsh. Chem.143201215291532
- B.SadeghiB.B.F.MirjaliliM.M.HashemiBF3·SiO2: an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazolesTetrahedron Lett.49200825752577
- Z.TavakoliM.BagherneghadK.NiknamSynthesis of 1,2,4,5-tetrasubstituted imidazoles using sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl]ester as a recyclable solid acidJ. Heterocycl. Chem.492012634639
- X.B.WangL.HeT.Y.JianS.YeCyclic phosphoric acid catalyzed one-pot, four-component synthesis of 1,2,4,5-tetrasubstituted imidazolesChin. Chem. Lett.2320121316
- A.R.KarimiZ.AlimohammadiM.M.AminiWells–Dawson heteropolyacid supported on silica: a highly efficient catalyst for synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazolesMol. Divers.142010635641
- M.M.HeraviF.DerikvandF.F.BamoharramHighly efficient, four-component one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacids as green and reusable catalystsJ. Mol. Catal. A: Chem.2632007112114
- L.NagarapuS.ApuriS.KantevariPotassium dodecatugstocobaltate trihydrate (K5CoW12O40·3H2O): a mild and efficient reusable catalyst for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles under conventional heating and microwave irradiationJ. Mol. Catal. A: Chem.2662007104108
- H.ZangQ.SuY.MoB.W.ChengS.JunIonic liquid [EMIM]OAc under ultrasonic irradiation towards the first synthesis of trisubstituted imidazolesUltrason. Sonochem.172010749751
- A.HasaninejadA.ZareM.ShekouhyJ.A.RadCatalyst-free one-pot four component synthesis of polysubstituted imidazoles in neutral ionic liquid 1-butyl-3-methylimidazolium bromideJ. Comb. Chem.122010844849
- S.RostamniaA.ZabardastiSBA-15/TFE (SBA-15/2,2,2-trifluoroethanol) as a suitable and effective metal-free catalyst for the preparation of the tri- and tetra-substituted imidazoles via one-pot multicomponent methodJ. Fluorine Chem.14420126972
- V.KannanK.SreekumarClay supported titanium catalyst for the solvent free synthesis of tetrasubstituted imidazoles and benzimidazolesJ. Mol. Catal. A: Chem.37620133439
- S.BalalaieM.M.HashemiM.AkbariA novel one-pot synthesis of tetrasubstituted imidazoles under solvent-free conditions and microwave irradiationTetrahedron Lett.44200317091711
- S.A.SiddiquiU.C.NarkhedeS.S.PalimkarT.DanielR.J.LahotiK.V.SrinivasanRoom temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or α-hydroxyketoneTetrahedron Lett.61200535393546
- J.WangR.MasonD.V.DerveerK.FengX.R.BuConvenient preparation of a novel class of imidazo[1,5-a]pyridines: decisive role by ammonium acetate in chemoselectivityJ. Org. Chem.68200354155418
- A.ShaabaniA.RahmatiSilica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstituted imidazolesJ. Mol. Catal. A: Chem.2492006246248
- S.D.SharmaP.HazarikaD.KonwarAn efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by InCl3·3H2OTetrahedron Lett.49200822162220
- J.N.SangshettiN.D.KokareS.A.KothrkaraD.B.ShindeCeric ammonium nitrate catalysed three component one-pot efficient synthesis of 2,4,5-triaryl-1H-imidazolesJ. Chem. Sci.1202008463467
- M.M.HeraviK.BakhtiariH.A.OskooieS.TaheriSynthesis of 2,4,5-triaryl-imidazoles catalyzed by NiCl2·6H2O under heterogeneous systemJ. Mol. Catal. A: Chem.2632007279281
- S.BalalaieA.ArabanianOne-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiationGreen Chem.22000274276
- H.R.DarabiK.AghapoorF.MohsenzadehF.TaalaN.AsadollahnejadA.BadieiSilica-supported antimony(III) chloride as highly effective and reusable heterogeneous catalyst for the synthesis of quinoxalinesCatal. Lett.13320098489
- J.SafariZ.ZarnegarA highly efficient magnetic solid acid catalyst for synthesis of 2,4,5-trisubstituted imidazoles under ultrasound irradiationUltrason. Sonochem.202013740746
- A.SrinivasaB.P.NandeshwarappaB.M.KiranK.M.MahadevanAntimony (III) sulfate catalyzed one-pot synthesis of 2,3-disubstitutedindolesPhosphorus Sulfur Silicon182200722432266
- G.MaitiP.KunduAntimony trichloride-catalyzed Michael addition of indoles to the α,β-unsaturated ketonesSynth. Commun.37200823092316
- M.C.SinghR.K.PeddintiAntimony(III) chloride-catalyzed ring opening of epoxides with anilinesTetrahedron Lett.48200773547357
- L.F.ZhangS.T.YangSilica-supported antimony(III) chloride as an efficient heterogeneous catalyst for the synthesis of aminopropenones and 3-aminopropenoates under solvent-free conditionsRuss. J. Org. Chem.4520091821
- J.SafariS.H.BanitabaS.Dehghan KhaliliCellulose sulfuric acid catalyzed multicomponent reaction for efficient synthesis of 1,4-dihydropyridines via unsymmetrical Hantzsch reaction in aqueous mediaJ. Mol. Catal. A: Chem.33520115064
- J.SafariS.H.BanitabaS.Dehghan KhaliliCobalt nanoparticles promoted highly efficient one pot four-component synthesis of 1,4-dihydropyridines under solvent-free conditionsChin. J. Catal.12201118501855
- J.SafariZ.ZarnegarBiginelli reaction on Fe3O4–MWCNT nanocomposite: excellent reactivity and facile recyclability of the catalyst combined with ultrasound irradiationR. Soc. Chem. Adv.320131796217967
- B.KriegG.Z.ManeckeSynthese und Halbleitereigenschaften arylsubstituierter ImidaZoleNaturforschg22b1967132
- S.BalalaieA.ArabanianM.S.HashtroudiZeolite HY and silica gel as new and efficient heterogenous catalysts for the synthesis of triarylimidazoles under microwave irradiationMonatsh. Chem.1312000945948
- A.Y.UsyatinskyY.L.KhmelnitskyMicrowave-assisted synthesis of substituted imidazoles on a solid support under solvent-free conditionsTetrahedron Lett.41200050315035
- M.KidwaiP.MothsraV.BansalR.K.SomvanshiA.S.EthayathullaS.DeyT.P.SinghOne-pot synthesis of highly substituted imidazoles using molecular iodine: a versatile catalystJ. Mol. Catal. A: Chem.2652007177182
- S.Narayana MurthyB.MadhavY.V.D.NageswarDABCO as a mild and efficient catalytic system for the synthesis of highly substituted imidazoles via multi-component condensation strategyTetrahedron Lett.51201052525257
- A.R.KhosropourUltrasound-promoted greener synthesis of 2,4,5-trisubstituted imidazoles catalyzed by Zr(acac)4 under ambient conditionsUltrason. Sonochem.152008659664
- J.SafariZ.AkbariS.NasehNanocrystalline MgAl2O4 as an efficient catalyst for one-pot synthesis of multisubstituted imidazoles under solvent-free conditionsJ. Saudi Chem. Soc.52012419424