Abstract
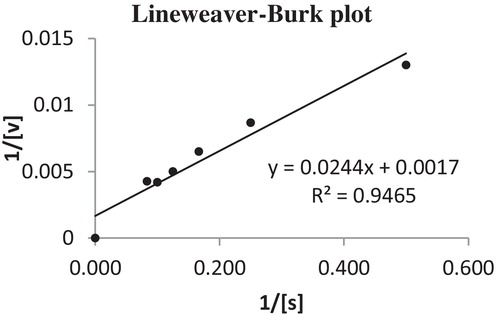
An efficient alkaline protease producer was isolated from the water of the hyperalkaline-saline Lonar soda lake and identified as Bacillus alcalophilus LW8 by culture-dependent techniques by its morphological, microscopic, biochemical, physiological and molecular characteristics. The 16S rRNA gene sequence was submitted to the GenBank nucleotide repository under accession number KC689353. Alkaline protease production was optimized by adopting a one-variable-at-a-time approach in a submerged fermentation system in modified fermentation medium (MFM). The optimized components of MFM were (w/w) casein (1%), sugarcane molasses (1%), NaCl (1%), ammonium sulphate (0.5%), KH2PO4 (0.05%), K2HPO4 (0.05%) and Na2CO3 (1%). Optimized culture conditions were used for alkaline protease production. The final yield of partially purified alkaline protease after dialysis was 53.35%. The molecular mass of the dialysed alkaline protease was 27 kDa. On a Lineweaver–Burk plot, the calculated Km and Vmax values were 24 mg/mL and 1000 U/mg, respectively. The enzyme was remarkably stable in the pH range 7.0–12.0, with optimum activity at pH 10.0. LW8 alkaline protease was completely inhibited by phenylmethylsulphonyl fluoride at 10 mmol/L, indicating that the enzyme belongs to the serine protease class. The metal ions Ca2+, Ba2+, Mg2+, Zn2+, Fe3+, Cu2+ and Mn2+ increased the catalytic activity of partially purified alkaline protease. The protease effectively decomposed the gelatinous coating on an X-ray film, hydrolysed blood clot, a blood-stain from a piece of cotton fabric and hairs from a piece of goat skin.
1 Introduction
Lonar crater (19°58’ N, 76°31’ E) is a simple, bowl-shaped, near-circular, remarkably well-preserved depression in the otherwise featureless Deccan Plateau in Buldhana district, Maharashtra State, India [Citation1]. It was first brought to international notice in 1823 by a British officer CJE Alexander [Citation2]. Lonar soda lake is a habitat conducive for alkaliphiles and alkali-stable extracellular enzyme producers.
Isolation of microorganisms to be used in industrial applications from sources such as alkaline habitats is much preferred, as these strains produce enzymes that are stable under high alkaline conditions and can resist chemical denaturants present in detergents. There is a growing need to develop a cost-effective, environment-friendly method for synthesizing such enzymes, to replace traditional carbon and nitrogen sources in various media. Use of agro-industrial residues as carbon and nitrogen sources has become popular in the synthesis of eco-enzymes. The aim of this study was to optimize alkaline protease production by Bacillus alcalophilus LW8 and to investigate its biotechnological applications.
2 Material and methods
2.1 Isolation and screening of alkaline protease producers
Water samples were collected from Lonar Lake, and their pH was measured. A composite water sample was inoculated into basal medium containing (g/L) glucose 2.0, casein 0.5, peptone 0.5, yeast extract 0.5, Na2CO3 10.0 and salt solution 50 mL [salt solution: (g/L) KH2PO4 5.0, MgSO4·7H2O 5.0 and FeSO4 0.1] enriched at 30 °C and 100 rpm for 24 h, then incubated in an orbital shaking incubator [Citation3]. The enriched broth was diluted and spread on Horikoshi and Akiba agar plates [Citation4], which were incubated at 30 °C for 48 h. Isolated colonies were cultivated on Horikoshi and Akiba agar slants. Cultures were incubated on alkaline casein agar [Citation5], and amido black solution was flooded onto the plates [Citation6] to screen for alkaline protease production. Cells (1%, v/v) from each isolate were grown overnight and inoculated into modified fermentation medium (MFM) comprising (g/L) casein 10.0, K2HPO4 0.5, KH2PO4 0.5 and Na2CO3 10.0 and incubated at 30 °C and 200 rpm for 72 h. The cultures were then centrifuged at 10 000 rpm for 10 min at 4 °C [Citation7]. Cell-free supernatants were subjected to the qualitative casein cup assay [Citation3,Citation6,Citation7], and a modified Anson’s quantitative assay for alkaline protease activity [Citation8] was carried out. One proteolytic unit was defined as the amount of the enzyme that releases 1 μmol/min of tyrosine under the assay conditions [Citation8,Citation9]. Total protein content was determined with bovine serum albumin as the standard [Citation10]. The isolate that yielded the highest alkaline protease activity, LW8, was selected for further studies.
2.2 Preliminary and molecular identification
The LW8 isolate was characterized by determining its morphological, microscopic and sugar use characteristics, enzyme profile, antibiotic sensitivity and physiological attributes [Citation4,Citation9,Citation11–Citation15]. Further, it was subjected to 16S rRNA molecular analysis. DNA was extracted by InstaGene Matrix (Bio-Rad, USA) treatment from cell pellets [Citation16], and the 16S rRNA gene was amplified in a thermocycler (Applied Biosystems, USA) with pair of primers (forward (27f) AGAGTTTGATCMTGGCTCAG and reverse (1492r) TACGGYTACCTTGTTACGACTT) [Citation7,Citation17,Citation18]. The amplified 16S rDNA PCR product was gel-purified with a QIAquick Gel Extraction kit (Qiagen, USA) [Citation19] and sequenced in an ABI Prism™ 377 automated DNA sequencer (Applied Biosystems, USA) [Citation18,Citation20]. The deduced sequence was compared with GenBank data by the BlastN search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) [Citation21,Citation22]. Phylogenetic and molecular evolutionary analyses were conducted with MEGA version 6.06 software [Citation23]. A phylogenetic tree was constructed by the neighbour joining method [Citation24]. Bootstrap analysis 1000 replication was applied [Citation25]. The evolutionary distances were computed by the maximum composite likelihood method [Citation26].
2.3 Optimization of physicochemical parameters for maximum alkaline protease production
Factors that affect protease production, such as inducers, carbon and nitrogen sources, pH, temperature and salt concentration, were optimized by a search technique, i.e. a one-variable-at-a-time approach in a submerged fermentation system in MFM. The range of optimum parameters achieved by one step was fixed subsequently in the next step [Citation3,Citation7]. The effect of time and agitation on protease production by the LW8 isolate was determined. A culture of LW8 grown overnight, with A600 = 0.9154 was inoculated into MFM and incubated with shaking (50–200 rpm) and under static conditions at 30 °C for 96 h. Samples were withdrawn aseptically every 24 h, and the catalytic activity of alkaline protease was determined under standard assay conditions [Citation3,Citation7,Citation8]. The parameters tested for maximum protease production were pH (8.0–12.0), temperature (20–60 °C), salt concentration (0–7% NaCl) and inoculum size (1–5%) [Citation3,Citation7]. The inducers tested were casein, skimmed milk, gelatin, peptone, tryptone, casein hydrolysate, yeast extract, beef extract, meat extract, flour made of seeds (corn, pumpkin, soybean, green gram, chick pea), dried powdered cow dung [Citation27,Citation28], feathers and defatted seed meals (soybean, mustard, corn, groundnut, cotton, sesame, sunflower and safflower), amended at a concentration of 1% (w/w) in MFM. The best inducer was evaluated for optimum concentration between 0.5% and 3.0% [Citation3,Citation7,Citation29]. The effects on alkaline protease production by the LW8 isolate of synthetic carbon sources (arabinose, glucose, glycerol, lactose, mannitol, ribose, sorbitol, sucrose and xylose), low-cost agricultural residues (dried powder of wheat bran, rice bran and sugarcane bagasse), industrial residue (sugarcane molasses), inorganic nitrogen sources (ammonium sulphate, ammonium nitrate, ammonium chloride) were investigated at a concentration of 1.0%. The optimum carbon and nitrogen source concentration for enhanced enzyme production was also determined [Citation3,Citation7,Citation29,Citation30]. For bulk production, 500 mL of optimized modified fermentation medium was prepared in a 1-L Erlenmeyer flask. After sterilization, an optimized quantity of prepared inoculum was inoculated and incubated under optimized culture conditions [Citation3,Citation7].
2.4 Purification and characterization of alkaline protease
After completion of production, a cell-free supernatant of LW8 was extracted by centrifuging whole fermented broth at 10 000 rpm and 4 °C for 10 min. Enzyme precipitation was carried out with concentrations of ammonium sulphate of 10–100% at 4 °C and kept overnight [Citation3]. The enzyme precipitate was collected by centrifuging the ammonium sulphate fractions at 10 000 rpm for 20 min. The catalytic activity of the enzyme solution was determined under standard assay conditions. The active fractions were dialysed against 0.2 mol/L glycine–NaOH buffer (pH 10.0), and the enzyme activity was determined. The total protein content of the enzyme was determined by the method of Folin and Lowery [Citation10]. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to determine the molecular mass by comparison with the standard broad-range protein markers (Merck Biosciences) [Citation7,Citation31,Citation32]. The hydrolytic activity of the partially purified alkaline protease was examined with 1% (w/v) of various proteinaceous substrates, such as casein, bovine serum albumin, egg albumin, feather meal and haemoglobin [Citation7]. Concentrations of casein substrate solution ranging from 5 to 20 mg/mL were prepared in 0.2 mol/l glycine–NaOH buffer (pH 10) and used to determine the optimum substrate concentration for the catalytic activity of alkaline protease. Km and Vmax values were calculated from a Lineweaver–Burk plot [Citation33,Citation34]. The concentration of casein at which maximum enzyme activity was obtained was considered to be 100%, and relative enzyme activity was determined. The catalytic activity of the partially purified enzyme was screened at pH 6.0–13.0 at increments of 1.0 unit in an appropriate buffer system [Citation21,Citation32]. pH stability was determined by pre-incubating partially purified enzyme for 15–60 min at room temperature with appropriate buffers (pH 6.0–13.0, 200 mmol/L), and the residual activity was measured under standard assay conditions.
The catalytic activity of the partially purified alkaline protease was measured at temperatures of 20–70 °C at increments of 10 units. Thermostability was determined by pre-incubating the partially purified alkaline protease at these temperatures for 15–60 min, and residual activity was determined as described previously [Citation21,Citation32]. The effects of various protease inhibitors, oxidizing agent, chelator, surfactants, solvents, metal ions and laundry detergents on enzyme activity were determined by pre-incubating the enzyme in these chemicals for 30 min at room temperature; residual activity was measured under standard assay conditions. The laundry detergents were boiled for 15 min and cooled before use [Citation7,Citation21,Citation32]. Partially purified alkaline protease derived from LW8 was also tested for blood-stain removal on cotton fabric [Citation21], degradation of the gelatinous coating of X-ray film [Citation21], liquefaction of goat blood clots and removal of hairs from goat skin [Citation7].
2.5 Statistical analysis
Mean values were derived from experiments performed in triplicate and used for graphical and tabular representation of data. Error is indicated as standard deviation (n = 3) calculated with MS-Excel 2013 software [Citation7].
3 Results and discussion
3.1 Isolation, screening and identification of protease-producing bacteria
The pH of the Lonar Lake water samples was 10. After enrichment of samples, 87 colonies were found on the agar plates, and 15 morphologically distinct colonies were isolated. Of these, seven showed a zone of clearance on alkaline casein agar plates. The isolate with the largest clearance zone and with significantly higher activity (396.67 ± 15.27 U/mL) was designated LW8. A cell-free supernatant of LW8 showed a zone of clearance around the well in a casein agar plate. The phenotypic, biochemical and physiological characteristics of the LW8 isolate are shown in . The nucleotide sequence of the 16S rRNA gene of the isolate showed greatest homology (100%) with the previously published sequence of Bacillus alcalophilus strain IARI-IHD-22. The sequence was deposited in the NCBI nucleotide sequence repository with accession number KC689353. On the basis of the morphological, microscopic, biochemical and physiological attributes, antibiotic sensitivity and molecular analysis, LW8 was identified as B. alcalophilus. Phylogenetic affiliation of B. alcalophilus strain LW8 and some other members of the phylum Firmicute and class Bacilli showed distinct clustering of isolates based on partial sequences of the 16S rRNA gene ().
Fig. 1 Phylogenetic tree, phylogenetic affiliation of some taxa in phylum Firmicute and class Bacilli showing distinct clustering of isolates based on 16S rRNA partial gene sequences. The tree was constructed with the neighbour-joining method with 1000 bootstrap replications. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The analysis involved 24 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 376 positions in the final dataset. The optimal tree with the sum of branch length = 0.80749455 is shown. The scale bar represents 0.01 nucleotide substitutions per site.
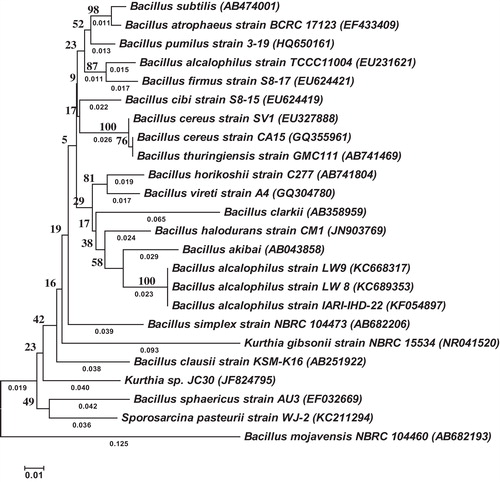
Table 1 Characterization of Bacillus alcalophilus LW8 isolate.
3.2 Optimization of physicochemical parameters for maximum alkaline protease production
B. alcalophilus LW8 exhibited maximum growth and alkaline protease production (510 ± 10 U/mL) at pH 10 and 30 °C after 48 h of incubation with shaking at 100 rpm. Further enhanced production (526.67 ± 7.63 U/mL) was recorded when 1% NaCl added to the MFM. Prolonged incubation and static conditions reduced protease production. Alkaline protease production and growth of LW8 were increased (548.33 ± 10.40 U/mL) when 4% of inoculum was added; a further increase in inoculum size (5%) enhanced growth but reduced protease production.
The effects of inducers on alkaline protease production are shown in A. Significant growth and protease production were seen with casein (548.33 ± 10.40 U/mL), followed by skimmed milk, gelatin, casein hydrolysate, soybean seed flour, feather meal, pumpkin seed flour, defatted soybean meal, chick pea seed flour, powdered cow dung, defatted groundnut meal, groundnut flour, defatted corn meal, yeast extract, defatted sunflower seed meal, defatted safflower seed meal, defatted cotton seed meal, corn seed flour, beef extract, defatted sesame seed meal, tryptone, peptone, meat extract and defatted mustard seed meal. The maximum level of protease production (548.33 ± 10.40 U/mL) was obtained when the medium was supplemented with 1% (w/w) casein as an inducer; further increases in concentration did not increase production further.
Fig. 2 Effects of various inducers and carbon and nitrogen sources on growth of LW8 strain and production of alkaline protease.
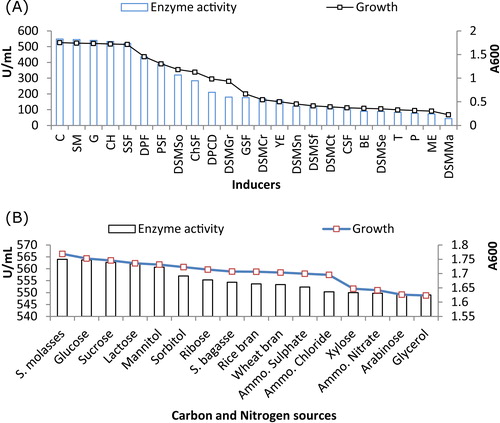
Extracellular alkaline protease production varied with the carbon and nitrogen source (B). Enhanced growth and production were seen in medium supplemented with sugarcane molasses (564 ± 1.73 U/mL), followed by glucose, sucrose, lactose, mannitol, sorbitol, ribose, sugarcane bagasse, rice bran, wheat bran, xylose, arabinose and glycerol. Of the inorganic nitrogen sources tested, maximum growth and alkaline protease production were found with ammonium sulphate (552.33 ± 13.65 U/mL), followed by ammonium chloride and ammonium nitrate. The greatest production of alkaline protease (569 ± 1.73 U/mL) was seen at optimum concentrations of sugarcane molasses (1.0%, w/w) and ammonium sulphate (0.5%, w/w); further increases decreased production.
3.3 Production, partial purification and characterization of alkaline protease
When concentrations of ammonium sulphate of 10–100% were added to 10 batches of 50 mL cell-free supernatant, a concentration of 60% have maximum alkaline protease activity (450 U/mL).
The steps involved in partial purification of the alkaline protease produced by strain LW8 are summarized in . A consistent increase in fold purification and specific activity at each step was observed. After dialysis, the enzyme resulted in a 1.24-fold increase, with 230.76 U/mg specific activity and 53.35% yield. The molecular mass of the dialysed alkaline protease was 27 kDa. Of the substrates tested, alkaline protease had a better hydrolysing capacity against casein, followed by bovine serum albumin, egg albumin, feather meal and haemoglobin (). The optimum casein concentration for the catalytic activity of the protease was 10 mg/mL. As per a Lineweaver–Burk plot, the calculated Km and Vmax were 24 mg/mL and 1000 U/mg, respectively (). The enzyme was remarkably stable in the pH range 7.0–12.0, with optimum activity at pH 10.0; residual activity greater than 50% was seen at pH 6.0–12.0 but was reduced to 33.3% at pH 13.0 after 60 min of pre-incubation in appropriate buffers. The enzyme had optimum catalytic activity at 50 °C (350 U/mL), followed by 40 °C, 30 °C, 20 °C, 60 °C and 70 °C before pre-incubation. The residual activity of alkaline protease from the LW8 strain in the presence of various chemicals, metal ions, solvents and detergents is shown in . The catalytic activity of partially purified LW8 alkaline protease was completely inhibited by 10 mmol/L phenylmethylsulfonyl fluoride, indicating that it is a serine alkaline protease.
Table 2 Characteristics of partially purified alkaline protease from Bacillus alcalophilus LW8.
Table 3 Effect of various chemicals, metal ions, solvents, detergents and substrates on activity of alkaline protease from LW8 strain.
3.4 Wash performance, degradation of gelatinous coating and liquefaction
Ariel™ alone in tap water did not completely remove a blood-stain from a piece of cotton cloth, but addition of alkaline protease from LW8 effectively removed the stain. It could therefore be used as an additive in laundry detergents. The protease also completely decomposed the gelatinous coating on a piece of X-ray film after 30 min of incubation, hydrolysed a blood clot effectively after 10 min at 30 °C and effectively removed hair from a goat skin after 7 h of incubation.
4 Conclusions
An efficient alkaline protease producer was isolated from Lonar soda lake and identified as B. alcalophilus LW8. Maximum alkaline protease production was achieved in optimized modified fermentation medium with various agro-industrial residues. The protease was remarkably stable in the presence of various chemical additives, including commercial laundry detergents. It also decomposed the gelatinous coating on an X-ray film, hydrolysed a blood clot, removed a blood-stain from cotton fabric and removed hair from a goat skin. Hence, the LW8 isolate and its alkaline protease could find potential applications in biotechnological industries.
Acknowledgements
The authors are thankful to the Honourable Vice-Chancellor, Swami Ramanand Teerth Marathwada University for providing the infrastructure and necessary facilities.
Notes
Peer review under responsibility of Taibah University
References
- C.P.AntonyD.KumaresanS.HungerH.L.DrakeJ.C.MurrellY.S.ShoucheMicrobiology of Lonar Lake and other soda lakesISME J.72013468476
- S.B.BorulStudy of water quality of Lonar LakeJ. Chem. Pharm. Res.4201217161718
- S.S.BalanR.NethajiS.SankarS.JayalakshmiProduction of gelatinase enzyme from Bacillus spp. isolated from the sediment sample of Porto Novo Coastal sitesAsian Pac. J. Trop. Biomed.2201218111816
- P.H.A.SneathN.S.MairM.E.SharpeJ.G.HoltBergey’s Manual of Systematic BacteriologyVol. I1986Williams & WilkinsBaltimore
- M.A.RonaldHandbook of Media for Environmental Microbiology2nd ed.2005CRC PressBoca Raton, Florida
- A.B.VermelhoM.N.L.MeirellesA.LopesS.D.G.PetinateA.A.ChaiaM.H.BranquinhaDetection of extracellular proteases from microorganisms on agar platesMem. Inst. Oswaldo Cruz911996755760
- J.RenganathanJ.ShanmugamA.BalumuriS.SundaramCharacterization of thermostable serine alkaline protease from an alkaliphilic strain Bacillus pumilus MCAS8 and its applicationsAppl. Biochem. Biotechnol.168201218491866
- S.S.YangJ.Y.WangProtease and amylase production of Streptomyces rimosus in submerged and solid state cultivationsBot. Bull. Acad. Sin.401999259265
- A.P.PathakM.G.RathodProduction and characterization of alkaline protease by Bacillus pasteurii: a Lonar soda lake isolateInnov. Res. Chem.120132226
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ. Biol. Chem.1931951265275
- K.R.AnejaExperiments in Microbiology, Plant Pathology and Biotechnology4th ed.2007New Age International PublishersNew Delhi
- K.HorikoshiT.AkibaAlkalophilic Microorganisms: A New Microbial World1982Springer-VerlagNew York
- A.P.PathakA.G.SardarIsolation and characterization of carotenoid producing Haloarchaea from solar saltern of Mulund, Mumbai, IndiaIndian J. Nat. Prod. Resour.32012483488
- A.G.SardarA.P.PathakExploring the microbiota of solar saltern of Mulund, Mumbai, IndiaIndian J. Mar. Sci.4342014634641
- A.P.PathakA.G.SardarIsolation and characterization of salt stable protease producing archaea from marine solar saltern of Mulund, MumbaiIndian J. Mar. Sci.432014412417
- A.BarbaroP.CormaciG.FalconeA.BarbaroM.RizzoGenetic study of 11 Y-STRs in the populations of Reggio Calabria, Catanzaro, Cosenza (Calabria-South of Italy)Forensic Sci. Int.1462004129131
- C.A.OsborneM.GalicP.SangwanP.H.JanssenPCR-generated artefact from 16S rRNA gene-specific primersFEMS Microbiol. Lett.2482005183187
- M.PradhapV.SelvisabhanayakamV.MathivananJ.V.A.ParthasarathyS.S.Ayyappan KumarStudy on 16S rRNA based PCR method for specific detection of Salmonella enterica typhi from gut of infected silkworm Bombyx mori (Linn.)J. Sci. Ind. Res.702011909911
- N.M.NathaniA.K.PatelP.S.DhamannapatilR.K.KothariK.M.SinghC.G.JoshiComparative evaluation of rumen metagenome community using qPCR and MG-RASTAMB Express3201318
- J.E.B.StewartP.J.AagaardE.G.PokorakD.PolanskeyB.BudowleEvaluation of a multicapillary electrophoresis instrument for mitochondrial DNA typingJ. Forensic Sci.48200319
- A.P.PathakK.B.DeshmukhAlkaline protease production extraction and characterization from alkaliphilic Bacillus licheniformis KBDL4: a Lonar soda lake isolateIndian J. Exp. Biol.502012569576
- K.B.DeshmukhA.P.PathakM.S.KaruppayilBacterial diversity of Lonar soda lake of IndiaIndian J. Microbiol.512011107113
- K.TamuraG.StecherD.PetersonA.FilipskiS.KumarMEGA6: molecular evolutionary genetics analysis version 6.0Mol. Biol. Evol.30201327252729
- N.SaitouM.NeiThe neighbor-joining method: a new method for reconstructing phylogenetic treesMol. Biol. Evol.41987406425
- J.FelsensteinConfidence limits on phylogenies: an approach using the bootstrapEvolution391985783791
- K.TamuraD.PetersonN.PetersonG.StecherM.NeiS.KumarMEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methodsMol. Biol. Evol.28201127312739
- P.VijayaraghavanA.VijayanA.ArunJ.K.JenishaS.G.P.VincentCow dung: a potential biomass substrate for the production of detergent-stable dehairing protease by alkaliphilic Bacillus subtilis strain VVSpringerplus1201219
- P.VijayaraghavanS.SaranyaS.G.P.VincentCow dung substrate for the potential production of alkaline proteases by Pseudomonas putida strain AT in solid-state fermentationChin. J. Biol.201416
- M.NadeemJ.I.QaziS.BaigQ.SyedEffect of medium composition on commercially important alkaline protease production by Bacillus licheniformis N-2Food Technol. Biotech.462008388394
- M.G.AdsulM.S.SinghviS.A.GaikaiwariD.V.GokhaleDevelopment of biocatalysts for production of commodity chemicals from lignocellulosic biomassBioresourc. Technol.102201143044312
- Q.K.BegR.GuptaPurification and characterization of an oxidation-stable, thiol dependent serine alkaline protease from Bacillus mojavensisEnzyme Microb. Tech.322003294304
- A.P.PathakM.G.RathodExploration of Unkeshwar hot springs in Maharashtra for thermostable amylase producersRes. Rev. Biosci.82014269276
- A.P.PathakB.N.RekadwadIsolation of thermophilic Bacillus sp. strain EF_TYK1-5 and production of industrially important thermostable α-amylase using suspended solids for fermentationJ. Sci. Ind. Res.722013685689
- H.LineweaverD.BurkThe determination of enzyme dissociation constantsJ. Am. Chem. Soc.561934658666