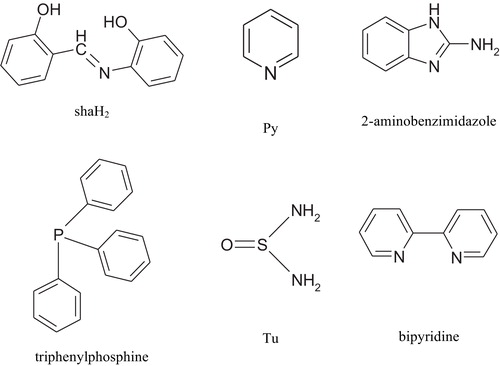
Abstract
The mononuclear ruthenium tricarbonyl derivative mer-[Ru(CO)3(sha)], 1, was synthesized from the reaction of [Ru3(CO)12] with N-salicylidene-2-hydroxyaniline (shaH2) Schiff base. The corresponding reactions of the ruthenium cluster with shaH2 in the presence of a secondary ligand L, L = 2-aminobenzimidazole, thiourea, pyridine, and triphenylphosphine, resulted in formation of the dicarbonyl derivatives [Ru(CO)2(sha)(L)], 2–5. The two carbonyl groups in these complexes were attached to the metal in a cis arrangement. In case of bipyridine (bpy) as a secondary ligand the [Ru(CO)2(shaH)(bpy)], 6, where the two CO moieties in a trans position. All the complexes were characterized by elemental analysis, mass, infrared and 1H nuclear magnetic resonance spectra. The ultraviolet–vis spectra of the complexes showed broad visible bands due to metal-to-ligand charge transfer. The stereochemistry and theoretical optimization of the three-dimensional geometry of the ligand and the complexes were determined.
1 Introduction
Much attention is being directed to the chemistry of ruthenium because of its promising photochemical and catalytic properties [Citation1,Citation2]. Ru(II) carbonyl complexes containing O,N-donor ligands and heterocyclic bases show redox properties as well as biological activity [Citation3]. For example, Ru(II) carbonyl complexes containing bidentate Schiff bases and triphenylphosphine or pyridine were found to be efficient catalysts in the oxidation of alcohols to aldehydes in the presence of N-methylmorpholine-N-oxide as a co-oxidant [Citation4,Citation5]. Furthermore, ruthenium carbonyl carboxylate complexes containing bipyridine ligands have good catalytic activity in the homogeneous hydrogenation of C=C and C=O bonds in hydroalcoholic solvents, which is an environmentally friendly process [Citation6].
Although Ru(I) complexes usually exist in dimeric forms, such as [Ru(OAc)(CO)2(dpa)]2, dpa = di(2-pyridyl)amine and [Ru2(C6H5CO2)2(CO)4(pyridine)2] [Citation7,Citation8], few such complexes have been reported with a mononuclear structure. Reaction of [Ru3(CO)12] with salicylideneimine-2-thiophenol (satpH2) Schiff base gave [RuI(CO)4(satpH)] complex and spectroscopic and magnetic studies of the complex revealed that it was paramagnetic with an octahedral arrangement [Citation9]. Our interest in the coordination chemistry of low-valent ruthenium complexes, especially with molecularly designed Schiff base ligands, prompted us to investigate the reactions of [Ru3(CO)12] with N-salicylidene-2-hydroxyaniline (shaH2) alone and in the presence of mono and bidentate secondary ligand ().
2 Materials and Methods
2.1 Reagents
[Ru3(CO)12], pyridine, triphenylphosphine, 2-aminobenzimidazole, thiourea and 2,2′-bipyridine were purchased from Aldrich. N-Salicylidene-2-hydroxyaniline was prepared as described elsewhere [Citation10]. All the solvents used were of analytical grade and were purified by distillation according to standard methods.
2.2 Instruments
Infrared (IR) measurements (KBr pellets) were carried out on a Shimadzu 8000 Fourier transform IR spectrometer. Electronic absorption spectra were measured on a Unicam UV2-300 ultraviolet (UV)–vis spectrophotometer. The spectra of the complexes were determined in various solvents (dimethylformamide, benzene, tetrahydrofuran and CH2Cl2) at concentrations of about 1 × 10−5 M. 1H Nuclear magnetic resonance spectra measurements were determined on a Varian-Mercury 300 MHz spectrometer. Samples were dissolved in (CD3)2SO with tetramethylsilane as the internal reference. The complexes were also characterized by elemental analysis (Perkin-Elmer 2400 CHN) and mass spectrometry (70 eV, EI, Finnigan MAT SSQ 7000 spectrometer). lists the color, yield, elemental analysis, and mass spectrometry data for each complex.
Table 1 Elemental analysis and mass spectrometry data for the ruthenium complexes.
2.3 Synthesis of complexes
2.3.1 Synthesis of [Ru(CO)3(sha)] complex (1)
A mixture of [Ru3(CO)12] (0.1 g, 0.16 mmol), shaH2 (0.1 g, 0.47 mmol) and benzene (about 30 cm3) was heated to reflux for 2 h. The color of the solution changed from orange to dark gray. The reaction mixture was cooled and the solvent was removed by evaporation. The solid residue was washed several times with boiling petroleum ether and then recrystallized from hot benzene.
2.3.2 Syntheses of [Ru(CO)2(sha)L] complexes (2–5)
A mixture of [Ru3(CO)12] (0.1 g, 0.16 mmol), shaH2 (0.1 g, 0.47 mmol) and L (pyridine, triphenylphosphine, thiourea and 2-aminobenzimidazole in benzene (about 30 cm3) was heated to reflux for 3–4 h. The reaction mixture was then cooled, and the solvent was removed by evaporation. The complex was washed several times with boiling petroleum ether and then recrystallized from hot benzene.
2.3.3 Synthesis of [Ru(CO)2(shaH)(bipyridine)] complex (6)
The procedure used was similar to that for the synthesis of complexes 2–5.
2.4 Computational details
All the calculations were performed as described previously [Citation11].
3 Results and discussion
3.1 IR and NMR studies
A series of ruthenium carbonyl Schiff base complexes (1–6) was synthesized from the reactions of [Ru3(CO)12] with N-salicylidene-2-hydroxyaniline (shaH2) alone or in presence of a secondary ligand (triphenylphosphine, pyridine, 2-aminobenzimidazole, thiourea, and bipyridine). The Schiff base shaH2 ligand can act as a bidentate or a tridentate donor ligand, depending on the reaction conditions (e.g. oxidation state of the metal ion) [Citation10,Citation12]. The IR spectrum of the free Schiff base exhibited a strong band at 1631 cm−1 due to the azomethine group. In the spectra of all complexes, this band was shifted to the region 1602–1611 cm−1, indicating the coordination of the Schiff base through the azomethine nitrogen atom [Citation12] (). The bands at 1274 and 1243 cm−1 in the shaH2 ligand have been attributed to phenolic C–O stretching. On complexation, these bands shift to higher frequencies. ν(Ru–O) and ν(Ru–N) bands were observed in the 533–574 and 426–500 cm−1 regions, respectively. In addition, all other characteristic bands due to triphenylphosphine, pyridine, 2-aminobenzimidazole, thiourea, and bipyridine were shifted to higher frequencies indicating coordination of these ligands to ruthenium species (). The 1H NMR spectra of the ligand and complexes 1–5 also confirmed the binding of the Schiff base and the secondary ligand to ruthenium (). The spectra showed lower field shifts in the region 6.00–8.96 ppm assigned to the aromatic protons of the sha, pyridine, triphenylphosphine, thiourea, and 2-aminobenzimidazole moieties [Citation13,Citation14].
Table 2 Important IR data for shaH2 and its ruthenium complexes.
Table 3 Important 1H NMR data for shaH2 and its ruthenium complexes.
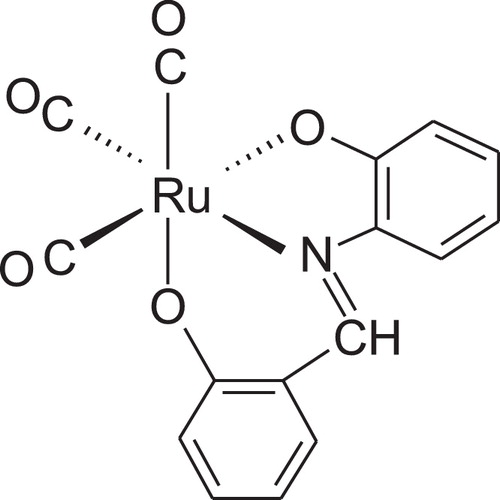
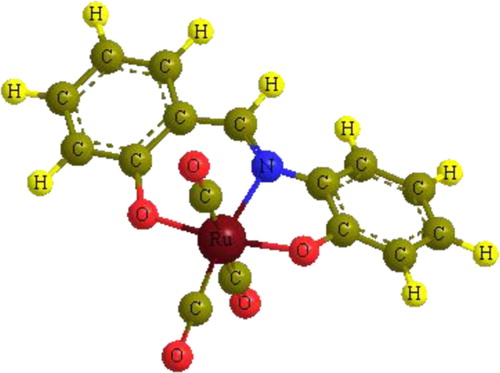
The IR spectrum of [Ru(CO)3(sha)] complex, 1, exhibited three bands in the terminal metal carbonyl region at 2052, 1981, and 1941 cm−1, indicating the presence of three CO groups attached terminally to ruthenium [Citation15]. The number and pattern of bands in the IR spectrum indicated that the complex exists in a mer-[Ru(CO)3(sha)] structure with a symmetry 2a1 + b1 [Citation16]. On comparison, the IR spectrum of the previously reported [Ru(CO)3(maleic hydrazide)] complex showed three bands at 2054, 1987, and 1920 cm−1 due to three CO groups bound to the metal [Citation17]. Interestingly, the band due to the two OH groups of the shaH2 ligand disappeared from the spectrum of the complex, as also confirmed by 1H NMR spectroscopy. Elimination of the hydrogen from the OH groups indicated that the metal coordinated to the ligand oxidatively [Citation18–Citation20]. From the elemental analysis and spectroscopic data, it can be concluded that the ruthenium species exists in an octahedral environment with a 2+ oxidation state (d6) and a low spin electronic configuration ().
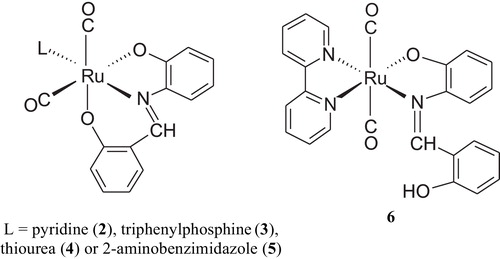
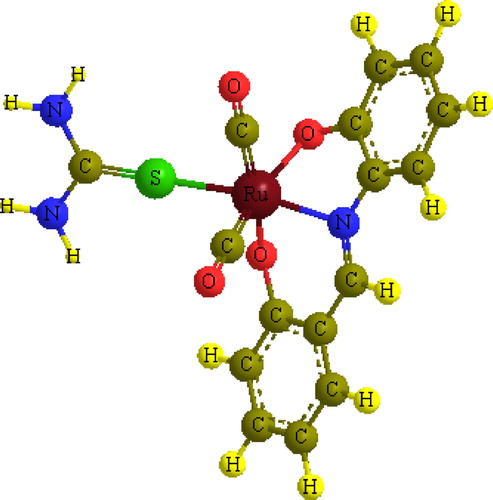
The IR spectra of the four complexes [Ru(CO)2(sha)L], where L = pyridine, 2, triphenylphosphine, 3, thiourea, 4, and 2-aminobenzimidazole, 5, exhibited symmetric and asymmetric stretching vibrations (a1 + b1) in the terminal metal carbonyl region due to two CO groups attached to ruthenium (). The existence of two bands for two CO groups bound to a metal indicates that they are in a cis position. The IR and NMR spectra of the complexes showed disappearance of the OH band-signal of the Schiff base, indicating that the ligand bound to the metal with the displacement of the two OH protons, i.e., the metal was in a 2+ oxidation state. Therefore, the ruthenium metal would expect to have a six coordinate arrangement in which the Schiff base bound from its tridentate donor atoms in addition to two terminal carbonyl groups and the secondary monodentate ligand. shows the proposed structure of the complexes.
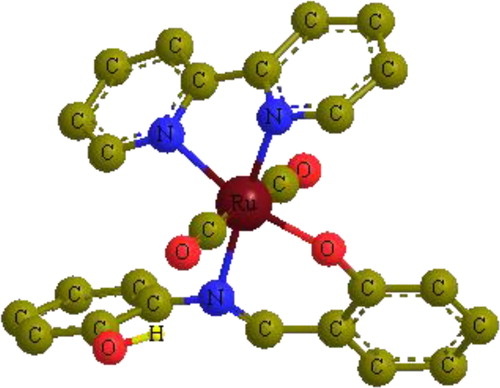
The IR spectrum of the complex [Ru(CO)2(shaH)((bipyridine)], 6, showed a ν(OH) stretching frequency at 3420 cm−1. It also displayed a strong band (a1g) in the terminal metal carbonyl region due to two CO ligands attached to the metal [Citation21,Citation22]. The 1H NMR spectrum of 6 showed no signals for the ligands protons, which is a characteristic feature of paramagnetic species. Magnetic susceptibility measurement of the complex at 298 K showed an effective magnetic moment μeff of 1.56 μB. The μeff value is slightly smaller than the spin-only moment of an unpaired electron (1.73 μB). Thus, it can be concluded that a change in the formal oxidation state of the ruthenium atom from zero to 1+ was achieved via oxidative addition of the shaH2 ligand to ruthenium with a proton displacement to give a low-spin d7 electronic configuration [Citation19]. Therefore, according to the spectroscopic studies, the complex might have the structure proposed in .
3.2 Stereochemistry of complexes
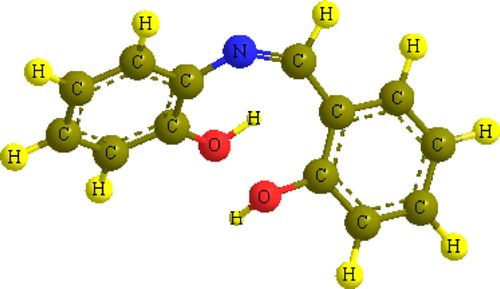
In investigating the stereochemistry of the most stable complexes, we first focused on the structure of the shaH2 molecule and specifically on the orientation of its terminal functional groups with respect to each other and to the central azomethine moiety. An energetically stable model (19.45 kcal/mol) showed a specific feature, whereby one of the hydroxyl groups was oriented towards the azomethine nitrogen to form a hydrogen bond (N–H distance = 2.53 Å, ). In addition, it can be observed that the structure is not planar; the hydroxyl group that is not involved in the hydrogen bonding is bent away from the plane and contains the imine and the other hydroxyl group in order to reduce the steric repulsion. The dihedral angle between the two planes was found to be 65.2°. This orientation suggests that the ligand coordinates to the metal through the azomethine nitrogen and one hydroxyl group acts as a bidentate ligand or acts as a tridentate with the coordination of additional hydroxyl oxygen atoms.
For the [Ru(CO)3(sha)] complex, an energetically stable model with distorted octahedral geometry was found to have about 99.23 kcal/mol, which is consistent with the spectroscopic findings (). The bonding between Ru and the sha moiety was determined from distance and angle analysis, showing bond lengths for Ru–N and the two Ru–O of 1.94, 1.89, and 1.90 Å, respectively. Optimized angles for N–Ru–O (six membered cycle), N–Ru–O (five-membered cycle) and O–Ru–O are 96.1, 75.8, and 171.1°, respectively. The three carbonyl groups bound to the central Ru species form a T-shape to complete the octahedral coordination. The bond angle between the two trans COs is 175.6°, while the angles between the CO groups in the cis positions are 91.5̊ and 91.4°.
The four Ru(CO)2(sha)L complexes were optimized at the same level of theory. illustrates the minimum energy optimized structure of Ru(CO)2(sha)(thiourea) complex as an example. The significant feature of these structures is the distorted octahedral coordination around the ruthenium species. For example, the bond angles involving ruthenium in the Ru(CO)2(sha)(thiourea) complex are: S–Ru–N = 163.3°, S–Ru–O = 107.3°, S–Ru–CO = 85.5° and C–Ru–O = 82.5°. To release the steric effect, the two CO groups were bent, forming an OC–Ru–CO angle of 89.4° with a cis configuration. The cis configuration is consistent with the order of the symmetric and asymmetric stretching frequencies in the IR spectrum of the complex.
In the case of the Ru(CO)2(shaH)(bipyridine) complex, where the secondary ligand is the bidentate bipyridine ligand, the energetically stable model (476.17 kcal/mol) shows different structural arrangements from those of the monodentate ligands (). The shaH moiety is attached to Ru as a bidentate ligand and the two CO groups are bound to the metal in a trans fashion (OC–Ru–CO bond angle, 173.2°), which is consistent with the IR studies. Also, the hydroxyl group of the shaH moiety was oriented toward the azomethine nitrogen to form a hydrogen bond (N–H distance = 2.30 Å).
3.3 Electronic absorption spectra
The UV–vis spectra of the shaH2 ligand and the complexes were measured in various solvents (tetrahydrofuran, CH2Cl2, dimethylformamide, and C6H6)(). The electronic absorption spectrum of shaH2 consisted of two high intensity bands in the range 262–272 and 352–357 nm, due to π–π* and n–π* transitions, respectively. A bathochromic shift in the π–π* electronic transitions was observed in the complexes, while the n–π* bands exhibited hypsochromic shift with a considerable change in absorbance (). These orders of shifts in electronic transitions are consistent with complex formation. The solvent had a slight effect on the band positions. All the spectra of the complexes exhibited bands around 400–500 nm, which could be assigned to charge transfer transitions [Citation23]. The spectrum of 1 showed a broad absorption band around 400 nm in all solvents, which could be due to M→L charge transfer arising from excitation of an electron from the metal t2g level to the unfilled molecular orbitals derived from the π* level of the ligands, in accordance with the assignments made for similar octahedral ruthenium (II) complexes [Citation5]. Complexes 2–6, however, exhibited broad absorption bands, with shoulders at lower energies than complex 1. The presence of different acceptor levels in these complexes may be responsible for the observed multiple absorptions [Citation9,Citation24,Citation25].
Table 4 The UV–vis data for shaH2 and its ruthenium complexes.
Notes
Peer review under responsibility of Taibah University
References
- J.R.ReimersN.S.HushElectron transfer and energy transfer through bridged systems. 4. Intermetallic coupling and electronic spectra of the bis(pentaammineruthenium) complexes of α,ω-dipyridyl trans-polyenes in deuterium oxideInorg. Chem.29199036863697
- P.FredianiM.BianchiA.SalviniR.GuarducciL.C.CarluccioF.PiacentiRuthenium carbonyl carboxylate complexes with nitrogen-containing ligands III. Catalytic activity in hydrogenationJ. Organomet. Chem.4981995187197
- K.K.NareshR.RameshSynthesis, characterization, redox property and biological activity of Ru(II) carbonyl complexes containing O, N-donor ligands and heterocyclic basesSpectrochim. Acta A60200429132918
- A.M.El-HendawyA.El-KourashyM.M.ShanabSchiff base complexes of ruthenium (III), molybdenum (VI) and uranium (VI) and use of the former as catalytic organic oxidantsPolyhedron111992523530
- P.ViswanathamurthiR.KarvembuV.TharaneeswaranK.NatarajanRuthenium (II) complexes containing bidentate Schiff bases and triphenylphosphine or triphenylarsineJ. Chem. Sci.1172005235238
- A.SalviniP.FredianiE.RivaltaRuthenium carbonyl carboxylates with nitrogen containing ligands: V. On the syntheses and catalytic activity of new ruthenium complexes containing bicarboxylate ligandsInorg. Chim. Acta3512003225234
- C.M.KepertG.B.DeaconL.SpicciaSynthesis and structures of di(2-pyridyl)amine diruthenium(I) complexes, including an example of monodentate coordinationInorg. Chim. Acta3552003213222
- B.TherrienG.Süss-FinkSawhorse-type diruthenium tetracarbonyl complexesCoord. Chem. Rev.253200926392664
- M.M.H.KhalilM.M.AboalyR.M.RamadanSpectroscopic and electrochemical studies of ruthenium and osmium complexes of salicylideneimine-2-thiophenol Schiff baseSpectrochim. Acta A612005157161
- S.M.El-MedaniStructural studies of some chromium, molybdenum and tungsten complexes of N-salicylidene-2-hydroxyanilineJ. Coord. Chem.572004115122
- R.M.RamadanL.H.Abdel-RahmanM.IsmaelT.A.YoussefS.A.AliSynthesis and spectroscopic studies of some chromium and molybdenum derivatives of bis-(acetylacetone)ethylenediimine ligandJ. Mol. Struct.10492013712
- S.M.El-MedaniO.A.M.AliR.M.RamadanPhotochemical reactions of group 6 metal carbonyls with N-salicylidene-2-hydroxyaniline and bis-(salicylaldehyde)phenylenediimineJ. Mol. Struct.7382005171177
- T.J.BatterhamNMR Spectra of Simple Heterocycles1982R.E. KriegerNew York
- N.ChandrashekharB.ThomasV.GayathriK.V.RamanathanN.M.GowdaSynthesis and NMR spectral assignments of novel nitrogen and sulfur heterocyclic compoundsMagn. Reson. Chem.462008769774
- K.NakamotoInfrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic and Bioinorganic Chemistry6th ed.2009WileyNew York
- K.Veera ReddySymmetry and Spectroscopy of Molecules2009New Age InternationalNew Delhi
- H.A.MohamedS.A.AliR.M.RamadanSpectroscopic and thermal studies of chromium (III), molybdenum (VI) and ruthenium (0) complexes of maleic hydrazideSpectrochim. Acta A642006913917
- J.CollmanL.S.HegedusPrinciples and Application of Organotransition Metal Chemistry1980University Science BookHerndon, Virginia
- O.A.M.AliM.M.H.KhalilG.M.AttiaR.M.RamadanGroup VI dinuclear oxo metal complexes of salicylideneimine-2-anisole Schiff baseSpectrosc. Lett.3620037182
- D.Y.SabryT.A.YoussefS.M.El-MedaniR.M.RamadanReactions of chromium and molybdenum carbonyls with bis-(salicylaldehyde)ethylenediamine Schiff baseJ. Coord. Chem.56200313751381
- M.A.TaherS.E.JarelnabbiA.G.M.Al-SehemiS.M.El-MedaniR.M.RamadanSynthesis and spectroscopic studies of some new metal carbonyl derivatives of 1-(2-pyridylazo)-2-naphtholJ. Coord. Chem.62200912931301
- R.M.RamadanSynthesis and physicochemical studies of new dimethylglyoxime-tungsten complexesTransit. Met. Chem.231998507510
- A.B.P.LeverInorganic Electronic Spectroscopy2nd ed.1984ElsevierNew York
- T.MathurJ.DindaP.DattaG.MostafaT.-H.LuC.SinhaRu(0)-azoimine-carbonyl and Ru(II)-pyridyl-azo-imidazole complexesPolyhedron25200625032512
- M.M.H.KhalilH.A.MohamedS.M.El-MedaniR.M.RamadanNew group 6 metal carbonyl derivatives of 2-(2′(-pyridyl)benzimidazole: synthesis and spectroscopic studiesSpectrochim. Acta A59200313411347