Abstract
Malaria and lymphatic filariasis are two of the most common identifiable mosquito-borne parasitic diseases worldwide. Mosquito-borne disease is believed to be responsible for approximately 1 million deaths per year. Such diseases are controlled by use of insecticides; however, these may have undesired effects on non-target organisms. We therefore sought a suitable larvicidal compound that could replace insecticides by evaluating copper(II) complexes for larvicidal activity against Anopheles subpictus and Culex quinquefasciatus.
1 Introduction
Mosquitoes can transmit pathogens to human beings and are responsible for numerous infectious diseases, including malaria, filariasis, Japanese encephalitis, yellow fever, dengue and chikungunya [Citation1]. Mosquito-borne diseases are endemic in over 100 countries, causing nearly 2 million deaths each year, including at least 1 million children, with as many as 2100 million people at risk around the world [Citation2,Citation3]. Mosquitoes also cause allergic responses in humans that include local skin and systemic reactions such as angioedema [Citation3,Citation4]. Culex quinquefasciatus and its complex isomorphic sibling species Anopheles subpictus are major public health problems in India, with around 31 million cases of microfilaraemia, 23 million cases of symptomatic filariasis and about 473 million individuals potentially at risk of infection [Citation4–Citation9]. The most medically important species C. quinquefasciatus and A. subpictus breed in water polluted with organic debris, such as rotting vegetation, household refuse and excreta. Larvae of these vector species are commonly found in partially blocked drains, soak-away pits, septic tanks and village pots, especially abandoned receptacles in which the water is polluted and unfit for drinking. Mosquitoes are associated with urbanization and towns with poor drainage and sanitation. Under these conditions, their populations increase rapidly. C. quinquefasciatus commonly rest indoors both before and after feeding but also shelter in outdoor resting places [Citation5–Citation11].
An obvious method for the control of mosquito-borne diseases is the use of insecticides, and many synthetic agents have been used in the field with considerable success. They also provoke undesirable effects, including toxicity to non-target organisms, giving rise to environmental and human health concerns.
Free copper ions Cu+ and Cu2+ are highly bioavailable and thus toxic to aquatic organisms, whereas copper bound to organic matter is widely considered to be non-bioavailable [Citation6–Citation18]. We synthesized four new copper(II) complexes and evaluated them for larvicidal activity and determined their mode of toxicity to contribute to the design of better, safer materials.
2 Materials and methods
2.1 General and instrumental
Common reagents such as ethanol, Cu(NO3)2·3H2O, NaOH, NaClO4·H2O and 1,10-phenanthroline monohydrate were of analytical grade and used as received. l-Threonine, urea, thiourea, semicarbazide and thiosemicarbazide were purchased from Aldrich Chemicals Co. The precursor Cu(II) complex [Cu(phen)(l-Thr)(H2O)](Cl04) was prepared as described in the literature [Citation19]. A. subpictus and C. quinquefasciatus were collected from insect-rearing cages and identified in the Zonal Entomological Research Centre, Vellore. For the bioassay, mosquito larvae were taken in five batches of 20 in 249 mL of water. Elemental analysis was performed in a Thermo Finnigan Flash EA 1112 CHNSO analyser, and Fourier transform-infrared (FT-IR) spectra were obtained on a Perkin Elmer FT-IR spectrometer with samples prepared as KBr pellets. Molar conductivity was measured in an Elico conductivity bridge type CM82 and a dip-type cell and with a cell constant 1.0 cm−1 conductivity meter.
2.2 Preparation of complexes
2.2.1 Synthesis of [Cu(phen)(l-Thr)(Sc)](ClO4)
The precursor complex [Cu(phen)(l-Thr(H2O)](ClO4) (0.9566 g, 2 mmol) was dissolved in an aqueous ethanol solution to which 0.223 g (2 mmol) of semicarbazide was added slowly and stirred for 3 h at room temperature. The resulting sky-blue precipitate was filtered and washed with ethanol and ether, yielding 77%. Data for this complex: calculated for C17H20ClCuN6O8 (%): C 38.14; H 3.77; N 15.70; found (%) C 38.42; H 3.64; N 15.51; IR (KBr, cm−1): 3485br, 3051s, 2008w, 1625s, 1606m, 1585m, 1519s, 1429s, 1147w, 1087vs (ClO4−), 850s, 839m, 776w, 723m, 622s, cm−1. ΛM (Ω−1 cm2 M−1) in methanol at 25 °C: 95.
2.2.2 Synthesis of [Cu(phen)(l-Thr)(TSc)](ClO4)
The precursor complex Cu(phen)(l-Thr)(H2O)](ClO4) (0.9566 g, 2 mmol) was dissolved in an aqueous ethanol solution to which 0.1822 g (2 mmol) of thiosemicarbazide was added slowly and stirred for 3 h at room temperature. The resulting light-blue precipitate was filtered and washed with ethanol and ether, yielding 69%. Data for this complex: calculated for C17H20ClCuN6O7S (%): C 37.03; H 3.66; N 15.24; found (%) C 37.14; H 3.42; N 15.14; IR (KBr, cm−1): 3456br, 3053s, 2082s, 1585w, 1518m, 1429s, 1342w, 1226m, 1147s, 1087vs (ClO4−), 850m, 777w, 722m, 622s, cm−1. ΛM (Ω−1 cm2 M−1) in methanol at 25 °C: 98.
2.2.3 Synthesis of [Cu (phen)(l-Thr)(U)](ClO4)
The precursor complex [Cu(phen)(l-Thr)(H2O)](ClO4) (0.9566 g, 2 mmol) was dissolved in an aqueous ethanol solution to which 0.1201 g (2 mmol) of urea was added slowly and stirred for 3 h at room temperature. The resulting light-blue precipitate was filtered and washed with ethanol and ether, yielding 81%. Data for this complex: calculated for C17H19ClCuN5O8 (%): C 39.24; H 3.68; N 13.46; found (%) C 39.49; H 3.42; N 15.14; IR (KBr, cm−1): 3410br, 3059s, 2011m, 1625w, 1586s, 1519s, 1428s, 1223w, 1144w, 1091vs (ClO4−), 869w, 849m, 778w, 722s, 622s, cm−1. ΛM (Ω−1 cm2 M−1) in methanol at 25 °C: 91.
2.2.4 Synthesis of [Cu(phen)(l-Thr)(TU)](ClO4)
The precursor complex Cu(phen)(l-Thr)(H2O)](ClO4) (0.9566 g, 2 mmol) was dissolved in an aqueous ethanol solution to which 0.1522 g (2 mmol) of thiourea was added slowly and stirred for 3 h at room temperature. The resulting light-yellow precipitate was filtered and washed with ethanol and ether, yielding 84%. Data for this complex: calculated for C17H19ClCuN5O7S (%): C 38.06; H 3.57; N 13.06; found (%) C 38.14; H 3.42; N 13.11; IR (KBr, cm−1): 3418br, 3065 m, 2006w, 1625w, 1518s, 1428s, 1343w, 1224m, 1143w, 1090vs (ClO4−), 868w, 846s, 770w, 723m, 623s, 546w cm−1. ΛM (Ω−1 cm2 M−1) in methanol at 25 °C: 89.
Although no problems were encountered, perchlorate salts of transition metal complexes with organic ligands are potentially explosive. Only small amounts of material should be prepared, and they should be handled with caution.
2.3 Parasite rearing and collection
C. quinquefasciatus and A. subpictus larvae were collected from rice fields and areas of stagnant water around Vellore (12°55′48″ N, 79°7′48″ E) and identified in the Zonal Entomological Research Centre, Vellore, Tamil Nadu, to start the colony; larvae were kept in plastic and enamel trays containing tap water and maintained and reared in the laboratory as described elsewhere [Citation20].
2.4 Larvicidal bioassay
Mosquito larvae were taken in five batches of 20 in 249 mL of water and 1.0 mL of complexes 1–4. The control was dechlorinated tap water. The number of dead larvae was counted after 24 h of exposure, and the percentage mortality was reported for an average of five replicates. Experimental media in which 100% mortality of larvae occurred were selected for the dose–response bioassay.
2.5 Dose–response bioassay
All four complexes were assayed for dose–response larvicidal activity against larvae of A. subpictus and C. quinquefasciatus. Concentrations of complexes 1–4 ranging from 10.0 to 0.625 mg/L were used. The number of dead larvae was counted after 24 h of exposure, and the percentage mortality was reported for an average of five replicates. The test samples were found to have equivalent toxic potential.
2.6 Data analysis
Mean percentage larval mortality was analyzed by analysis of variance and compared with Duncan's multiple range tests to determine differences among complexes 1–4 in and within-species and concentration differences. Before analysis, mortality was corrected for control mortality using Abbott's formula [Citation21]. The median lethal concentrations (LC50) and the associated confidence intervals were estimated from 24-h mortality data by probit analysis [Citation22]. LC50 and slopes were considered significantly different if their confidence intervals did not overlap. All differences were considered significant at p < 0.05.
3 Results and discussion
3.1 Synthesis and characterization
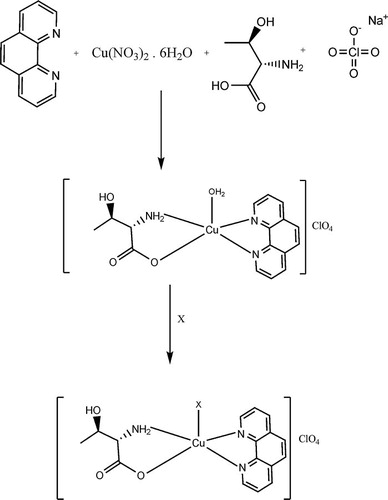
With [Cu(phen)(l-Thr)(H2O)](ClO4) copper(II) complex as the precursor, [Cu(phen)(l-Thr)(X)](ClO4) was prepared by reaction with X = semicarbazide (4), thiosemicarbazide (2), urea (1) and thiourea (3) by ligand substitution in water–ethanol to obtain coloured complexes. These complexes are stable in the solid state and soluble in water and common organic solvents. Elemental analysis of the copper(II) complexes agreed with the theoretical values. The synthetic strategy is outlined in . The molar conductance (80–102 Ω−1 cm2 M−1) in methanol indicates that the complex is of a 1:1 electrolytic nature, giving [Cu(phen)(l-Thr)(X)]+ in solution [Citation23].
3.2 Infrared spectra
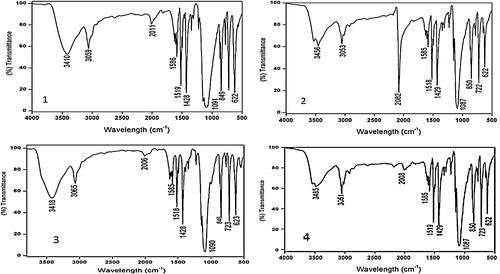
In the IR spectra (), the complexes showed asymmetric νas (COO−) around 1625 (1), 1626 (2), 1625 (3) and 1625 (4) cm−1 and symmetric stretching vibration νs (COO−) around 1342 (1), 1342 (2), 1342 (3) and 1343 (4) cm−1, respectively. The difference between νas (COO−) and νs (COO−) frequencies is >200 cm−1, which indicates that the carboxylate group is coordinated to the metal ion in a monodentate fashion [Citation24]. The peaks corresponding to the ring-stretching frequencies ν (C=C) and ν (C=N)] at 1503 and 1421 cm−1 of free phen is shifted to higher frequencies upon complexation (1519, 1429 (1), 1518, 1429 (2), 1519, 1428 (3) and 1518, 1428 (4) cm−1), indicating coordination of the heterocyclic nitrogen atoms to the metal ion. The characteristic out-of-plane hydrogen bending modes of free phenanthroline observed at 853 cm−1 and 738 cm−1 is shifted to 850, 723 (1), 850, 722 (2), 849, 722 (3) and 846, 723 (4) cm−1. These shifts can be explained by the fact that each of the two nitrogen atoms of phenanthroline ligands donates a pair of electrons to the central copper metal, forming a coordinate covalent bond [Citation25]. The broad band observed around 3485 (1), 3456 (2), 3410 (3) and 3418 (4) cm−1 is assigned to N–H asymmetric stretching of the amine group and 3051 (1), 3053 (2), 3059 (3) and 3065 (4) cm−1 is assigned to N–H symmetric stretching. The very strong band at 1087 (1), 1087 (2), 1091 (3) and 1090 (4) cm−1 was assigned to ν (Cl–O) of perchlorate anion [Citation19].
3.3 Percentage mortality and LC50 values of C. quinquefasciatus larvae
The percentage mortality due to complexes 1–4 was 89, 76, 54, 34 and 21; 84, 74, 67, 33 and 21; 100, 89, 76, 61 and 31; and 80, 69, 36, 41 and 31 against larvae of C. quinquefasciatus at concentrations of 10, 5, 2.5, 1.25 and 0.625 mg/L, respectively.
The LC50 values of complexes 1–4 against larvae of C. quinquefasciatus were 2.09, 1.85, 0.79 and 0.61 mg/L (r2 = 0.857, 0.710, 0.897 and 0.897), respectively (). The control showed no mortality.
Table 1 Mortality of C. quinquefasciatus larvae at various concentrations of test samples.
3.4 Percentage mortality and LC50 values of A. subpictus larvae
The percentage mortality due to complexes 1–4 was 96, 81, 54, 39 and 29; 80, 71, 56, 35 and 27; 100, 88, 76, 54 and 31; and 83, 70, 65, 30 and 22 against larvae of A. subpictus at concentrations of 10, 5, 2.5, 1.25 and 0.625 mg/L, respectively.
The LC50 values of complexes 1–4 against larvae of A. subpictus were 1.88, 1.72, 0.89 and 1.78 mg/L (r2 = 0.903, 0.722, 0.935 and 0.710), respectively (). The control showed no mortality.
Table 2 Mortality of A. subpictus at various concentrations of different test samples.
All four complexes showed larvicidal activity; however, urea and thiourea showed greater larvicidal activity against C. quinquefasciatus, even at low doses, than semicarbazide and thiosemicarbazide. Only the urea complex had significant larvicidal activity against A. subpictus. The complexes are therefore effective larvicides against these two species of mosquito, with activities similar to those reported in the literature [Citation26,Citation27]. We provide preliminary data supporting the effectiveness of the urea complex against A. subpictus and C. quinquefasciatus larvae. Further evaluation of this complex for its effect on non-target organisms is necessary before it can be used for the control of mosquito larvae.
4 Conclusion
The precursor complex Cu(phen)(l-Thr (ClO4) complex with urea had better larvicidal activity against C. quinquefasciatus and A. subpictus larvae in a dose dependent manner. Works are in progress to ascertain the toxicity to non-target organisms.
Notes
Peer review under responsibility of Taibah University
References
- R.NauenInsecticide resistance in disease vectors of public health importancePest Manage. Sci.632007628633
- N.S.KorgaonkarA.KumarR.S.YadavD.KabadiA.P.DashMosquito biting activity on humans and detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, IndiaInd. J. Med. Res.1352012120126
- M.S.KlempnerT.R.UnnaschL.T.HuTaking a bite out of vector-transmitted infectious diseasesN. Engl. J. Med.356200725672569
- Z.PengJ.YangH.WangF.E.SimonsProduction and characterization of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteinsInsect Biochem. Mol. Biol.291999909914
- S.Ravi KiranP.Sita DeviEvaluation of mosquitocidal activity of essential oil and sesquiterpenes from leaves of Chloroxylon swietenia DCParasitol. Res.1012007413418
- A.KumarN.ValechaT.JainA.P.DashBurden of malaria in India: retrospective and prospective viewAm. J. Trop. Med. Hyg.776 Suppl.20076978
- K.P.PailyS.L.HotiK.BalaramanDevelopment of lymphatic filarial parasite Wuchereria bancrofti (Spirurida: Onchocercidae) in mosquito species (Diptera: Culicidae) fed artificially on microfilaremic bloodJ. Med. Entomol.43200612221226
- T.SolomonControl of Japanese encephalitis within our graspN. Engl. J. Med.3552006869871
- V.K.AgrawalV.K.SashindranLymphatic filariasis in India: problems, challenges and new initiativesMed. J. Armed Forces India622006359362
- E.A.OttesenP.J.HooperM.BradleyG.BiswasThe global programme to eliminate lymphatic filariasis: health impact after 8 yearsPLOS Negl. Trop. Dis.22008317
- M.ServiceMedical Entomology for Students2012Cambridge University PressCambridge
- S.M.EricksonMosquito infection responses to developing filarial wormsPLOS Negl. Trop. Dis.32009529
- G.ChandraI.BhattacharjeeS.ChatterjeeA review on Anopheles subpictus Grassi—a biological vectorActa Trop.1152010142154
- L.A.GutierrezNatural infectivity of Anopheles species from the Pacific and Atlantic regions of ColombiaActa Trop.107200899105
- E.MichaelD.A.BundyB.T.GrenfellRe-assessing the global prevalence and distribution of lymphatic filariasisParasitology1121996409428
- S.ManguinM.J.BangsJ.PothikasikornT.ChareonviriyaphapReview on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoesJ. Mol. Epidemiol. Evol. Genet. Infect. Dis.102010159177
- V.P.SharmaMalaria and poverty in IndiaCurr. Sci.842003513515
- W.R.ArnoldR.C.SantoreJ.S.CotsifasPredicting copper toxicity in estuarine and marine waters using the biotic ligand modelMar. Pollut. Bull.50200516341640
- S.ZhangY.ZhuC.TuH.WeiZ.YangL.LinJ.DingJ.ZangZ.GuoA novel cytotoxic ternary copper(II) complex of 1, 10-phenanthroline and l-threonine with DNA nuclease activityJ. Inorg. Biochem.98200420992106
- C.KamarajA.BagavanA.A.RahumanA.A.ZahirG.ElangoG.PandiyanLarvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae)Parasitol. Res.104200911631171
- W.S.AbbottA method of computing the effectiveness of an insecticideJ. Econ. Entomol.181925265267
- D.J.FinneyW.L.StevensA table for the calculation of working probits and weights in probit analysisBiometrika351948191201
- W.J.GearyThe use of conductivity measurements in organic solvents for the characterization of coordination compoundsCoord. Chem. Rev.7197181122
- L.H.Abdel RahmanL.P.BattagliaM.R.MahmoudSynthesis, characterization and stability constant determination of l-phenylalanine ternary complexes of cobalt(II), nickel(II), copper(II) with N-heterocyclic aromatic bases and X-ray crystal structure of aqua-1,10-phenanthroline-l-phenylalaninato copper (II) perchlorate complexPolyhedron151996327334
- L.JinP.YangSynthesis and DNA binding studies of CoIII mixed–ligand complex containing dipyrido [3, 2-a:2′,3′-c] phenazine and phenPolyhedron1619973395
- T.T.NguyenN.OgwuruG.EngTolerance of Aedes aegypti larvae to triorganotinsAppl. Organometal. Chem.142000345348
- T.S.Basu BaulS.DharE.RivarolaF.E.SmithR.ButcherX.SongM.McCainG.EngSynthesis and characterization of some dibutylbis{5-[(E)-2-(aryl)-1-diazenyl]-2-hydroxybenzoato}tin(IV) compounds. Toxicity studies of di- and tri-organotin complexes on the second instar of Aedes aegypti mosquito larvaeAppl. Organometal. Chem.172003261267