Abstract
The objective of this study was to investigate the in vitro antioxidant activities of polyphenolic extract of Terminalia chebula Retzius (Combretaceae) fruits. The polyphenolic extract of T. chebula fruits was evaluated for antioxidant activity by determining the reducing power, total antioxidant capacity, DPPH radical concentration (IC50 14 μg/mL), nitric oxide radical concentration (IC50 30.51 μg/mL) and hydrogen peroxide scavenging activity (IC50 265.53 μg/mL) under in vitro conditions. Moreover, the phytochemical characterisation of the extract was also measured by determining the total phenolic, flavonoid, tannin and ascorbic acid contents. Characterisation of the extract was also performed by HPLC profiling with the standard gallic acid. The present study demonstrated that the extract has significant reducing capacity and nitric oxide scavenging activity. It also scavenges hydrogen peroxide-induced radicals. The activity of the extract may be due to the total polyphenolic content. The antioxidant activity of the extract is significantly higher than the standard ascorbic acid, and its activity is concentration-dependent. It is concluded that a polyphenolic-rich fraction of T. chebula fruits is a potential source of natural antioxidants.
1 Introduction
It is currently hypothesised that many diseases are due to oxidative stress that results from an imbalance between the formation and detoxification of pro-oxidants. Oxidative stress is initiated by reactive oxygen species (ROS), which are produced as a by-product of electron transport in mitochondria [Citation1]. The increase in ROS generation or decreased antioxidant availability can result in a net increase in intracellular ROS. Normally, intracellular molecules including mitochondrial antioxidants prevent cellular damage produced by endogenous ROS. In living systems, free radicals and ROS are constantly generated and cause extensive damage to tissues and in various disease conditions, particularly degenerative diseases, and also lead to extensive lysis [Citation2]. Therefore, the mechanism of action of many synthetic drugs involves free radical scavenging, which protects against oxidative damage but has adverse side effects [Citation3]. An alternative is the consumption of natural antioxidants from various food supplements and traditional medicines [Citation4], and there is increased interest among phytotherapy researchers to use medicinal plants with antioxidant activity for protection against oxidative stress.
Terminalia chebula Retzius (Combretaceae), commonly known as haritaki, exhibits a number of medicinal activities due to the presence of a large number of different phyto-constituents. The plant has a round crown and spreading branches. The bark is dark brown with some longitudinal cracks. The leaves are ovate and elliptical, with two large glands at the top of the petiole. The fruit or drupe is approximately 1–2 inches in size. It has five lines or five ribs on the outer skin. The fruits of T. chebula are reported to have hepatoprotective activity against CCl4 and tert-butyl hydroperoxide [Citation5]. The fruits also display cytoprotective [Citation6], antidiabetic [Citation7,Citation8], antioxidant [Citation9], antibacterial [Citation10,Citation11], anti-arthritic [Citation12], hypo-cholesterolaemic [Citation13] and anti-inflammatory activities [Citation14].
Antioxidant activities increase proportionally with the polyphenol content, primarily because of their redox properties [Citation15]. Among the diverse roles of polyphenols, they protect cell constituents against destructive oxidative damage, thus limiting the risk of various degenerative diseases associated with oxidative stress by acting as potent free radical scavengers. The polyphenol antioxidant activity is due to the chemical structure and ability to donate/accept electrons, thereby delocalising the unpaired electron within the aromatic structure [Citation16]. Recent studies showed the antioxidant properties of different extracts of T. chebula fruits. In an earlier report, a 70% methanol extract of T. chebula fruits was found to have good efficacy in radical scavenging abilities [Citation17]. In another report, chloroform, ethanolic, n-butanolic and organic aqueous extracts were investigated for anti-lipid peroxidation, anti-superoxide radical formation and free radical scavenging activities [Citation18]. The results showed that all tested extracts exhibited antioxidant activity at different magnitudes of potency [Citation18]. Casuarinin, chebulanin, chebulinic acid and 1,6-di-O-galloyl-b-d-glucose, isolated from T. chebula, were also tested in this study and showed significant antioxidant activity [Citation18]. Chang and Lin [Citation19] reported antioxidant activities of water, methanol and 95% ethanol extracts of the air-dried fruit of T. chebula. However, the antioxidant activity of the polyphenolic-rich extract of T. chebula fruit has not been previously evaluated. Therefore, the objective of this study was to assess the free radical scavenging potential of the polyphenolic extract of T. chebula fruits.
2 Materials and methods
2.1 Chemicals
Gallic acid standard (Batch No. 90618) was procured from Himedia Laboratories Pvt. Ltd., Mumbai, India. Ascorbic acid was obtained from Merck Specialties Pvt. Ltd., Mumbai, India. HPLC-grade methanol and acetonitrile, as well as acetic acid were obtained from Merck Specialties Pvt. Ltd., Mumbai, India. All of the chemicals used in this study were of analytical reagent grade, unless specified.
2.2 Plant material and extract preparation
The T. chebula fruits were collected from a local market of Ahmedabad, India, during January 2012 and were taxonomically identified and authenticated as T. chebula fruits by Dr. Yogesh T. Jasrai, Department of Botany, Gujarat University, India. A voucher specimen was deposited in the herbarium for future reference (Ref. No. GU/2013/53).
The T. chebula fruits were thoroughly cleaned, dried under the shade and coarsely powdered. The polyphenolic-rich extract was prepared according to a previously reported method [Citation20]. The powdered plant material was mixed with 70% methanol and stored at room temperature for 5 days. After 5 days, it was filtered and the solvent was evaporated. The residue was dissolved in water, and the aqueous layer was washed with petroleum ether several times until a clear upper layer of petroleum ether was obtained. The lower layer was then treated with ethyl acetate containing glacial acetic acid (10 mL/L). Extraction of the polyphenols was performed for 36 h at room temperature, and the combined ethyl acetate layer was then concentrated. The residue was lyophilised. The extract obtained was dried and stored in an airtight container at 4 °C. The yield of the dry polyphenolic extract was 30.5% (w/w). The dried extract was dissolved in Milli-Q water and used for further study.
2.3 Phytochemical studies
Qualitative analysis for determining the presence of tannins, saponins, glycosides, flavonoids, steroids and alkaloids in the polyphenolic extract was performed using standard methods [Citation21].
2.3.1 Total phenolic content (TPC)
The total phenolic content of the polyphenolic extract of T. chebula was estimated by the reported method [Citation22]. Various concentrations of gallic acid were used to plot the standard curve. The total phenolic content of the extract is expressed as mg gallic acid equivalents/g dry weight of extract.
2.3.2 Flavonoid content
The total flavonoid content in the T. chebula polyphenolic extract was estimated by the standard method [Citation23]. The flavonoid content of the extract is expressed as mg quercetin equivalents/g dry weight of extract.
2.3.3 Tannin content
The tannin content of the T. chebula polyphenolic extract was estimated by the method described by Hagerman and Butler [Citation24]. The tannin content of the extract is expressed as mg rutin equivalents/g dry weight of extract.
2.3.4 Ascorbic acid content
Ascorbic acid, also called vitamin C, is one of the most abundant antioxidants in T. chebula extract and was quantified according to the previously reported method [Citation25]. The ascorbic content of the extract is expressed as μg/g dry weight of extract.
2.4 Standardisation of extract
The polyphenolic extract of T. chebula fruits was standardised using gallic acid as a standard. For reverse-phase separation of gallic acid from the other constituents, a Spectra-Physics HPLC-UV system (San Jose, CA, USA) with a Spectra system P1000 pump, a Spectra system UV1000 detector and a Rheodyne 7125 injector with a 20-μL loop was used. Chromatographic separation was achieved on a COSMOSIL C18 column (250 mm × 4.6 mm, length × inner diameter with 5-μm particle size), maintained at 35 °C in a column oven. The mobile phase consisted of deionised water:acetonitrile:acetic acid (88:10:2, v/v/v). The flow rate of the mobile phase was maintained at 1.0 mL/min, and the wavelength of the detector was set at 280 nm.
2.5 Reducing power assay
The reducing power was determined based on the ability of the antioxidant to form a coloured complex with potassium ferricyanide, TCA and FeCl3. It was measured by a modified method [Citation26] in which the extract of T. chebula fruits (1 mL) at various concentrations (25, 50, 100, 150, 200 and 250 μg/mL) was mixed with potassium ferricyanide and phosphate buffer (pH 6.6) and incubated at 50 °C for 20 min. Then, TCA (10%) was added and centrifuged at 3000 rpm for 10 min. The supernatant was removed and mixed with FeCl3 (0.1%). The absorbance was then measured at 700 nm. A higher absorbance of the reaction mixture indicates a higher reducing power.
2.6 Total antioxidant capacity
The total antioxidant capacity of the polyphenolic extract of T. chebula was measured using a spectrophotometer method at concentrations of 50–500 μg/mL [Citation27]. Ascorbic acid equivalents were calculated using a standard curve of ascorbic acid. The experiment was conducted in triplicate, and the values are expressed as μg equivalents of ascorbic acid per μg/mL of extract.
2.7 DPPH radical scavenging assay
The ability of T. chebula polyphenolic extract/ascorbic acid to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was measured by the reported method Shimada et al. [Citation27]. The addition of 0.1 mM DPPH solution in various concentrations (10, 25, 50, 75, 100 and 150 μg/mL) of plant extract/ascorbic acid in the presence of Tris–HCl buffer (50 mM, pH 7.4) resulted in decreased absorbance, which was measured at 517 nm. A mixture of methanol and extract served as the blank. The per cent inhibition was calculated by measuring the absorbance of extract/ascorbic acid treated samples against the blank. The IC50 values for the polyphenolic extract were calculated and compared with the standard reference compound ascorbic acid.
2.8 Nitric oxide radical scavenging assay
The ability of the polyphenolic extract of T. chebula at graded concentrations of 50–500 μg/mL to scavenge nitric oxide radical was determined by the modified method [Citation28]. Various concentrations of polyphenolic extract were added to 10 mM sodium nitroprusside and incubated for 2.5 h. After incubation, Griess reagent was added to the tubes and the absorbance of the chromophore formed was read at 590 nm. The blank solution contained a mixture of 0.5 mL PBS and 0.5 mL extract. The IC50 values and per cent inhibition by various concentrations of extract were calculated by comparing the absorbance values of the control and test compounds.
2.9 Hydrogen peroxide scavenging assay
The hydrogen peroxide scavenging activity of T. chebula at concentrations of 50–500 μg/mL was estimated using a hydrogen peroxide solution [Citation29]. A solution of hydrogen peroxide (2 mmol/L) was prepared in phosphate buffer (pH 7.4). The polyphenolic extract/ascorbic acid (25, 50, 75, 100, 125 and 150 μg/mL) was added to the hydrogen peroxide solution (0.6 mL). A mixture of phosphate buffer (3.3 mL) and extract (0.5 mL) served as the blank. The absorbance of hydrogen peroxide at 230 nm was determined after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide and compared to ascorbic acid, which was the reference compound.
2.10 Statistical analysis
The results are expressed as the mean ± standard error of the mean (SEM). The statistical analysis and linear regression analysis were performed using GraphPad Instat, software, version 5.0. The IC50 values were calculated and compared by paired t tests. p < 0.05 was considered significant.
3 Results and discussion
3.1 Phytochemical screening
The qualitative phytochemical screening of the polyphenolic extract revealed the presence of tannins, saponins, flavonoids and alkaloids. The total phenolic content of the extract of T. chebula was 134.47 mg gallic acid equivalent/g dry weight. The flavonoid content of the extract was 7.934 mg of quercetin equivalent/g dry weight. The tannin content of the polyphenolic extract of T. chebula was 31.47 mg rutin equivalent/g dry weight. The ascorbic acid content of the extract was 8.74 μg/g dry weight of extract. Phytochemical analysis of T. chebula showed the presence of gallic acid, ellagic acid, tannic acid, ethyl gallate, chebulic acid, chebulagic acid, corilagin, mannitol, ascorbic acid (vitamin C), and other compounds [Citation30]. Another report found T. chebula with 32% tannin content [Citation31]. Polyphenols are widely distributed in plants, and phenolic antioxidants act as free radical scavengers and metal chelators [Citation32]. Recently, bioflavonoids and polyphenols of plant origin have been used extensively for free radical scavenging and to inhibit membrane lipid peroxidation [Citation33].
3.2 Standardisation of extract
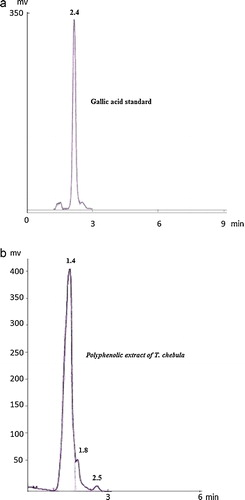
The polyphenolic extract of T. chebula fruits showed the presence of gallic acid with a retention factor of 2.50 (b), which is comparable to the retention factor of 2.40 of the pure standard gallic acid (a). Moreover, the polyphenolic extract of T. chebula fruits also showed the presence of other polyphenolic compounds, with retention factors of 1.40 and 1.80. Recently, many natural antioxidants have been isolated from different plant materials [Citation34,Citation35], and some types of polyphenols, such as chebulanin, corilagin, neochebulinic acid, ellagic acid, gallic acid, chebulagic acid, and chebulinic acid, are present in the fruits of T. chebula [Citation36]. In an earlier study, Rangsriwong et al. [Citation37] investigated the separation of polyphenolic compounds, such as gallic acid and ellagic acid, from T. chebula fruits by subcritical water extraction. The decoction of T. chebula fruit contained 3,4,6-tri-O-galloyl-d-glucose, chebulic acid, β-punicalagin, corilagin, α-punicalagin, chebulagic acid, gallic acid, 1,3,4,6-tri-O-galloyl-β-d-glucose, chebulinic acid, 1,2,3,4,6-penta-O-galloyl-d-glucose, ellagic acid, and 1,6-di-O-galloyl-d-glucose [Citation38]. Flavonoids also have significant antioxidant activity under both in vivo and in vitro conditions [Citation39].
3.3 Reducing power assay
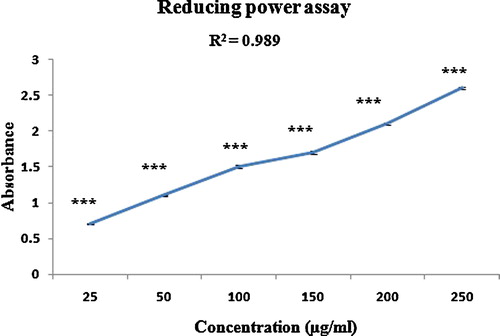
The reducing capacity of the polyphenolic extract of the T. chebula fruits was measured by its ability to transform Fe3+ to Fe2+ at various concentrations (25, 50, 100, 150, 200 and 250 μg/mL). The results revealed that the reducing activity significantly increased as the concentration of the extract was increased (r2 = 0.989), with a maximum increase at 250 μg/mL (). The reductive capacity of a compound depends on the presence of reductones, which exhibit antioxidative potential by breaking the free radical chain and donating a hydrogen atom. Therefore, reducing activity leads to the termination of the radical chain reactions that may otherwise be very damaging [Citation40]. The presence of antioxidant reductants in the polyphenolic extract of T. chebula causes the reduction of the Fe3+/ferricyanide complex to the ferrous form, indicating that the polyphenolic extract of T. chebula has significant reducing power. Li et al. [Citation41] reported the existence of a similar linear co-relationship between the reducing power and total phenolic content (TPC).
3.4 Total antioxidant capacity
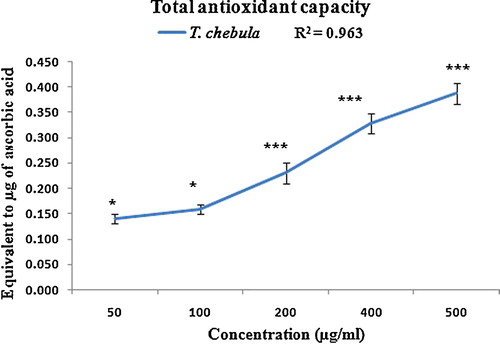
The total antioxidant capacity of the polyphenolic extract of T. chebula fruits ranged from 0.140 to 0.387 at graded concentrations of 50–500 μg/mL (r2 = 0.963; ). Gallic acid, a polyphenyl present in T. chebula, was previously evaluated for free radical scavenging activity and has significant reducing power and antioxidant activity [Citation42].
3.5 DPPH radical scavenging assay
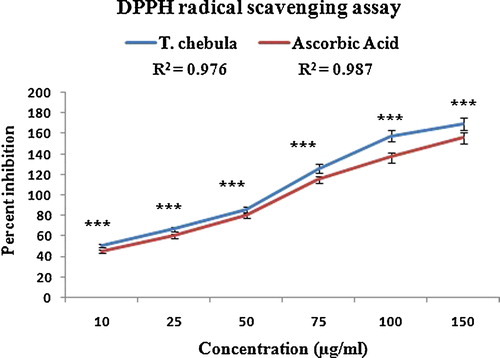
The DPPH radical scavenging activity of the polyphenolic extract of T. chebula at different concentrations is shown in and is compared to the reference compound ascorbic acid. The radical scavenging activity of the extract was the highest at the maximum dose of 150 μg/mL, with an IC50 value of 14 ± 0.05 μg/mL (r2 = 0.976). Ascorbic acid showed an IC50 value of 16 ± 0.06 μg/mL (r2 = 0.987). The effect was concentration-dependent. DPPH is a stable, nitrogen-centred free radical, which, upon accepting hydrogen from the antioxidants present in the polyphenolic extract, is converted into a stable diamagnetic molecule, diphenyl-picryl hydrazine [Citation43]. The observed reduction of DPPH by the extract was either due to the transfer of a hydrogen atom or the transfer of an electron. Phenolic compounds are also effective hydrogen donors, which makes them good antioxidants [Citation44].
3.6 Nitric oxide radical scavenging assay
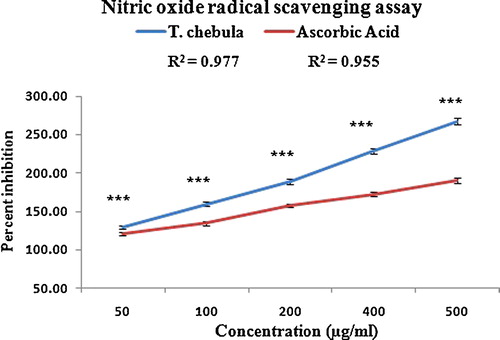
In the present study, the nitric oxide radical quenching activity of the polyphenolic extract was detected and compared with the standard ascorbic acid. The extract exhibited the maximum per cent inhibition of 267% at a concentration of 500 μg/mL, with an IC50 value of 30.51 μg/mL, in a concentration-dependent manner (r2 = 0.977; ). However, ascorbic acid exhibited maximum per cent inhibition of 189%, with an IC50 value of 42 μg/mL (r2 = 0.955; ). The scavenging activity of the extract against nitric oxide was detected by its ability to inhibit the formation of nitrite through direct competition with oxygen and oxides of nitrogen in the reaction mixture [Citation40]. The decrease in the concentration of the nitric oxide radical was more significant than ascorbic acid, which is due to the antioxidant activity of the polyphenolic extract. Nitric oxide is a potent pleiotropic mediator of physiological processes, such as smooth muscle relaxation, neuronal signalling, inhibition of platelet aggregation and regulation of cell-mediated toxicity. It is a diffusible free radical that plays many roles as an effector molecule in diverse biological systems, including neuronal communication, vasodilatation and antimicrobial and antitumor activities [Citation45]. Moreover, in pathological conditions, nitric oxide reacts with superoxide anion and forms potentially cytotoxic molecules, such as peroxynitrite.
3.7 Hydrogen peroxide scavenging activity
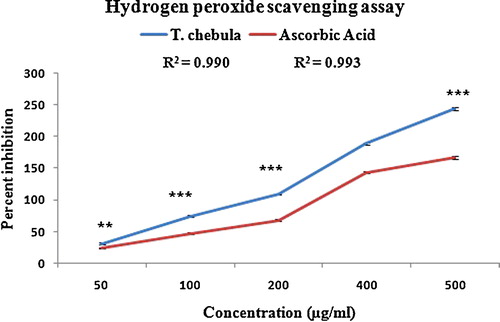
The polyphenolic-rich extract of T. chebula showed significant scavenging activity of H2O2 in a concentration-dependent manner (r2 = 0.990, ), with an IC50 value of 265.53 μg/mL, whereas the IC50 value for ascorbic acid was 278 μg/mL (r2 = 0.993, ). Hydrogen peroxide is a weak oxidising agent and can directly inactivate a few enzymes, usually by oxidation of essential thiol groups. Hydrogen peroxide can cross cell membranes rapidly, and once inside the cell, H2O2 likely reacts with Fe2+, and possibly Cu2+ ions, to form hydroxyl radicals, which then become powerful oxidising agents. This may cause many toxic effects. The phenolic compounds may act as free radical scavengers because of their hydrogen-donating ability and scavenging ability [Citation46].
3.8 Correlation between the total phenolic content and antioxidant activity
The total phenolic content of the polyphenolic extract of T. chebula is significantly correlated with its total antioxidant capacity (R = 0.992, p < 0.05), DPPH radical scavenging activity (R = 0.971, p < 0.05), nitric oxide radical quenching activity (R = 0.995, p < 0.05) and hydrogen peroxide scavenging activity (R = 0.990, p < 0.05). This result indicates that the phenolic contents of T. chebula are responsible for its antioxidant activity. It has been proposed that the antioxidant activity of plants may be due to their phenolic compounds [Citation47]. Many plants exhibit efficient antioxidant properties because of their phenolic constituents [Citation48].
4 Conclusion
Polyphenols are valuable plant constituents for the scavenging of free radicals because of their phenolic hydroxyl groups [Citation49]. This, together with the obtained results, suggests that as the amount of polyphenolic compounds increases, the antioxidant activity also increases. In conclusion, the present study demonstrates that the polyphenolic extract of T. chebula fruits can protect the body from oxidative stress from ROS, which may be due to the phyto-chemicals in the form of polyphenols that occur in the plant. These may be used in nutraceuticals and the food industry. However, additional studies are necessary to develop a method for the fractionation and identification of polyphenols and to determine the most active antioxidant compounds in the polyphenolic extract.
Acknowledgement
The financial assistance to Sarmistha Saha from Lady Tata Memorial Trust (LTMT/AD/Q2/2011-2013) in the form of a Senior Research Fellowship is gratefully acknowledged.
Notes
Peer review under responsibility of Taibah University.
References
- Y.LiuG.FiskumD.SchubertGeneration of reactive oxygen species by the mitochondrial electron transport chainJ. Neurochem.802002780787
- B.HalliwellJ.M.GutteridgeFree Radicals in Biology and Medicine1998Oxford University PressOxford
- R.YazdanparastA.ArdestaniIn vitro antioxidant and free radical scavenging activity of Cyperus rotundusJ. Med. Food102007667674
- R.YazdanparastS.BahramikiasA.ArdestaniNasturtium oficinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic ratsChem. Biol. Interact.1722008176184
- S.A.TasaduqK.SinghS.SethiS.C.SharmaK.L.BediJ.SinghB.S.JaggiR.K.JohriHepatocurative and antioxidant profile of HP-1, a polyherbal phytomedicineHum. Exp. Toxicol.222003639645
- N.A.MinkyunB.A.E.WanS.S.KangB.S.MinJ.K.YooO.K.YukS.Yu-IchiroY.SciichiM.NobuhikoCytoprotective effect on oxidative stress and inhibitory effect on cellular aging of Terminialia chebula fruitPhytother. Res.182004737741
- G.P.SenthilkumarS.P.SubramanianBiochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in streptozotocin-induced experimental diabetes in ratsJ. Appl. Biomed.62008105115
- V.R.KannanG.S.RajasekarP.RajeshV.BalasubramanianN.RameshE.K.SolomonD.NivasS.ChandruAnti-diabetic activity on ethanolic extracts of fruits of Terminalia chebula Retz. alloxan induced diabetic ratsAm. J. Drug Discov. Dev.22012135142
- H.S.LeeS.H.JungB.S.YunK.W.LeeIsolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytesArch. Toxicol.812007211218
- F.MalckzadehH.EhsanifarN.ShahamatM.LevinR.R.ColwellAntibacterial activity of black myrobalan (Terminalia chebula Retz.) against Helicobactor pyroliInt. J. Antimicrobiol. Agents1820018588
- D.LeeK.BooJ.WooF.DuanK.LeeT.KwonH.Y.LeeK.Z.RiuD.LeeAnti-bacterial and anti-viral activities of extracts from Terminalia chebula barksJ. Korean Soc. Appl. Biol. Chem.542011295298
- V.NairS.SinghY.K.GuptaAnti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental modelsJ. Pharm. Pharmacol.62201018011806
- D.A.IsraniK.V.PatelT.R.GandhiAnti-hyperlipidemic activity of aqueous extract of Terminalia chebula and Gaumutra in high cholesterol diet fed ratsInt. J. Pharm. Sci.120104859
- N.PratibhaV.S.SaxenaA.AmitP.D'SouzaM.BagchiD.BagchiAnti-inflammatory activities of Aller-7, a novel polyherbal formulation for allergic rhinitisInt. J. Tissue React.2620044351
- G.K.RasineniD.SiddavattamA.R.ReddyFree radical quenching activity and polyphenols in three species of ColeusJ. Med. Plants Res.22008285291
- J.A.RossC.M.KasumDietary flavonoids: bioavailability, metabolic effects, and safetyAnn. Rev. Nutr.2220021934
- B.HazraR.SarkarS.BiswasN.MandalComparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalisBMC Complement. Altern. Med.10201020
- H.ChengT.LinK.YuC.YangC.LinAntioxidant and free radical scavenging activities of Terminalia chebulaBiol. Pharm. Bull.26200313311335
- C.L.ChangC.S.LinPhytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extractsEvid. Based Complement. Altern. Med.2012201217
- C.T.KumarappanE.ThilagamS.C.MandalAntioxidant activity of polyphenolic extracts of Ichnocarpus frutescensSaudi J. Biol. Sci.192012349355
- A.SofowaraMedicinal Plants and Traditional Medicine in Africa2009Spectrum Books Ltd.Ibadan, Nigeria289
- V.L.SingletonR.OrthoferR.M.Lamuela-RaventosAnalysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagentMethods Enzymol.2991999152178
- A.MedaC.E.LamienM.RomitoJ.MillogoO.G.NacoulmaDetermination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activityFood Chem.912005571577
- A.E.HagermanL.G.ButlerProtein precipitation method for the quantitative determination of tanninsJ. Agric. Food Chem.261978809812
- B.C.AdedayoG.ObohA.A.AkindahunsiChanges in the total phenol content and antioxidant properties of pepperfruit (Dennettia tripetala) with ripeningAfr. J. Food Sci.42010403409
- G.K.JayaprakashR.P.SinghK.K.SakariahAntioxidant activity of grape seed extracts on peroxidation models in vitroJ. Agric. Food Chem.5520011018
- K.ShimadaK.FujikawaK.YaharaT.NakamuraAntioxidative properties of xanthan on the anti-oxidation of soybean oil in cyclodextrin emulsionJ. Agric. Food Chem.401992945948
- N.SreejayanM.N.A.RaoNitric oxide scavenging by curcuminoidsJ. Pharm. Pharmacol.491997105107
- R.J.RuchS.J.ChengJ.E.KlaunigPrevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green teaCarcinogenesis10198910031008
- I.S.GroverS.BalaAntimutagenic activity of T. chebula (myroblan) in Salmonella typhimuriumInd. J. Exp. Biol.301992339341
- R.R.ChattopadhyayS.K.BhattacharyyaPlant review Terminalia chebula: an updatePharmacog. Rev.112007151156
- L.J.JuangS.J.SheuT.C.LinDetermination of hydrolyzable tannins in the fruit of Terminalia chebula by high-performance liquid chromatography and capillary electrophoresisJ. Sep. Sci.272004718724
- A.A.NewairyH.M.AbdouProtective role of flax lignans against lead acetate-induced oxidative damage and hyperlipidemia in ratsFood Chem. Toxicol.472009813818
- L.PackerA.S.H.OngBiological Oxidants and Antioxidants: Molecular Mechanisms and Health Effects1997AOCS PressChampaign, IL
- S.V.JovanovicM.G.SimicAntioxidants in nutritionAnn. N.Y. Acad. Sci.8992000326334
- T.BhaumikP.C.JoshiA.K.DeyA.B.KunduChemical investigation of Terminalia chebula Retz.Bull. Med. Ethnobot. Res.101989190192
- P.RangsriwongN.RangkadilokJ.SatayavivadM.GotoA.ShotiprukSubcritical water extraction of polyphenolic compounds from Terminalia chebula Retz. fruitsSep. Purif. Technol.6620095156
- F.PellatiR.BruniD.RighiA.GrandiniM.TognoliniF.P.PioF.PoliS.BenvenutiD.D.RioD.RossiMetabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activityJ. Ethnopharmacol.1472013277285
- P.G.PiettaFlavonoids as antioxidantsJ. Nat. Prod.63200010351042
- C.AlasalvarM.KaramacR.AmarowiczF.ShahidiAntioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy coverJ. Agric. Food Chem.54200648264832
- H.LiZ.HaoX.WangL.HuangJ.LiAntioxidant activities of extracts and fractions from Lysimachia foenum-graecum HanceBioresour. Technol.1002009970974
- G.YenP.DuhH.TsaiAntioxidant and pro-oxidant properties of ascorbic acid and gallic acidFood Chem.792002307313
- S.V.KnezevicB.BlazekovicM.B.StefanA.AlegroT.KoszegiJ.PetrikAntioxidant activities and polyphenolic contents of three selected Micromeria species from CroatiaMolecules16201114541470
- A.MichalakPhenolic compounds and their antioxidant activity in plants growing under heavy metal stressPolish J. Environ. Stud.152006523530
- F.ShahidiC.AlasalvarC.M.Liyana-PathiranaAntioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproductJ. Agric. Food Chem.55200712121220
- O.BeyhanM.ElmastasF.GedikliTotal phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae)J. Med. Plants Res.4201010651072
- N.C.CookS.SammanFlavonoids – chemistry, metabolism, cardioprotective effects, and dietary sourcesJ. Nutr. Biochem.719966676
- R.A.LarsonThe antioxidants of higher plantsPhytochemistry271988969978
- T.HatanoH.KagawaT.YasuharaT.OkudaTwo new flavonoids and other constituents in licorice root their relative astringency and radical scavenging effectsChem. Pharm. Bull.36198820902097