 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We evaluated the antioxidant and antimicrobial activity of a methanolic extract of the roots of Arisaema jacquemontii. Antioxidant activity was determined in the 1,1-diphenyl-2-picryl-hydrazyl (DPPH), nitroblue tetrazolium (NBT) and ferric reducing power tests. The extract had significant antioxidant activity in all assays, with values of 64.16 ± 0.19% in the DPPH and 62.16 ± 0.17% in the NBT assays, and reduced Fe3+ ferricyanide complex to the ferrous form (Fe2+). Antibacterial activity and minimum inhibitory concentrations were calculated by the broth dilution method. The root extract prevented the growth of both Gram-positive and Gram-negative bacteria, at a minimum inhibitory concentration of 0.24–0.41 mg/mL. Antifungal activity, measured as inhibition of mycelium growth, was 28.32–36.50%. The antimicrobial and antioxidant activities of the extracts were positively associated with the total phenolic and flavonoid contents of the extract.
1 Introduction
Changing environmental conditions are giving rise to a variety of free radicals, which plants have to deal with them in order to survive. Reactive oxygen species, such as singlet oxygen, superoxide ion, hydroxyl ion and hydrogen peroxide, are highly reactive, toxic molecules, which are generated normally in cells during metabolism. They cause severe oxidative damage to proteins, lipids, enzymes and DNA by covalent binding and lipid peroxidation, with subsequent tissue injury. Natural antioxidant agents have attracted much interest because of their ability to scavenge free radicals [Citation1]. Free radicals have been implicated in the development of a number of disorders, including cancer, neurodegeneration and inflammation [Citation2–Citation4], giving rise to studies of antioxidants for the prevention and treatment of diseases. The presence of antioxidants such as phenolics, flavonoids, tannins and proanthocyanidins in plants may provide protection against a number of diseases; for example, ingestion of natural antioxidants has been inversely associated with morbidity and mortality from degenerative disorders [Citation5]. Medicinal plants are therefore being investigated for their antioxidant properties, and the demand for natural antioxidants and food preservatives is increasing [Citation6].
Table 2a Antibacterial activity of methanolic extract of A. jacquemontii of roots (MIC values expressed as mg/ml).
Table 2b Antifungal activity of methanolic extract of A. jacquemontii roots (expressed as % inhibition of mycelium growth).
Arisaema jacquemontii is an important medicinal plant of Kashmir, which has been used to cure various ailments in traditional systems of medicine. The plant belongs to the family Araceae and is commonly known as cobra lily. It is used as a food, an anthelmenthic and in the treatment of respiratory infections, dermatitis and as an antidote for snakebites [Citation7]. A lectin from A. jacquemontii tuber was reported to have anti-insect and anti-proliferative properties [Citation8]. Anticonvulsant activity and an effect on platelet aggregation have also been reported [Citation9], and the leaves have been reported to have antioxidant and immunomodulating potential [Citation10]. Although the plant is widely used in traditional medicine, few studies have been conducted of the pharmacological activities of the plant. Phenolic and flavonoid compounds are widespread in plant kingdom where they act as antioxidants and free radical scavengers. The objective of this study was to determine the total phenolic and flavonoid content and the antioxidant and antimicrobial activity of a methanolic extract of roots of A. jacquemontii.
2 Materials and methods
2.1 Plant material and preparation of extract
Roots of A. jacquemontii were collected in Uri, Jammu and Kashmir. The identity of plant was confirmed at the Centre of Plant Taxonomy and Biodiversity, University of Kashmir. The roots were dried in shade at room temperature, then chopped and ground to a fine powder in a mechanical blender. Dried root powder (20 g) was packed into a Soxhlet apparatus and extracted with 300 mL methanol at 60–65 °C for 3–4 h. The extract was filtered through Whatman filter paper No. 1, and the filtrate was concentrated under reduced pressure at 40 °C. The extract was dried, weighed (2.6 g) and stored at 4 °C in storage vials for experimental use.
2.2 Total phenolic content
The total phenolic content of the extract was determined by the Folin–Ciocalteu method [Citation11]. Briefly, 200 μL of crude extract (1 mg/mL) were made up to 3 mL with distilled water, mixed thoroughly with 0.5 mL of Folin–Ciocalteu reagent for 3 min, followed by the addition of 2 mL of 20% (w/v) sodium carbonate. The mixture was allowed to stand for a further 60 min in the dark, and absorbance was measured at 650 nm. The total phenolic content was calculated from the calibration curve, and the results were expressed as mg of gallic acid equivalent per g dry weight.
2.3 Total flavonoid content
The total flavonoid content of crude extract was determined by the aluminium chloride colorimetric method [Citation12]. In brief, 50 μL of crude extract (1 mg/mL ethanol) were made up to 1 mL with methanol, mixed with 4 mL of distilled water and then 0.3 mL of 5% NaNO2 solution; 0.3 mL of 10% AlCl3 solution was added after 5 min of incubation, and the mixture was allowed to stand for 6 min. Then, 2 mL of 1 mol/L NaOH solution were added, and the final volume of the mixture was brought to 10 mL with double-distilled water. The mixture was allowed to stand for 15 min, and absorbance was measured at 510 nm. The total flavonoid content was calculated from a calibration curve, and the result was expressed as mg rutin equivalent per g dry weight.
2.4 Antioxidant properties
2.4.1 1,1-Diphenyl-2-picryl-hydrazyl assay
The antioxidant activity of the extract was determined by the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay, as described earlier with some modifications [Citation13]. Briefly, 200 μL of each extract (100–500 μg/mL) were mixed with 3.8 mL DPPH solution and incubated in the dark at room temperature for 1 h. The absorbance of the mixture was then measured at 517 nm. Ascorbic acid was used as a positive control. The ability of the sample to scavenge DPPH radical was determined from:
2.4.2 Nitroblue tetrazolium assay
Superoxide anion scavenging activity was determined as described earlier [Citation14]. The reaction was performed in 50 mmol/L phosphate buffer (pH 7.8) containing concentrations of 100–500 μg/mL of the extract, 1.5 mmol/L riboflavin, 50 mmol/L nitroblue tetrazolium (NBT), 10 mmol/L d,l-methionine, and 0.025% (v/v) Triton X-100. The reaction was initiated by illuminating the reaction mixture; the absorbance of formazan was recorded at 560 nm, and the percentage scavenging activity was described as the inverse of the produced formazan. Ascorbic acid was used as a positive control.
2.4.3 Ferric reducing power assay
Ferric reducing or antioxidant power was determined as described earlier [Citation15]. Briefly, 100 μL of the extract (100–500 μg/mL) were mixed with 2.5 mL of 200 mmol/L phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide and incubated at 50 °C for 20 min. Then, 2.5 mL of 10% trichloroacetic acid were added, and the tubes were centrifuged at 10,000 rpm for 10 min. Then, 5 mL of the upper layer were mixed with 5.0 mL distilled water and 1 mL of 0.1% ferric chloride, and the absorbance of the reaction mixtures was measured at 700 nm. Ascorbic acid was used as a positive control.
2.5 Antimicrobial property
2.5.1 Antibacterial activity
Antimicrobial activity was tested in both Gram-negative and Gram-positive bacteria obtained from the Department of Microbiology, RTM Nagpur University, Nagpur, India. The strains were maintained by periodic subculture on nutrient agar and preserved at 4 °C prior to use. They were grown overnight in 10 mL broth at 37 °C, which was then centrifuged at 150 rpm. Minimum inhibitory concentrations (MICs) were determined by the broth microdilution method [Citation16–Citation18]. Serial dilutions of the stock solutions of the crude extract in broth medium were prepared on a microtitre plate, and microbial suspensions were added to the microwells at 5 × 105 microorganisms/mL. The microtitre plates were then incubated at 37 °C for 24 h. Activity was recorded as blue colouration in the wells after addition of resazurin. MICs were determined as the lowest concentrations that prevented visible growth. Streptomycin was used as a positive control. Each assay was repeated three times.
2.5.2 Antifungal activity
The antifungal activity of the root extract of A. jacquemontii was determined by the disc diffusion method [Citation19]. A conidial suspension (1 mL) of each fungus was added to each Petri dish, followed by 15 mL of potato dextrose agar supplemented with streptomycin sulfate (100 mg/L). After solidification of the substrate, a 5-mm disc of Whatman paper No. 3 was soaked with 20 μL of root extract, allowed to dry and placed on the inoculated Petri dishes. For the control, the disc was moistened with methanol. The plates were then incubated at 28 °C for 7 days. Antifungal activity was evaluated as the percentage inhibition of mycelium growth according to the formula:where C and T are the mean mycelium growth (mm) of controls and treated discs. All tests were performed in triplicate.
2.6 Statistical analysis
Statistical analysis was carried out with GraphPad Prism 6 software (Graph Pad Software, Inc., USA), and results are expressed as means ± standard deviation.
3 Results and discussion
3.1 Phenolic and flavonoid contents
The total phenolic content of the methanolic root extract, calculated from the calibration curve (R2 = 0.998), was 45.17 ± 1.70 gallic acid equivalents/g, and the total flavonoid content (R2 = 0.999) was 35 ± 2.20 rutin equivalents/g (). Phenolic compounds have redox properties, which allow them to act as antioxidants [Citation20]. As their free radical scavenging ability is facilitated by their hydroxyl groups, the total phenolic concentration could be used as a basis for rapid screening of antioxidant activity. Flavonoids, including flavones, flavanols and condensed tannins, are plant secondary metabolites, the antioxidant activity of which depends on the presence of free OH groups, especially 3-OH. Plant flavonoids have antioxidant activity in vitro and also act as antioxidants in vivo [Citation21,Citation22]. As this is the first report on the antioxidant activity of A. jacquemontii, thorough phytochemical analyses should be done to identify the active phenolic and flavonoid components.
Table 1 Total phenolics and flavonoids content of methanolic extract of A. jacquemontii roots.
3.2 Antioxidant activity
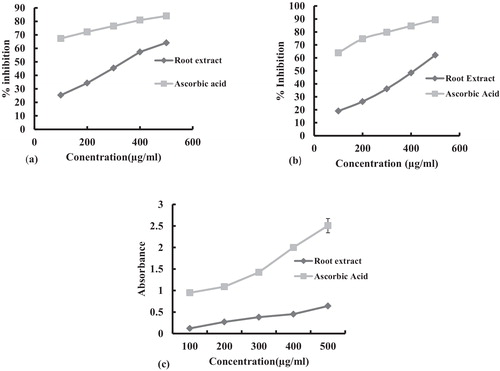
Plants rich in secondary metabolites, including phenolics, flavonoids and carotenoids, have antioxidant activity due to their redox properties and chemical structures. The methanolic root extract of A. jacquemontii had strong antioxidant activity against all the free radicals investigated. The DPPH radical is widely used in assessing free radical scavenging activity because of the ease of the reaction. DPPH scavenging activity was 64.16% at a concentration of 500 μg/mL root extract, while that of the control, ascorbic acid, was 84% (). Superoxide is a reactive oxygen species that can damage cells and DNA, leading to various diseases [Citation23]. Superoxide scavenging activity, determined in the NBT assay, was 62.16% for 500 μg/mL of the root extract and 89.36% for ascorbic acid (). In assays of the reducing power of the crude extract, significant changes in absorbance at 700 nm were observed (0.12–0.64) with increasing concentrations of extract (100–500 μg/mL) ().
Fig. 1 (a) Free radical scavenging activity, (b) superoxide scavenging activity, (c) ferrous reducing capacity of methanolic extracts of the roots of Arisaema jacquemontii. Ascorbic acid was included as a positive control. Each value is the mean ± standard deviation.
The high phenolic and flavonoid content is responsible for the bioactivity of these crude extracts. Flavonoids are highly effective scavengers of most oxidizing molecules, including singlet oxygen, and various other free radicals implicated in several diseases [Citation24]. Flavonoids suppress reactive oxygen formation, chelate trace elements involved in free-radical production, scavenge reactive species and up-regulate and protect antioxidant defenses [Citation25]. Similarly, phenolics conferring oxidative stress tolerance on plants. Crude extracts of fruits, herbs, vegetables, cereals and other plant materials rich in phenolics are increasingly being used in the food industry for their antioxidative properties and health benefits.
3.3 Antimicrobial activity
The antibacterial properties of methanolic extract of the roots of A. jacquemontii in vitro are presented in . The extracts had antibacterial activity against both Gram-positive and Gram-negative bacteria, with MICs of 0.24–0.41 mg/mL. The extract had the greatest activity against Salmonella enteritidis and Micrococcus luteus and the least against Streptococcus faecalis and Staphylococcus aureus. The root extract also had significant antifungal activity (), with values of 28.32–36.50%, the greatest activity being seen against Fusarium oxysporum and the least against Aspergillus flavus.
These results suggest that A. jacquemontii is a potential source of broad-spectrum antimicrobial agents. The antimicrobial activity of the extract may be attributed to the high content of flavonoids, which have been reported to be involved in inhibition of nucleic acid biosynthesis and other metabolic processes [Citation26]. Flavonoids have also been reported to inhibit spore germination of plant pathogens [Citation27]. Moreover, flavonoids are synthesized by plants in response to microbial infection. Phenolic compounds with a C3 side chain at a lower level of oxidation and containing no oxygen have often been reported to be antimicrobials [Citation28]. The partially hydrophobic nature of their phenolic compounds has also been reported to be responsible for their antimicrobial activity. The mechanism of the toxicity of polyphenols against microbes may be related to inhibition of hydrolytic enzymes (proteases) or other interactions that inactivate microbial adhesins, cell envelope transport proteins and non-specific interactions with carbohydrates [Citation29]. The antifungal and antimicrobial activity of phenolic and flavonoid compounds has been reported previously [Citation30–Citation32]. Isolation of the responsible elements is necessary for fully elucidating the antibacterial activity of these crude extracts. This might also provide insight about their possible use in food and non-food systems.
4 Conclusion
Our results suggest that A. jacquemontii is a potential source of antioxidant and antimicrobial agents and could be used as a natural antioxidant and preservative in food and non-food systems. Further phtyochemical analysis is required to isolate the elements of the plant that show a broad spectrum of pharmacological activity.
Notes
Peer review under responsibility of Taibah University.
References
- N.SaeedM.R.KhanM.ShabbirAntioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L.BMC Complement. Altern. Med.122012221
- B.HalliwellOxidative stress and cancer: have we moved forward?Biochem. J.4012007111
- B.HalliwellOxidative stress and neurodegeneration: where are we now?J. Neurochem.97200616341658
- L.R.FergusonChronic inflammation and mutagenesisMutat. Res. Fund. Mol.6902010311
- I.GulcinAntioxidant activity of food constituents: an overviewArch. Toxicol.862012345391
- W.PeschelF.Sanchez-RabanedaW.DieckmannA.PlescherI.Gartziaet alAn industrial approach in the search of natural antioxidants from vegetable and fruits wastesFood Chem.972006137150
- H.VermaV.K.LalK.K.PantN.SoniA review on Arisaema jacquemontiiJ. Pharm. Res.5201214801482
- M.KaurK.SinghJ.P.RupS.S.KambojA.K.SaxenaM.SharmaM.BhagatS.K.SoodJ.SinghA tuber lectin from Arisaema jacquemontii Blume with anti-insect and anti-proliferative propertiesJ. Biochem. Mol. Biol.392006432440
- S.JeelaniM.A.KhurooT.K.RazadanNew triterpenoids from Arisaema jacquemontiiJ. Asian Nat. Prod. Res.22010157161
- R.R.SudanM.BhagatS.GuptaJ.SinghA.KoulIron (FEII) chealtion, ferric reducing antioxidant power, and immune modulating potential of Arisaema jacquemontii (Himalayan cobra lily)Biomed. Res. Int.20142014179865
- C.KaurH.C.KapoorAnti-oxidant activity and total phenolic content of some Asian vegetablesInt. J. Food Sci. Technol.372002153161
- C.ChangM.YangH.WenJ.ChernEstimation of total flavonoid content in propolis by two complementary colorimetric methodsJ. Food Drug Anal.102002178182
- D.VillanoM.S.Fernandez-PachonM.L.MoyaA.M.TroncosoM.C.Garcıa-ParrillaRadical scavenging ability of polyphenolic compounds towards DPPH free radicalTalanta712007230
- D.VyasS.KumarPurification and partial characterization of a low temperature responsive Mn-SOD from tea (Camellia sinensis (L.) O. Kuntze)Biochem. Biophys. Res. Commun.3292005831838
- H.ZhaoW.FanJ.DongJ.LuJ.Chenet alEvaluation of antioxidant activities and total phenolic contents of typical malting barley varietiesFood Chem.1072008296304
- M.J.SalvadorE.O.FerreiraE.M.F.PralS.C.AlfieriS.AlbuquerqueI.Y.Itoet alBioactivity of crude extracts and some constituents of Blutaparon portulacoides (Amaranthaceae)Phytomedicine92002566571
- H.M.EriccsonJ.C.SherrisAntibiotic sensitivity testing: report of an international collaborative studyActa Pathol. Microbiol. Scand.21719711
- W.C.EvansTrease and Evans’ Pharmacognosy14th ed.1996W.B. Saunders Co. Ltd.Singapore290
- B.NaimaN.DébbabiH.B.JannetZ.MighriM.E.MahjoubAntifungal activity of volatile components extracted from leaves, stems and flowers of four plants growing in TunisiaPhytopathol. Mediterr.442005307312
- M.A.SoobratteeV.S.NeergheenA.Luximon-RammaO.I.AruomaO.T.BahorunPhenolics as potential antioxidant therapeutic agents: mechanism and actionsMutat. Res. Fundam. Mol.5792005200213
- S.GeethaM.Sai-RamS.S.MongiaV.SinghG.Ilavazhaganet alEvaluation of antioxidant activity of leaf extract of sea buckthorn (Hippophae rhamnoides L.) on chromium (VI) induced oxidative stress in albino ratsJ. Ethnopharmacol.872003247251
- K.ShimoiS.MasudaB.ShenM.FurugoriN.KinzeRadioprotective effects of antioxidative plant flavonoids in miceMutat. Res. Fund. Mol.3501996153161
- S.ShuklaA.MehtaK.V.BajpaiS.ShuklaIn vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert.Food Chem. Toxicol.47200923382343
- L.BravoPolyphenols: chemistry, dietary sources, metabolism and nutritional significanceNutr. Rev.561998317333
- G.AgatiE.AzzarelloS.PollastriM.TattiniFlavonoids as antioxidants in plants: location and functional significancePlant Sci.19620126776
- T.P.T.CushnieA.J.LambAntimicrobial activity of flavonoidsInt. J. Antimicrob. Agents262005343356
- J.B.HarborneC.A.WilliamsAdvances in flavonoid research since 1992Phytochemistry552000481504
- B.BerkadaPreliminary report on warfarin for the treatment of herpes 210 simplexJ. Irish Coll. Phys. Surg.22197856
- R.PylaT.J.KimJ.L.SilvaY.S.JungEnhanced antimicrobial activity of starch-based film impregnated with thermally processed tannic acid, a strong antioxidantInt. J. Food Microbiol.282010154160
- W.F.ZhengR.X.TanL.YangZ.L.LiuTwo flavones from Artemisia giraldii and their antimicrobial activityPlanta Med.621996160162
- C.CafarchiaN.De LaurentisM.A.MililloV.LosaccoV.PucciniAntifungal activity of Apulia region propolisParassitologia1411999587590
- A.J.AfolayanJ.J.MeyerThe antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitensJ. Ethnopharmacol.1571997177181
