Abstract
An efficient, convenient method for the synthesis of 3,4-dihydropyrano[3,2-c]chromene derivatives by one-pot, three-component reaction of aldehydes, malononitrile/cyanoacetate, and 4-hydroxycoumarin in the presence of a catalytic amount of thiourea dioxide, an efficient, reusable organic catalyst, is described. A variety of 3,4-dihydropyrano[3,2-c]chromene derivatives were obtained, and 6-amino-5-cyano-4-phenyl-2-methyl-4H-pyran-3-carboxylic acid ethyl esters were obtained by condensation of aldehydes, malononitrile and ethyl acetoacetate in the presence of thiourea dioxide in aqueous medium. The salient features of the protocol are mild reaction conditions, high yields, short reaction time, safety, high atom-economy, eco-friendly standards, easy isolation of products, no column chromatographic separation and reusability of the catalyst.
1 Introduction
Multicomponent reactions have emerged as an efficient, powerful tool in modern organic chemistry for the generation of highly diverse, complex products from readily available substrates in a single operation, without isolation of intermediates, in minimal time, with maximum selectivity, high atom-economy, high purity and excellent yields. Multicomponent reactions are widely used in medicinal chemistry and modern organic synthesis because they are one-pot processes for assembling three or more components [Citation1,Citation2].
Dihydropyrano[c]chromenes and their derivatives are of considerable interest as they have a wide range of biological properties, including diuretic, analgesic, myorelaxant [Citation3], anticoagulant [Citation4], anticancer [Citation5], antitumour [Citation6], cytotoxic [Citation7] and anti-HIV [Citation8,Citation9] activities. They are also used as antimicrobial and anti-tuberculosis agents [Citation10].
A number of methods have been reported for the synthesis of 3,4-dihydropyrano[c]chromenes with the catalysts silica-bonded N-propylpiperazine sodium n-propionate [Citation11], hexamethylenetetramine [Citation12], tetrabutylammonium bromide [Citation13], 1,8-diazabicyclo[5.4.0]undec-7-ene [Citation14], 4-(dimethylamino) pyridine [Citation15], diammonium hydrogen phosphate [Citation16], CuO nanoparticles [Citation17], sodium dodecylsulfate [Citation18], triethylenetetraammonium trifluoroacetate [Citation19], α-Fe2O3 nanoparticles [Citation20], silica-grafted ionic liquid [Citation21], electrolysis in an undivided cell in the presence of sodium bromide as an electrolyte [Citation22], piperidine/triethylamine in aqueous media [Citation23], potassium phthalimide in aqueous media [Citation24], piperidine-functionalized poly(ethylene glycol) bridged dicationic ionic liquid [Citation25], polymer supported sulfanilic acid [Citation26], basic ionic liquid [Citation27], ammonium acetate [Citation28], cellulose-SO3H [Citation29] and H6P2W18O62·18H2O [Citation30]. Many of these procedures have merit; however, most require refluxing for hours in organic solvents, complex steps, use of expensive catalysts and tedious work-up. We decided to investigate thiourea dioxide for use as an organic catalyst for the synthesis of dihydropyrano[3,2-c]chromene derivatives in water.
Thiourea dioxide is a well-known reducing agent [Citation31]. It has received considerable attention as a catalyst for the construction of carbon–carbon and carbon–hetero-atom bonds [Citation32–Citation35] due to its eco-friendly nature, easy handling, high reactivity and easy work-up. It is a novel organic catalyst in the one-pot synthesis of a library of heterocyclic compounds [Citation32], hydrolysis of imines [Citation33], naphthopyran derivatives [Citation34] and catalytic oxidation of alcohols [Citation35]. We have reported that thiourea dioxide is an efficient catalyst in the reaction of aromatic aldehydes with malononitrile/cyanoacetate and 4-hydroxy-6-methylpyran-2-one in water at 80 °C. This reaction led only to the corresponding pyrano[4,3-c]pyran derivatives in excellent yields [Citation36].
Thiourea dioxide is easily prepared [Citation37] by oxidation of thiourea with hydrogen peroxide. This catalyst is also called formamidine-sulfinic acid or amino imino methanesulfinic acid. It is a stable powdered compound, which dissolves in water and decomposes gradually to exhibit reducing action. It can activate organic substrates by hydrogen bonding. Owing to the presence of two extra oxygen atoms, it forms strong hydrogen bonds and can provide higher activation than the corresponding thiourea. In addition, thiourea dioxide is insoluble in common organic solvents and can therefore easily be recovered at the end of a reaction for reuse.
During our studies to improve the eco-compatibility of certain organic processes [Citation38–Citation41], we have been particularly interested in synthesizing potentially active dihydropyrano[c]chromene derivatives in water to ensure environmentally benign reactions. Here, we report efficient preparation of dihydropyrano[c]chromene derivatives in a one-pot reaction of aromatic aldehyde, malononitrile or cyanoacetate and 4-hydroxycoumarin, catalysed by thiourea dioxide in water ().
Scheme 1 Synthesis of various 3,4-dihydropyrano[c]chromenes and 6-amino-5-cyano-4-aryl-2-methyl-4H-pyran-3-carboxylic acid ethyl esters.

4H-Pyrans have potent biological properties, such as antitumour, antibacterial, antiviral, anti-tubercular and spasmolytic activities [Citation42–Citation45]. In view of this broad spectrum, chemists have developed numerous protocols for their syntheses with various catalysts, such as SnCl2/nano SiO2 [Citation46], MgO [Citation47], hexadecyl dimethylbenzyl ammonium bromide [Citation48], potassium phthalimide [Citation49], silica nanoparticles [Citation50] and silica-bonded N-propylpiperazine sodium n-propionate [Citation51]. These methods however, have drawbacks, and give moderate yields even after prolonged reaction. This clearly indicates that there is still scope to develop an efficient, eco-sustainable method for the synthesis of 4H-pyrans. To extend the application of thiourea dioxide, we also synthesized a series of 6-amino-5-cyano-4-aryl-2-methyl-4H-pyran-3-carboxylic acid ethyl esters by the three-component condensation of ethyl acetoacetate, aldehydes with malononitrile using thiourea dioxide as the catalyst in aqueous medium at 70 °C ().
2 Experimental
2.1 Apparatus and analysis
Chemicals were purchased from Merck, Fluka and Aldrich chemical companies. All yields refer to isolated products unless otherwise stated. 1H nuclear magnetic resonance (NMR) (500 MHz) and 13C NMR (125 MHz) spectra were obtained on a Bruker DRX-500 Avance at ambient temperature, with tetramethylsilane as internal standard and dimethylsulfoxide (DMSO)-d6 as solvent. Fourier transform infrared (IR) spectra were obtained as KBr discs on a Shimadzu spectrometer. Mass spectra (MS) were determined on a Varion-Saturn 2000 GC/MS instrument. Elemental analyses were performed in a Perkin Elmer 2400 CHN elemental analyser flowchart.
2.2 General procedure for the synthesis of dihydropyrano[c]chromene derivatives (4a–t)
A dry 50-mL flask was charged with aromatic aldehyde 1 (1 mmol), malononitrile or cyanoacetate 2 (1 mmol), 4-hydroxycoumarin 3 (1 mmol), thiourea dioxide (10 mol%) and water (5 mL), and the resulting mixture was stirred at 70 °C for 8–30 min. After completion of the reaction, as indicated by thin-layer chromatography (TLC), ethanol (10 mL) was added, and the reaction mixture was filtered. The remaining solution was washed with warm ethanol (3× 5 mL) to separate the organic catalyst. After cooling, the crude products were precipitated. The remaining aqueous thiourea dioxide was collected and reused with no further processing for subsequent runs. The reaction products were identified by comparing their physical and spectral data (i.e. IR, 1H and 13C NMR and MS) with those reported in the literature for the same compounds. The crude products were purified by recrystallization from ethanol (95%) to give 4a–t.
2.3 Spectral data for the synthesized compounds (4a–t)
2.3.1 2-Amino-4-phenyl-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4a)
IR (KBr, cm−1): 3450 and 3370 (NH2), 3266 (Ar-H), 2211 (CN), 1715 (C=O), 1666, 1609, 1466, 1366, 1210, 1122, 1050, 1001, 778; 1H NMR (500 MHz, DMSO-d6) δ: 4.52 (s, 1H, CH), 7.15 (br s, 2H, NH2), 7.21–7.41 (m, 5H, Ar-H), 7.53 (d, J = 7.6 Hz, 1H, Ar-H), 7.69 (t, J = 7.6 Hz, 1H, Ar-H), 7.79 (t, J = 7.6 Hz, 1H, Ar-H), 7.94 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.4, 103.7, 113.0, 117.4, 120.3, 123.1, 124.2, 124.9, 125.8, 129.4, 130.0, 134.0, 146.5, 150.8, 152.9, 155.1, 157.2, 161.1 ppm; MS (ESI): m/z 317 (M+H)+. Anal. calculated for C19H12N2O3(%): C, 72.16; H, 3.82; N, 8.86. Found: C, 72.11; H, 3.77; N, 8.84.
2.3.2 2-Amino-4-(4-chlorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4b)
IR (KBr, cm−1): 3442 and 3374 (NH2), 3281 (Ar-H), 2210 (CN), 1710 (C=O), 1674, 1608, 1453, 1373, 1233, 1128, 1066, 1001, 772; 1H NMR (500 MHz, DMSO-d6) δ: 4.57 (s, 1H, CH), 7.11 (br s, 2H, NH2), 7.22 (d, 2H, J = 8.2 Hz, Ar-H), 7.41 (d, 2H, J = 8.2 Hz, Ar-H), 7.50 (d, J = 7.6 Hz, 1H, Ar-H), 7.72 (t, J = 7.6 Hz, 1H, Ar-H), 7.76 (t, J = 7.6 Hz, 1H, Ar-H), 7.89 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.7, 103.9, 113.3, 118.3, 119.8, 123.0, 123.7, 124.6, 125.7, 129.7, 130.2, 134.1, 146.7, 151.0, 152.9, 155.1, 157.7, 160.8 ppm; MS (ESI): m/z 351.5 (M+H)+. Anal. calculated for C19H11ClN2O3 (%): C, 65.06; H, 3.16; N, 7.99. Found: C, 64.92; H, 3.08; N, 7.92.
2.3.3 2-Amino-4-(4-methoxyphenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4c)
IR (KBr, cm−1): 3443 and 3373 (NH2), 3276 (Ar-H), 2217 (CN), 1714 (C=O), 1670, 1606, 1485, 1372, 1328, 1244, 1118, 1064, 874, 674; 1H NMR (500 MHz, DMSO-d6) δ: 3.63 (s, 3H, OCH3), 4.71 (s, 1H, CH), 7.20 (br s, 2H, NH2), 7.33 (d, 2H, J = 8.2 Hz, Ar-H), 7.44 (d, 2H, J = 8.2 Hz, Ar-H), 7.57 (d, J = 7.6 Hz, 1H, Ar-H), 7.69 (t, J = 7.6 Hz, 1H, Ar-H), 7.79 (t, J = 7.6 Hz, 1H, Ar-H), 7.92 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 54.4, 56.8, 104.2, 113.7, 117.3, 120.5, 122.9, 124.5, 124.9, 125.5, 129.7, 130.5, 134.2, 147.0, 151.0, 153.1, 154.7, 157.5, 160.8 ppm; MS (ESI): m/z 347 (M+H)+. Anal. calculated for C20H14N2O4 (%): C, 69.36; H, 4.07; N, 8.09. Found: C, 69.30; H, 4.02; N, 8.01.
2.3.4 2-Amino-4-(2-fluorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4d)
IR (KBr, cm−1): 3451 and 3374 (NH2), 3270 (Ar-H), 2212 (CN), 1712 (C=O), 1667, 1603, 1463, 1377, 1119, 1060, 952, 876, 622; 1H NMR (500 MHz, DMSO-d6) δ: 4.74 (s, 1H, CH), 7.11–7.15 (m, 2H, Ar-H), 7.21–7.26 (m, 2H, Ar-H), 7.29 (br s, 2H, NH2), 7.44 (d, J = 8.0 Hz, 1H, Ar-H), 7.56 (t, J = 7.5 Hz, 1H, Ar-H), 7.70 (t, J = 7.5 Hz, 1H, Ar-H), 7.88 (d, J = 7.5 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.9, 104.0, 114.2, 117.5, 120.4, 123.4, 124.3, 125.2, 125.8, 129.6, 130.4, 134.5, 147.2, 151.4, 153.3, 154.8, 157.4, 161.3 ppm; MS (ESI): m/z 335 (M+H)+. Anal. calculated for C19H11FN2O3 (%): C, 68.26; H, 3.32; N, 8.38. Found: C, 68.20; H, 3.27; N, 8.33.
2.3.5 2-Amino-4-(2-chlorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4e)
IR (KBr, cm−1): 3437 and 3381 (NH2), 3273 (Ar-H), 2211 (CN), 1710 (C=O), 1664, 1602, 1457, 1379, 1222, 1122, 1066, 1001, 780; 1H NMR (500 MHz, DMSO-d6) δ: 4.71 (s, 1H, CH), 7.00 (t, J = 7.2 Hz, 1H, Ar-H), 7.07 (d, J = 8.0 Hz, 1H, Ar-H), 7.16 (d, J = 7.4 Hz, 1H, Ar-H), 7.25 (t, J = 8.0 Hz, 1H, Ar-H), 7.28 (br s, 2H, NH2), 7.49 (d, J = 8.0 Hz, 1H, Ar-H), 7.53 (t, J = 7.5 Hz, 1H, Ar-H), 7.71 (t, J = 8.0 Hz, 1H, Ar-H), 7.87 (d, J = 7.5 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.5, 104.1, 114.0, 117.6, 119.6, 123.5, 124.2, 125.3, 125.8, 129.8, 129.8, 133.7, 147.1, 151.3, 153.1, 154.7, 157.6, 161.2 ppm; MS (ESI): m/z 351.5 (M+H)+. Anal. calculated for C19H11ClN2O3 (%): C, 65.06; H, 3.16; N, 7.99. Found: C, 64.94; H, 3.10; N, 7.94.
2.3.6 2-Amino-4-(2-bromophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4f)
IR (KBr, cm−1): 3444 and 3380 (NH2), 3272 (Ar-H), 2214 (CN), 1714 (C=O), 1669, 1622, 1608, 1458, 1376, 1113, 1067, 870, 633; 1H NMR (500 MHz, DMSO-d6) δ: 4.85 (s, 1H, CH), 7.19 (t, J = 8.0 Hz, 1H, Ar-H), 7.28–7.33 (m, 2H, Ar-H), 7.38 (br s, 2H, NH2), 7.47 (d, J = 7.5 Hz, 1H, Ar-H), 7.56 (t, J = 7.5 Hz, 1H, Ar-H), 7.62 (d, J = 7.8 Hz, 1H, Ar-H), 7.72 (t, J = 7.6 Hz, 1H, Ar-H), 7.90 (d, J = 7.8 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 57.0, 103.6, 114.1, 118.1, 119.4, 123.7, 124.1, 124.6, 125.0, 129.1, 129.7, 133.8, 147.4, 151.0, 153.2, 154.8, 158.0, 161.4 ppm; MS (ESI): m/z 396 (M+H)+. Anal. calculated for C19H11BrN2O3 (%): C, 57.74; H, 2.81; N, 7.09. Found: C, 57.62; H, 2.77; N, 7.04.
2.3.7 2-Amino-4-(3-chlorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4g)
IR (KBr, cm−1): 3452 and 3383 (NH2), 3281 (Ar-H), 2211 (CN), 1716 (C=O), 1677, 1617, 1457, 1377, 1224, 1125, 1064, 1001, 770; 1H NMR (500 MHz, DMSO-d6) δ: 4.57 (s, 1H, CH), 7.01–7.07 (m, 3H, Ar-H), 7.22 (t, J = 8.0 Hz, 1H, Ar-H), 7.31 (br s, 2H, NH2), 7.46 (d, J = 8.0 Hz, 1H, Ar-H), 7.56 (t, J = 7.5 Hz, 1H, Ar-H), 7.76 (t, J = 8.0 Hz, 1H, Ar-H), 7.88(d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 57.4, 103.5, 113.7, 118.2, 119.9, 123.3, 124.3, 124.8, 125.3, 129.3, 129.9, 133.5, 147.0, 150.8, 153.1, 154.6, 158.0, 161.2 ppm; MS (ESI): m/z 351.6 (M+H)+. Anal. calculated for C19H11ClN2O3 (%): C, 65.06; H, 3.16; N, 7.99. Found: C, 64.97; H, 3.12; N, 7.96.
2.3.8 2-Amino-4-(3-nitrophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4h)
IR (KBr, cm−1): 3437 and 3377 (NH2), 3277 (Ar-H), 2213 (CN), 1715 (C=O), 1673, 1612, 1458, 1375, 1218, 1119, 1058, 1001, 783; 1H NMR (500 MHz, DMSO-d6) δ: 4.62 (s, 1H, CH), 7.04–7.10 (m, 3H, Ar-H), 7.24 (t, J = 8.0 Hz, 1H, Ar-H), 7.33 (br s, 2H, NH2), 7.48 (d, J = 8.0 Hz, 1H, Ar-H), 7.54 (t, J = 7.5 Hz, 1H, Ar-H), 7.70 (t, J = 8.0 Hz, 1H, Ar-H), 7.93(d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 57.1, 104.0, 113.8, 117.6, 119.6, 122.8, 124.5, 124.8, 125.2, 129.2, 129.9, 134.3, 147.2, 150.8, 152.6, 155.0, 158.3, 160.7 ppm; MS (ESI): m/z 362 (M+H)+. Anal. calculated for C19H11N3O5 (%): C, 63.15; H, 3.07; N, 11.63. Found: C, 63.06; H, 3.01; N, 11.57.
2.3.9 2-Amino-4-(3-hydroxyphenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4i)
IR (KBr, cm−1): 3435 and 3375 (NH2), 3275 (Ar-H), 2215 (CN), 1709 (C=O), 1671, 1609, 1455, 1370, 1255, 1170, 1117, 1053, 990, 773; 1H NMR (500 MHz, DMSO-d6) δ: 4.66 (s, 1H, CH), 7.07–7.13 (m, 3H, Ar-H), 7.18 (t, J = 8.0 Hz, 1H, Ar-H), 7.30 (br s, 2H, NH2), 7.43 (d, J = 8.0 Hz, 1H, Ar-H), 7.57 (t, J = 7.5 Hz, 1H, Ar-H), 7.77 (t, J = 8.0 Hz, 1H, Ar-H), 7.90 (d, J = 8.0 Hz, 1H, Ar-H), 9.74 (s, 1H, OH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 57.3, 103.8, 113.6, 117.8, 119.7, 123.1, 123.9, 124.6, 125.4, 129.4, 129.8, 134.2, 146.8, 151.1, 152.4, 155.1, 158.2, 160.6 ppm; MS (ESI): m/z 333 (M+H)+. Anal. calculated for C19H12N2O4 (%): C, 68.67; H, 3.64; N, 8.43. Found: C, 68.60; H, 3.61; N, 8.38.
2.3.10 2-Amino-4-(4-methylphenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4j)
IR (KBr, cm−1): 3445 and 3381 (NH2), 3281 (Ar-H), 2213 (CN), 1707 (C=O), 1670, 1604, 1458, 1364, 1219, 1112, 1054, 1001, 769; 1H NMR (500 MHz, DMSO-d6) δ: 2.17 (s, 3H, CH3), 4.67 (s, 1H, CH), 7.19 (br s, 2H, NH2), 7.28 (d, 2H, J = 8.2 Hz, Ar-H), 7.37 (d, 2H, J = 8.2 Hz, Ar-H), 7.55 (t, J = 7.6 Hz, 1H, Ar-H), 7.70 (t, J = 7.6 Hz, 1H, Ar-H), 7.80 (t, J = 7.6 Hz, 2H, Ar-H), 7.90 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 20.3, 57.3, 103.8, 113.4, 117.6, 120.4, 123.3, 124.3, 124.8, 125.7, 129.5, 130.4, 133.8, 146.7, 150.7, 153.0, 155.0, 157.2, 160.7 ppm; MS (ESI): m/z 331 (M+H)+. Anal. calculated for C20H14N2O3(%): C, 72.72; H, 4.27; N, 8.48. Found: C, 72.63; H, 4.20; N, 8.44.
2.3.11 2-Amino-4-(4-bromophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4k)
IR (KBr, cm−1): 3448 and 3373 (NH2), 3280 (Ar-H), 2222 (CN), 1712 (C=O), 1675, 1623, 1608, 1450, 1377, 1113, 1065, 874, 636; 1H NMR (500 MHz, DMSO-d6) δ: 4.64 (s, 1H, CH), 7.16 (br s, 2H, NH2), 7.26 (d, 2H, J = 8.2 Hz, Ar-H), 7.45 (d, 2H, J = 8.2 Hz, Ar-H), 7.59 (d, J = 7.6 Hz, 1H, Ar-H), 7.67 (t, J = 7.6 Hz, 1H, Ar-H), 7.82 (t, J = 7.6 Hz, 1H, Ar-H), 7.93 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.8, 103.6, 114.2, 118.1, 120.2, 122.8, 123.7, 124.6, 125.7, 129.3, 130.4, 133.7, 146.8, 151.2, 152.4, 155.1, 157.7, 161.0 ppm; MS (ESI): m/z 396 (M+H)+. Anal. calculated for C19H11BrN2O3 (%): C, 57.74; H, 2.81; N, 7.09. Found: C, 57.68; H, 2.76; N, 7.03.
2.3.12 2-Amino-4-(4-nitrophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4l)
IR (KBr, cm−1): 3449 and 3380 (NH2), 3282 (Ar-H), 2223 (CN), 1717 (C=O), 1670, 1624, 1454, 1380, 1115, 1067, 1010, 752, 624; 1H NMR (500 MHz, DMSO-d6) δ: 4.59 (s, 1H, CH), 7.17 (br s, 2H, NH2), 7.29 (d, 2H, J = 8.2 Hz, Ar-H), 7.39 (d, 2H, J = 8.2 Hz, Ar-H), 7.54 (d, J = 7.6 Hz, 1H, Ar-H), 7.74 (t, J = 7.6 Hz, 1H, Ar-H), 7.77 (t, J = 7.6 Hz, 1H, Ar-H), 7.88 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 57.1, 104.1, 114.2, 117.7, 120.3, 123.5, 124.5, 124.9, 125.6, 129.5, 130.5, 133.9, 146.9, 151.3, 152.5, 155.1, 157.6, 161.2 ppm; MS (ESI): m/z 362 (M+H)+. Anal. calculated for C19H11N3O5 (%): C, 63.15; H, 3.07; N, 11.63. Found: C, 63.08; H, 3.03; N, 11.59.
2.3.13 2-Amino-4-(2-nitrophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4m)
IR (KBr, cm−1): 3455 and 3382 (NH2), 3278 (Ar-H), 2222 (CN), 1719 (C=O), 1666, 1621, 1454, 1385, 1118, 1065, 1010, 766, 628; 1H NMR (500 MHz, DMSO-d6) δ: 4.70 (s, 1H, CH), 7.10–7.16 (m, 2H, Ar-H), 7.24–7.30 (m, 2H, Ar-H), 7.36 (br s, 2H, NH2), 7.48 (d, J = 8.0 Hz, 1H, Ar-H), 7.54 (t, J = 7.5 Hz, 1H, Ar-H), 7.74 (t, J = 7.5 Hz, 1H, Ar-H), 7.94 (d, J = 7.5 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 57.3, 104.0, 113.7, 117.7, 120.2, 123.6, 124.2, 124.8, 125.6, 129.7, 130.4, 133.5, 147.2, 151.0, 152.8, 155.1, 157.5, 161.3 ppm; MS (ESI): m/z 362 (M+H)+. Anal. calculated for C19H11N3O5 (%): C, 63.15; H, 3.07; N, 11.63. Found: C, 63.04; H, 3.00; N, 11.54
2.3.14 2-Amino-4-(3,4-dichlorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4n)
IR (KBr, cm−1): 3437 and 3380 (NH2), 3278 (Ar-H), 2216 (CN), 1712 (C=O), 1664, 1627, 1459, 1370, 1216, 1108, 1064, 1001, 769; 1H NMR (500 MHz, DMSO-d6) δ: 4.59 (s, 1H, CH), 7.06–7.10 (m, 2H, Ar-H), 7.17 (t, J = 8.0 Hz, 1H, Ar-H), 7.36 (br s, 2H, NH2), 7.42 (d, J = 8.0 Hz, 1H, Ar-H), 7.54 (t, J = 7.5 Hz, 1H, Ar-H), 7.71 (t, J = 8.0 Hz, 1H, Ar-H), 7.87 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 57.0, 103.7, 113.8, 117.9, 119.5, 123.7, 124.1, 124.9, 125.5, 129.5, 130.3, 133.7, 147.2, 150.6, 152.9, 154.7, 157.5, 160.8 ppm; MS (ESI): m/z 386 (M+H)+. Anal. calculated for C19H10Cl2N2O3 (%): C, 59.24; H, 2.62; N, 7.27. Found: C, 59.18; H, 2.58; N, 7.26.
2.3.15 2-Amino-4-(4-fluorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4o)
IR (KBr, cm−1): 3439 and 3383 (NH2), 3282 (Ar-H), 2214 (CN), 1708 (C=O), 1668, 1607, 1457, 1376, 1210, 1107, 1063, 1001, 771; 1H NMR (500 MHz, DMSO-d6) δ: 4.63 (s, 1H, CH), 7.21 (br s, 2H, NH2), 7.30 (d, 2H, J = 8.2 Hz, Ar-H), 7.42 (d, 2H, J = 8.2 Hz, Ar-H), 7.60 (d, J = 7.6 Hz, 1H, Ar-H), 7.75 (t, J = 7.6 Hz, 1H, Ar-H), 7.80 (t, J = 7.6 Hz, 1H, Ar-H), 7.96 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.3, 103.2, 113.8, 117.5, 119.7, 122.8, 124.2, 124.8, 125.7, 129.4, 129.7, 133.8, 147.3, 150.4, 153.0, 154.8, 158.0, 160.5 ppm; MS (ESI): m/z 335 (M+H)+. Anal. calculated for C19H11FN2O3 (%): C, 68.26; H, 3.32; N, 8.38. Found: C, 68.17; H, 3.28; N, 8.30.
2.3.16 2-Amino-4-(4-hydroxyphenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile (4p)
IR (KBr, cm−1): 3443 and 3380 (NH2), 3274 (Ar-H), 2221 (CN), 1714 (C=O), 1667, 1602, 1458, 1377, 1262, 1170, 1124, 1059, 995, 783; 1H NMR (500 MHz, DMSO-d6) δ: 4.65 (s, 1H, CH), 7.15 (br s, 2H, NH2), 7.25 (d, 2H, J = 8.2 Hz, Ar-H), 7.41 (d, 2H, J = 8.2 Hz, Ar-H), 7.55 (d, J = 7.6 Hz, 1H, Ar-H), 7.71 (t, J = 7.6 Hz, 1H, Ar-H), 7.79 (t, J = 7.6 Hz, 1H, Ar-H), 7.93 (d, J = 8.0 Hz, 1H, Ar-H), 9.68 (s, 1H, OH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.7, 103.2, 113.7, 117.4, 119.8, 122.9, 124.3, 124.9, 125.7, 129.3, 129.9, 133.8, 146.6, 150.6, 153.4, 154.9, 158.3, 160.4 ppm; MS (ESI): m/z 333 (M+H)+. Anal. calculated for C19H12N2O4 (%): C, 68.67; H, 3.64; N, 8.43. Found: C, 68.55; H, 3.64; N, 8.35.
2.3.17 2-Amino-4-phenyl-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carboxylic acid ethyl ester (4q)
IR (KBr, cm−1): 3413 and 3338 (NH2), 3260 (Ar-H), 1700 (C=O), 1670 (C=O), 1640, 1607, 1444, 1366, 1257, 1153, 1114, 1044, 980, 750; 1H NMR (500 MHz, DMSO-d6) δ: 1.04 (t, 3H, J = 7.2 Hz, CH3), 3.72 (q, 2H, J = 7.2 Hz, CH2), 4.64 (s, 1H, CH), 7.14 (br s, 2H, NH2), 7.22–7.40 (m, 5H, Ar-H), 7.41 (d, 1H, J = 8.2 Hz, Ar-H), 7.55 (t, J = 7.6 Hz, 1H, Ar-H), 7.77 (t, J = 7.6 Hz, 1H, Ar-H), 7.90 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.8, 53.5, 56.1, 103.1, 113.2, 119.0, 122.4, 124.1, 124.4, 125.2, 128.7, 129.4, 134.2, 145.3, 150.6, 152.8, 154.4, 159.3, 162.4 ppm; MS (ESI): m/z 364 (M+H)+. Anal. calculated for C21H17NO5 (%): C, 69.41; H, 4.72; N, 3.85. Found: C, 69.33; H, 4.66; N, 3.80.
2.3.18 2-Amino-4-(4-chlorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carboxylic acid ethyl ester (4r)
IR (KBr, cm−1): 3418 and 3342 (NH2), 3255 (Ar-H), 1708 (C=O), 1678 (C=O), 1643, 1609, 1446, 1360, 1253, 1155, 1117, 1050, 986, 762; 1H NMR (500 MHz, DMSO-d6) δ: 0.98 (t, 3H, J = 7.2 Hz, CH3), 3.77 (q, 2H, J = 7.2 Hz, CH2), 4.60 (s, 1H, CH), 7.11 (br s, 2H, NH2), 7.19 (d, 2H, J = 8.2 Hz, Ar-H), 7.37 (d, 2H, J = 8.2 Hz, Ar-H), 7.47 (d, J = 7.6 Hz, 1H, Ar-H), 7.60 (t, J = 7.6 Hz, 1H, Ar-H), 7.75 (t, J = 7.6 Hz, 1H, Ar-H), 7.93 (d, J = 8.0 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.6, 53.0, 56.6, 103.2, 113.0, 119.3, 122.0, 124.0, 124.5, 125.7, 128.4, 129.9, 134.5, 145.0, 150.3, 152.2, 154.3, 159.6, 162.7 ppm; MS (ESI): m/z 398.5 (M+H)+. Anal. calculated for C21H16ClNO5 (%): C, 63.40; H, 4.05; N, 3.52. Found: C, 63.33; H, 4.00; N, 3.48.
2.3.19 2-Amino-4-(4-nitrophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carboxylic acid ethyl ester (4s)
IR (KBr, cm−1): 3422 and 3349 (NH2), 3268 (Ar-H), 1711 (C=O), 1682 (C=O), 1650, 1607, 1438, 1358, 1250, 1158, 1119, 1053, 983, 758; 1H NMR (500 MHz, DMSO-d6) δ: 1.05 (t, 3H, J = 7.2 Hz, CH3), 3.75 (q, 2H, J = 7.2 Hz, CH2), 4.62 (s, 1H, CH), 7.17 (br s, 2H, NH2), 7.26 (d, 2H, J = 8.2 Hz, Ar-H), 7.43 (d, 2H, J = 8.2 Hz, Ar-H), 7.57 (d, J = 7.6 Hz, 1H, Ar-H), 7.69 (t, J = 7.6 Hz, 1H, Ar-H), 7.80 (t, J = 7.6 Hz, 1H, Ar-H), 7.96 (d, J = 8.1 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.9, 53.2, 56.4, 103.0, 113.8, 119.6, 122.2, 124.5, 124.9, 125.5, 128.3, 129.3, 134.7, 145.3, 150.8, 152.4, 154.7, 159.4, 162.0 ppm; MS (ESI): m/z 409 (M+H)+. Anal. calculated for C21H16N2O7 (%): C, 61.77; H, 3.95; N, 6.86. Found: C, 61.72; H, 3.90; N, 6.80.
2.3.20 2-Amino-4-(4-methylphenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carboxylic acid ethyl ester (4t)
IR (KBr, cm−1): 3410 and 3352 (NH2), 3269 (Ar-H), 1704 (C=O), 1680 (C=O), 1645, 1605, 1451, 1363, 1260, 1150, 1112, 1055, 982, 757; 1H NMR (500 MHz, DMSO-d6) δ: 1.07 (t, 3H, J = 7.2 Hz, CH3), 2.19 (s, 3H, CH3), 3.73 (q, 2H, J = 7.2 Hz, CH2), 4.65 (s, 1H, CH), 7.19 (br s, 2H, NH2), 7.20 (d, 2H, J = 8.1 Hz, Ar-H), 7.39 (d, 2H, J = 8.1 Hz, Ar-H), 7.46 (d, J = 7.4 Hz, 1H, Ar-H), 7.65 (t, J = 7.4 Hz, 1H, Ar-H), 7.74 (t, J = 7.4 Hz, 1H, Ar-H), 7.98 (d, J = 8.1 Hz, 1H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.2, 20.5, 53.7, 56.8, 103.7, 113.5, 119.8, 122.1, 124.1, 124.8, 125.3, 128.7, 129.0, 134.0, 145.2, 150.1, 152.6, 154.1, 159.5, 162.8 ppm; MS (ESI): m/z 378 (M+H)+. Anal. calculated for C22H19NO5 (%): C, 70.02; H, 5.07; N, 3.71. Found: C, 70.00; H, 5.05; N, 3.68.
2.4 General procedure for the synthesis of 6-amino-5-cyano-2-methyl-4-aryl-4H-pyran-3-carboxylate ethyl esters
Thiourea dioxide (10 mol%) was added to a mixture of ethyl acetoacetate (1 mmol), aldehydes (1 mmol) and malononitrile (1 mmol) in water (5 mL), and the mixture was heated at 70 °C for the appropriate time (). The progress of the reaction was monitored by TLC. After completion of the reaction, the mass was cooled to 25 °C, and the mixture was dissolved in ethanol (20 mL). The solvent was concentrated under vacuum, and the crude residue was purified by recrystallization from ethanol. The remaining aqueous thiourea dioxide was collected and reused with no further processing for subsequent runs. The products were identified by IR, 1H NMR, 13C NMR, mass, elemental analysis and melting-points.
Table 5 Preparation of 6-amino-5-cyano-4-aryl-2-methyl-4H-pyran-3-carboxylic acid ethyl ester derivatives from benzaldehydes, malononitrile and ethyl acetoacetate catalysed by TUD in water.Table Footnotea
2.5 Spectral data for the synthesized compounds (6a–l)
2.5.1 6-Amino-5-cyano-4-phenyl-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6a)
IR (KBr, cm−1): 3426, 3357, 3230, 2210, 1677, 1652, 1494, 1222, 795; 1H NMR (500 MHz, DMSO-d6) δ: 1.12 (t, J = 7.4 Hz, 3H, CH3CH2), 2.33 (s, 3H, CH3), 4.13 (q, J = 7.4 Hz, 2H, CH3CH2), 4.72 (s, 1H, CH), 5.22 (s, 2H, NH2), 7.06–7.33 (m, 5H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 13.9, 19.5, 39.2, 58.9, 62.4, 105.8, 118.8, 127.3, 128.4, 133.0, 144.4, 149.5, 154.9, 166.2 ppm; MS (ESI): m/z 285 (M+H)+. Anal. calculated for C16H16N2O3 (%): C, 67.60; H, 5.67; N, 9.85. Found: C, 67.51; H, 5.62; N, 9.77.
2.5.2 6-Amino-5-cyano-4-(4-chlorophenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6b)
IR (KBr, cm−1): 3433, 3362, 3233, 2212, 1672, 1644, 1488, 1213, 822; 1H NMR (500 MHz, DMSO-d6) δ: 1.11 (t, J = 7.2 Hz, 3H, CH3CH2), 2.29 (s, 3H, CH3), 4.17 (q, J = 7.2 Hz, 2H, CH3CH2), 4.68 (s, 1H, CH), 5.27 (s, 2H, NH2), 7.13 (d, J = 7.2 Hz, 2H, Ar-H), 7.33 (d, J = 7.2 Hz, 2H Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.3, 18.7, 38.4, 59.3, 61.4, 106.3, 117.3, 126.8, 128.2, 132.4, 143.7, 148.9, 155.2, 165.7 ppm; MS (ESI): m/z 319.5 (M+H)+. Anal. calculated for C16H15ClN2O3 (%): C, 60.29; H, 4.74; N, 8.79. Found: C, 60.20; H, 4.70; N, 8.75.
2.5.3 6-Amino-5-cyano-4-(4-methoxyphenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6c)
IR (KBr, cm−1): 3403, 3355, 3240, 2207, 1674, 1639, 1480, 1221, 806. 1H NMR (500 MHz, DMSO-d6) δ: 1.15 (t, J = 7.2 Hz, 3H, CH3CH2), 2.32 (s, 3H, CH3), 3.67 (s, 3H, OCH3), 4.13 (q, J = 7.2 Hz, 2H, CH3CH2), 4.67 (s, 1H, CH), 5.28 (s, 2H, NH2), 7.09 (d, J = 7.2 Hz, 2H, Ar-H), 7.41 (d, J = 7.2 Hz, 2H Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.6, 18.6, 38.6, 59.6, 61.8, 106.5, 118.4, 126.7, 128.3, 132.6, 143.3, 149.7, 155.4, 165.9 ppm; MS (ESI): m/z 315 (M+H)+. Anal. calculated for C17H18N2O4 (%): C, 64.96; H, 5.74; N, 8.91. Found: C, 64.87; H, 5.68; N, 8.88.
2.5.4 6-Amino-5-cyano-4-(2-fluorophenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6d)
IR (KBr, cm−1): 3425, 3358, 3244, 2205, 1668, 1648, 1490, 1224, 799. 1H NMR (500 MHz, DMSO-d6) δ: 1.21 (t, J = 7.6 Hz, 3H, CH3CH2), 2.34 (s, 3H, CH3), 4.10 (q, J = 7.6 Hz, 2H, CH3CH2), 4.74 (s, 1H, CH), 5.19 (s, 2H, NH2), 7.12–7.32 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.4, 19.3, 39.3, 58.7, 62.6, 105.4, 118.3, 127.0, 128.4, 133.2, 144.6, 148.8, 154.6, 166.3 ppm; MS (ESI): m/z 303 (M+H)+. Anal. calculated for C16H15FN2O3 (%): C, 63.57; H, 5.00; N, 9.27. Found: C, 63.48; H, 4.93; N, 9.22.
2.5.5 6-Amino-5-cyano-4-(2-chlorophenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6e)
IR (KBr, cm−1): 3440, 3363, 3234, 2210, 1667, 1634, 1493, 1218, 777. 1H NMR (500 MHz, DMSO-d6) δ: 1.16 (t, J = 7.2 Hz, 3H, CH3CH2), 2.34 (s, 3H, CH3), 4.15 (q, J = 7.2 Hz, 2H, CH3CH2), 4.70 (s, 1H, CH), 5.32 (s, 2H, NH2), 7.16–7.38 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 13.8, 18.4, 38.8, 59.4, 61.8, 106.5, 117.4, 126.7, 128.6, 132.7, 143.6, 149.4, 155.7, 165.6 ppm; MS (ESI): m/z 319.6 (M+H)+. Anal. calculated for C16H15ClN2O3 (%):C, 60.29; H, 4.74; N, 8.79. Found: C, 60.22; H, 4.69; N, 8.77.
2.5.6 6-Amino-5-cyano-4-(2-bromophenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6f)
IR (KBr, cm−1): 3432, 3349, 3232, 2213, 1673, 1642, 1487, 1219, 762. 1H NMR (500 MHz, DMSO-d6) δ: 1.10 (t, J = 7.2 Hz, 3H, CH3CH2), 2.29 (s, 3H, CH3), 4.16 (q, J = 7.2 Hz, 2H, CH3CH2), 4.72 (s, 1H, CH), 5.27 (s, 2H, NH2), 7.11–7.29 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.1, 18.8, 38.5, 59.8, 61.5, 106.7, 118.6, 126.6, 128.7, 132.3, 143.0, 149.8, 155.3, 165.4 ppm; MS (ESI): m/z 364 (M+H)+. Anal. calculated for C16H15BrN2O3 (%): C, 52.90; H, 4.16; N, 7.71. Found: C, 52.82; H, 4.11; N, 7.66.
2.5.7 6-Amino-5-cyano-4-(3-chlorophenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6g)
IR (KBr, cm−1): 3427, 3348, 3245, 2215, 1675, 1641, 1486, 1223, 780. 1H NMR (500 MHz, DMSO-d6) δ: 1.07 (t, J = 7.4 Hz, 3H, CH3CH2), 2.29 (s, 3H, CH3), 4.10 (q, J = 7.4 Hz, 2H, CH3CH2), 4.74 (s, 1H, CH), 5.22 (s, 2H, NH2), 7.16–7.41 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 13.7, 19.7, 39.5, 58.8, 62.6, 105.6, 118.5, 127.4, 128.0, 133.1, 144.7, 149.8, 154.8, 166.4 ppm; MS (ESI): m/z 319.5 (M+H)+. Anal. calculated for C16H15ClN2O3 (%):C, 60.29; H, 4.74; N, 8.79. Found: C, 60.18; H, 4.66; N, 8.70
2.5.8 6-Amino-5-cyano-2-methyl-4-(3-nitrophenyl)-4H-pyran-3-carboxylic acid ethyl ester (6h)
IR (KBr, cm−1): 3443, 3352, 3232, 2214, 1677, 1637, 1489, 1227, 825. 1H NMR (500 MHz, DMSO-d6) δ: 1.05 (t, J = 7.2 Hz, 3H, CH3CH2), 2.33 (s, 3H, CH3), 4.13 (q, J = 7.2 Hz, 2H, CH3CH2), 4.71 (s, 1H, CH), 5.23 (s, 2H, NH2), 7.19–7.44 (m, 4H, Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.0, 18.7, 38.5, 59.5, 61.9, 106.4, 117.8, 126.4, 128.4, 132.4, 143.1, 149.2, 155.1, 165.9 ppm; MS (ESI): m/z 330 (M+H)+. Anal. calculated for C16H15N3O5 (%): C, 58.36; H, 4.58; N, 12.76. Found: C, 58.25; H, 4.56; N, 12.70.
2.5.9 6-Amino-5-cyano-4-(3-hydroxyphenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6i)
IR (KBr, cm−1): 3437, 3359, 3228, 2203, 1669, 1639, 1490, 1219, 815. 1H NMR (500 MHz, DMSO-d6) δ: 1.11 (t, J = 7.2 Hz, 3H, CH3CH2), 2.27 (s, 3H, CH3), 4.13 (q, J = 7.2 Hz, 2H, CH3CH2), 4.74 (s, 1H, CH), 5.22 (s, 2H, NH2), 7.15–7.42 (m, 4H, Ar-H), 9.77 (s, 1H, OH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.2, 18.9, 38.9, 59.3, 61.7, 106.8, 118.7, 126.8, 128.8, 132.5, 143.7, 149.6, 155.4, 165.5 ppm; MS (ESI): m/z 301 (M+H)+. Anal. calculated for C16H16N2O4 (%): C, 64.00; H, 5.37; N, 9.33. Found: C, 63.90; H, 5.30; N, 9.27.
2.5.10 6-Amino-5-cyano-4-(4-methylphenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6j)
IR (KBr, cm−1): 3432, 3353, 3239, 2219, 1680, 1640, 1495, 1217, 796. 1H NMR (500 MHz, DMSO-d6) δ: 1.14 (t, J = 7.4 Hz, 3H, CH3CH2), 2.17 (s, 3H, CH3), 2.34 (s, 3H, CH3), 4.15 (q, J = 7.4 Hz, 2H, CH3CH2), 4.66 (s, 1H, CH), 5.27 (s, 2H, NH2), 7.16 (d, J = 7.2 Hz, 2H, Ar-H), 7.33 (d, J = 7.2 Hz, 2H Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.4, 19.0, 39.4, 58.6, 62.3, 105.9, 118.6, 127.6, 128.3, 133.3, 144.5, 149.5, 154.7, 166.6 ppm; MS (ESI): m/z 299 (M+H)+. Anal. calculated for C17H18N2O3 (%): C, 68.45; H, 6.08; N, 9.39. Found: C, 68.38; H, 6.00; N, 9.30.
2.5.11 6-Amino-5-cyano-4-(4-bromophenyl)-2-methyl-4H-pyran-3-carboxylic acid ethyl ester (6k)
IR (KBr, cm−1): 3426, 3355, 3234, 2218, 1678, 1645, 1495, 1225, 809. 1H NMR (500 MHz, DMSO-d6) δ: 1.05 (t, J = 7.2 Hz, 3H, CH3CH2), 2.34 (s, 3H, CH3), 4.17 (q, J = 7.2 Hz, 2H, CH3CH2), 4.68 (s, 1H, CH), 5.27 (s, 2H, NH2), 7.11 (d, J = 7.2 Hz, 2H, Ar-H), 7.42 (d, J = 7.2 Hz, 2H Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 13.8, 18.6, 38.3, 59.4, 61.8, 106.5, 117.7, 126.9, 128.9, 132.8, 143.8, 150.0, 155.3, 165.8 ppm; MS (ESI): m/z 364 (M+H)+. Anal. calculated for C16H15BrN2O3 (%): C, 52.90; H, 4.16; N, 7.71. Found: C, 52.85; H, 4.09; N, 7.63.
2.5.12 6-Amino-5-cyano-2-methyl-4-(4-nitrophenyl)-4H-pyran-3-carboxylic acid ethyl ester (6l)
IR (KBr, cm−1): 3440, 3349, 3233, 2221, 1669, 1645, 1490, 1223, 745. 1H NMR (500 MHz, DMSO-d6) δ: 1.09 (t, J = 7.3 Hz, 3H, CH3CH2), 2.27 (s, 3H, CH3), 4.15 (q, J = 7.3 Hz, 2H, CH3CH2), 4.69 (s, 1H, CH), 5.28 (s, 2H, NH2), 7.21 (d, J = 7.2 Hz, 2H, Ar-H), 7.43 (d, J = 7.2 Hz, 2H Ar-H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 14.8, 19.3, 38.4, 59.7, 61.3, 106.7, 118.7, 126.5, 128.4, 132.2, 143.6, 150.2, 155.9, 165.3 ppm; MS (ESI): m/z 330 (M+H)+. Anal. calculated for C16H15N3O5 (%): C, 58.36; H, 4.58; N, 12.76. Found: C, 58.28; H, 4.53; N, 12.67.
3 Results and discussion
In order to find the most appropriate reaction conditions and to evaluate the catalytic efficiency of thiourea dioxide, a model study was conducted to determine the best conditions for the synthesis of 2-amino-4-(chlorophenyl)-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile 4b (). The solvents CHCl3, CH3CN, 1,4-dioxane, methanol, ethanol and water were tested (, entries 1–6); condensation of 4-chlorobenzaldehyde, 4-hydroxycoumarin and malononitrile was easier and gave the highest yield in the presence of water as solvent (, entry 6).
Table 1 Optimization of reaction conditions for the synthesis of 4b.Table Footnotea
The effect of reaction temperature was also examined, and the reaction was found to proceed smoothly at 70 °C (, entry 6). The model reaction was conducted at a range of temperatures, including room temperature, 50, 60, 70 and 80 °C, in the presence of 10 mol% thiourea dioxide catalyst in water (, entries 6–10). The reaction proceeded slowly at room temperature, and the reaction yield was increased with increasing temperature to 70 °C; when the reaction was heated above 70 °C, the time of reaction decreased. The greatest yield in the shortest reaction time was obtained in water at 70 °C (, entry 6).
We also evaluated the quantity of catalyst required for the synthesis of compound 4b. Catalyst loadings in the range of 0–15 mol% were tested (, entries 11–14). A low yield of the product was observed in the absence of the catalyst, and smaller amounts, such as 2 mol% and 5 mol%, afforded poorer yields. Loading of thiourea dioxide to 15 mol% did not improve yields to a greater extent. Thus, loading of 10 mol% thiourea dioxide in water was sufficient to push the reaction forward (). Of the catalysts tested, including KH2PO4, HBF4, LiBr, p-toluenesulfonic acid and thiourea dioxide, the last was the most efficient in terms of reaction time and yield of product (, entries 6 and 15–18).
Having established the reaction conditions for the multi-component reaction, the scope and limitations of the reaction with different aldehydes, malononitrile/cyano ester and 4-hydroxycoumarin were investigated. (entries 1–20) indicates that all the reactions proceeded efficiently, and the desired products were produced in high yields in short reaction times. In an investigation of the effect of electron-withdrawing substituents, electron-releasing substituents and halogens on the aromatic ring of aldehydes on the reaction results (), electron-withdrawing substituents and halogens produced higher yields of products than their electron-rich counterparts. We also found that the reaction of aromatic aldehydes with electron-withdrawing groups was more rapid than that of aldehydes with electron-donating groups.
Table 2 Preparation of 2-amino-4-phenyl-5-oxo-4H,5H-pyrano-[3,2-c]chromene-3-carbonitrile derivatives from benzaldehydes, malononitrile/ethyl cyanoacetate and 4-hydroxycoumarin catalysed by TUD in water.Table Footnotea
A comparison of the reaction times and yields of thiourea dioxide-catalysed synthesis of 3,4-dihydropyrano[3,2-c]chromene derivatives with those reported shows the merit of our method. Thiourea dioxide resulted in much greater activity, with a short reaction time and mild conditions () than the other catalysts studied, which were silica-bonded N-propylpiperazine sodium n-propionate, silica-bonded N-propylpiperazine and silica-bonded N-propylmorpholine, p-toluenesulfonic acid, silicotungstic acid, nano zinc oxide, nano aluminium hydroxide, nano aluminium oxide, hexamethylenetetramine, tetrabutylammonium bromide, 1,8-diazabicyclo[5.4.0]undec-7-ene, 4-(dimethylamino)pyridine, diammonium hydrogen phosphate, (S)-proline, CuO nanoparticles, sodium dodecyl sulfate, triethylenetetraammonium trifluoroacetate and α-Fe2O3 (, entries 1–20).
Table 3 Effect of different catalysts for the synthesis of 3,4-dihydropyrano[c]chromenes from the condensation of on the reaction of benzaldehyde, 4-hydroxycoumarin and malononitrile.
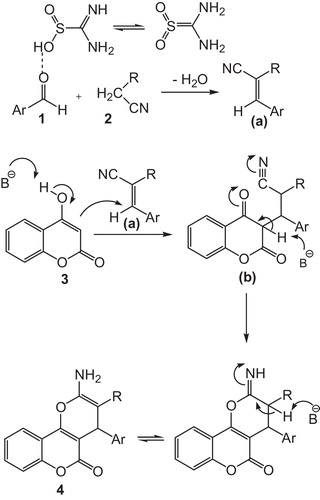
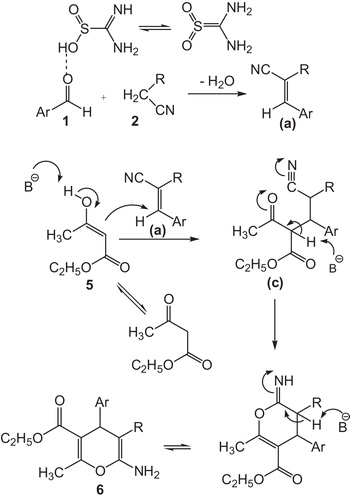
shows the efficiency of thiourea dioxide for the synthesis of 3,4-dihydropyrano[c]chromenes in comparison with our previous reported results for synthesis of pyrano[4,3-b]pyran derivatives in terms of reaction times and yields. Encouraged by these results, we extended the catalytic activity of thiourea dioxide to condensation reactions of aromatic aldehydes, malononitrile and ethyl acetoacetate to afford 6-amino-5-cyano-2-methyl-4-aryl-4H-pyran-3-carboxylate derivatives (). A series of 6-amino-5-cyano-2-methyl-4-aryl-4H-pyran-3-carboxylate derivatives with different substituents was prepared from different aromatic aldehydes bearing electron-withdrawing and electron-donating groups with malononitrile and ethyl acetoacetate in water at 70 °C (, entries 1–12). A probable mechanism for the formation of 3,4-dihydropyrano[3,2-c]chromene derivatives is outlined in . We assume that thiourea dioxide is an effective catalyst for the formation of the olefin a, which is formed in situ by Knoevenagel condensation of aryl aldehyde 1 and the active methylene compound 2. Olefin a subsequently reacts with 4-hydroxycoumarin to give intermediate b. Further, cyclization of b and subsequent tautomerization yielded the corresponding 3,4-dihydropyrano[3,2-c]chromene derivatives 4. 6-Amino-5-cyano-2-methyl-4-aryl-4H-pyran-3-carboxylate derivatives were obtained similarly ().
Table 4 Comparison of synthesis of various pyrano[4,3-b]pyran derivativesTable Footnotea with the synthesis of various 2-amino-4-phenyl-5-oxo-4H,5H-pyrano-[3,2-c]chromene derivativesTable Footnotec using TUD in water.
Scheme 3 A possible mechanism for the formation of 6-amino-5-cyano-4-aryl-2-methyl-4H-pyran-3-carboxylic acid ethyl esters.
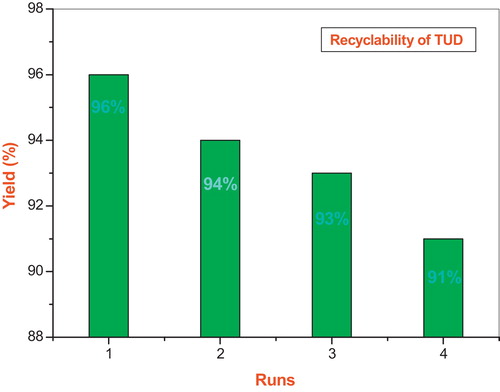
The possibility of recycling the catalyst was examined by performing the reaction of 4-chlorobenzaldehyde, malononitrile and 4-hydroxycoumarin in the presence of 10 mol% of thiourea dioxide in water. Upon completion of the reaction, as indicated by TLC, ethanol (10 mL) was added, and the reaction mixture was filtered. The remaining aqueous solution of thiourea dioxide was reused with no further treatment for the subsequent run. As shown in , thiourea dioxide can be recycled at least four times with no significant decrease in catalytic activity, the yields ranging from 96% to 91%.
4 Conclusions
We have developed a simple, highly efficient one-pot three-component method for the synthesis of various 3,4-dihydropyrano[3,2-c]chromene derivatives by reaction of aldehydes, malononitrile/cyano ester and 4-hydroxycoumarin catalysed by thiourea dioxide and also for the synthesis of 6-amino-5-cyano-4-phenyl-2-methyl-4H-pyran-3-carboxylic acid ethyl esters by one-pot condensation of aldehydes, malononitrile and ethyl acetoacetate in the presence of thiourea dioxide in aqueous medium. This procedure has many attractive features, such as operational simplicity, high product yield and easy work-up and purification. Furthermore, thiourea dioxide is inexpensive and non-volatile making the method environmentally friendly and economically acceptable.
Acknowledgement
Syed Sheik Mansoor expresses his gratitude to the management of C. Abdul Hakeem College, Melvisharam, India, for facilities and support.
Notes
Peer review under responsibility of Taibah University.
References
- J.ZhuH.BienaymeMulticomponent Reactions2005Wiley-VCHWeinheim
- J.SafariZ.ZarnegarM.HeydarianPractical, ecofriendly, and highly efficient synthesis of 2-amino-4H-chromenes using nanocrystalline MgO as a reusable heterogeneous catalyst in aqueous mediaJ. Taibah Univ. Sci.720131725
- L.BonsignoreG.LoyD.SecciA.CalignanoSynthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivativesEur. J. Med. Chem.281993517520
- G.CingolaniF.GualtieriM.PiginiNotes. Researches in the field of antiviral compounds. Mannich bases of 3-hydroxycoumarinJ. Med. Chem.121969531532
- J.Y.C.WuW.F.FongJ.X.ZhangC.H.LeungH.L.KwongM.S.YangD.LiH.Y.CheungReversal of multidrug resistance in cancer cells by pyranocoumarins isolated from Radix peucedaniEur. J. Pharmacol.4732003917
- F.W.PerrellaS.F.ChenD.L.BehrensR.F.KaltenbachIIIS.P.SeitzPhospholipase C inhibitors: a new class of agentsJ. Med. Chem.37199422322237
- N.R.EmmadiK.AtmakurG.K.ChityalS.PombalaJ.B.NanuboluSynthesis and cytotoxicity evaluation of highly functionalized pyranochromenes and pyranopyransBioorg. Med. Chem. Lett.22201272617264
- Y.KashmanK.R.GustafsonR.W.FullerJ.H.CardellinaJ.B.McmahonM.J.CurrensR.W.BuckheitS.H.HughesG.M.CraggM.R.BoydHIV inhibitory natural products. Part 7. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerumJ. Med. Chem.35199227352743
- A.D.PatilA.J.FreyerD.S.EgglestonR.C.HaltiwangerM.F.BeanP.B.TaylorM.J.CaranfaA.L.BreenH.R.BartusThe inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn.J. Med. Chem.36199341314138
- D.C.MungraM.P.PatelD.P.RajaniR.G.PatelSynthesis and identification of β-aryloxyquinolines and their pyrano[3,2-c]chromene derivatives as a new class of antimicrobial and antituberculosis agentsEur. J. Med. Chem.46201141924200
- K.NiknamA.JamaliSilica-bonded N-propylpiperazine sodium n-propionate as recyclable basic catalyst for synthesis of 3,4-dihydropyrano[c]chromene derivatives and biscoumarinsChin. J. Catal.33201218401849
- H.J.WangJ.LuZ.H.ZhangHighly efficient three component, one-pot synthesis of dihydropyrano[3,2-c]chromene derivativesMonatsh. Chem.141201011071112
- J.M.KhuranaS.KumarTetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditionsTetrahedron Lett.50200941254127
- J.M.KhuranaB.NandP.SalujaDBU: a highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano[4,3-b]pyranes, 2-amino-4H-benzo[h]chromenes and 2-amino-4H-benzo[g]chromenes in aqueous mediumTetrahedron66201056375641
- A.T.KhanM.LalS.AliM.M.KhanOne-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalystTetrahedron Lett.52201153275332
- S.AbdolmohammadiS.BalalaieNovel and efficient catalysts for the one-pot synthesis of 3,4-dihydropyrano[c]chromene derivatives in aqueous mediaTetrahedron Lett.48200732993303
- H.MehrabiM.Kazemi-MirekiCuO nanoparticles: an efficient and recyclable nanocatalyst for the rapid and green synthesis of 3,4-dihydropyrano[c]chromenesChin. Chem. Lett.22201114191422
- H.MehrabiH.AbusaidiSynthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives catalysed by sodium dodecyl sulfate (SDS) in neat waterJ. Iran. Chem. Soc.72010890894
- J.ZhengY.LiBasic ionic liquid-catalyzed multicomponent synthesis of tetrahydrobenzo[b]pyrans and pyrano[c]chromenesMendeleev Commun.212011280281
- H.NagabhushanaS.S.SaundalkarL.MuralidharB.M.NagabhushanaC.R.GirijaD.NagarajaM.A.PashaV.P.Jayashankaraα-Fe2O3 nanoparticles: an efficient, inexpensive catalyst for the one-pot preparation of 3,4-dihydropyrano[c]chromenesChin. Chem. Lett.222011143146
- K.NiknamA.PiranSilica-grafted ionic liquids as recyclable catalysts for the synthesis of 3,4-dihydropyrano[c]chromenes and pyrano[2,3-c]pyrazolesGreen Sustain. Chem.3201318
- Z.VafajooH.VeisiM.T.MaghsoodlouH.AhmadianElectrocatalytic multicomponent assembling of aldehydes, 4-hydroxycoumarin and malononitrile: an efficient approach to 2-amino-5-oxo-4,5-dihydropyrano [3,2-c]chromene-3-carbonitrile derivativesC. R. Chim.172014301304
- G.BrahmachariB.BanerjeeFacile and one-pot access to diverse and densely functionalized 2-amino-3-cyano-4H-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalystSustain. Chem. Eng.22014411422
- H.KiyaniF.GhorbaniPotassium phthalimide: an efficient and simple organocatalyst for the one-pot synthesis of dihydropyrano[3,2-c]chromenes in aqueous mediaRes. Chem. Intermed.201310.1007/s11164-013-1508-2
- Y.WangJ.LuoT.XingZ.LiuSynthesis of a novel piperidine-functionalized poly(ethylene glycol) bridged dicationic ionic liquid and its application in one-pot synthesis of substituted 2-amino-2-chromenes and 3,4-dihydropyrano[3,2-c]chromenes in aqueous mediaMonatsh. Chem.144201318711876
- J.P.PatelJ.R.AvalaniD.K.RavalPolymer supported sulphanilic acid: a highly efficient and recyclable green heterogeneous catalyst for the construction of 4,5-dihydropyrano[3,2-c] chromenes under solvent-free conditionsJ. Chem. Sci.1252013531536
- D.S.PatelJ.R.AvalaniD.K.RavalOne-pot solvent-free rapid and green synthesis of 3,4-dihydropyrano[c]chromenes using grindstone chemistryJ. Saudi Chem. Soc.201310.1016/j.jscs.2012.12.008
- S.KanakarajuB.PrasannaS.BasavojuG.V.P.ChandramouliAmmonium acetate catalyzed an efficient one-pot three component synthesis of pyrano[3,2-c]chromene derivativesArab. J. Chem.201310.1016/j.arabjc.2013.10.014
- H.R.ShaterianF.RigiNew applications of cellulose-SO3H as a bio-supported and biodegradable catalyst for the one-pot synthesis of some three-component reactionsRes. Chem. Intermed.201310.1007/s11164-013-1145-9
- M.M.HeraviB.A.JaniF.DerikvandF.F.BamoharramH.A.OskooieThree component, one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives in the presence of H6P2W18O62·18H2O as a green and recyclable catalystCatal. Commun.102008272275
- S.V.MakarovRecent trends in the chemistry of sulfur-containing reducing agentsRuss. Chem. Rev.702001885895
- S.VermaS.KumarS.L.JainB.SainThiourea dioxide promoted efficient organocatalytic one-pot synthesis of a library of novel heterocyclic compoundsOrg. Biomol. Chem.9201169436948
- S.KumarS.L.JainB.SainThiourea dioxide promoted cobalt-catalyzed hydrolysis of imines: dual activation via organocatalysis and metal catalysisRSC Adv.22012s789s791
- S.VermaS.L.JainThiourea dioxide catalyzed multi-component coupling reaction for the one step synthesis of naphthopyran derivativesTetrahedron Lett.53201260556058
- S.VermaR.SinghD.TripathiP.GuptaG.M.BahugunaS.L.JainThiourea dioxide with TBHP: a fruitful and greener recipe for the catalytic oxidation of alcoholsRSC Adv.3201341844188
- M.GhashangS.S.MansoorK.AswinThiourea dioxide: an efficient and reusable organocatalyst for the rapid one-pot synthesis of pyrano[4,3-b]pyran derivatives in waterChin. J. Catal.352014127133
- O. Ohura, O. Fujimoto, US Patent 4,233,238 (1980).
- S.S.MansoorK.AswinK.LogaiyaS.P.N.SudhanS.MalikAqueous media preparation of 2-amino-4,6-diphenylnicotinonitrile using cellulose sulfuric acidRes. Chem. Intermed.402014871885
- S.S.MansoorK.AswinK.LogaiyaS.P.N.SudhanH.RamadossMelamine trisulfonic acid: a new, efficient and reusable catalyst for the synthesis of some fused pyranopyrrole derivativesJ. Saudi Chem. Soc.201210.1016/j.jscs.2012.12.009
- K.AswinK.LogaiyaS.P.N.SudhanS.S.MansoorAn efficient one-pot synthesis of 1,4-dihydropyridine derivatives through Hantzsch reaction catalysed by melamine trisulfonic acidJ. Taibah Univ. Sci.6201219
- S.S.MansoorK.AswinK.LogaiyaS.P.N.SudhanAqua mediated synthesis of acridinediones using reusable silica supported sulfuric acid (SSA) as an efficient catalystJ. Taibah Univ. Sci.201410.1016/j.jtusci.2014.03.003
- J.L.WangD.LiuZ.J.ZhangS.ShanX.HanS.M.SrinivasulaC.M.CroceE.S.AlnemriZ.HuangStructure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cellsProc. Natl. Acad. Sci. U. S. A.97200071247129
- N.R.KamdarD.D.HaveliwalaP.T.MistryS.K.PatelDesign, synthesis and in vitro evaluation of antitubercular and antimicrobial activity of some novel pyranopyrimidinesEur. J. Med. Chem.45201050565063
- A.G.MartinezL.J.MarcoFriedlander reaction on 2-amino-3-cyano-4H-pyrans: synthesis of derivatives of 4H-pyran[2,3-b]quinoline, new tacrine analoguesBioorg. Med. Chem. Lett.7199731653170
- H.ValizadehaA.A.AzimiZnO/MgO containing ZnO nanoparticles as a highly effective heterogeneous base catalyst for the synthesis of 4H-pyrans and coumarins in [bmim]BF4J. Iran. Chem. Soc.82011123130
- J.Safaei-GhomiR.TeymuriH.Shahbazi-AlaviA.ZiaratiSnCl2/nano SiO2: a green and reusable heterogeneous catalyst for the synthesis of polyfunctionalized 4H-pyransChin. Chem. Lett.242013921925
- D.KumarV.B.ReddyS.SharadU.DubeS.KapurA facile one-pot green synthesis and antibacterial activity of 2-amino-4H-pyrans and 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromenesEur. J. Med. Chem.44200938053809
- T.-S.JinA.-Q.WangF.ShiL.-S.HanL.-B.LiuT.-S.LiHexadecyldimethyl benzyl ammonium bromide: an efficient catalyst for a clean one-pot synthesis of tetrahydrobenzopyran derivatives in waterARKIVOC1420067886
- H.KiyaniF.GhorbaniPotassium phthalimide promoted green multicomponent tandem synthesis of 2-amino-4H-chromenes and 6-amino-4H-pyran-3-carboxylatesJ. Saudi Chem. Soc.201410.1016/j.jscs.2014.02.004
- S.BanerjeeA.HornH.KhatriG.SeredaA green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalystTetrahedron Lett.52201118781881
- K.NiknamN.BorazjaniR.RashidianA.JamaliSilica-bonded N-propylpiperazine sodium n-propionate as recyclable catalyst for synthesis of 4H-pyran derivativesChin. J. Catal.34201322452254