Abstract
The reverse phase high performance liquid chromatographic (RP-HPLC) method has been developed for simultaneous estimation of starting material, impurities and final product in bulk API forms. The proposed method utilizes waters symmetry C18 (250 cm × 4.6 mm, 5 μm) column, mobile phase consisting of buffer and acetonitrile in the proportion of 40:60 (v/v) with apparent pH adjusted to 3.2, and UV detection at 245 nm. The described method was linear (r2 ≥ 0.99) over a range of 1–50 μg/mL and relative standard deviation values for intra-day and inter-day precision studies were less than 1.0% for all ingredients. The analytical mean recoveries of salicylic acid, salicylamide and deferasirox by the developed RP-HPLC method were 99.74%, 99.52% and 99.40% respectively. This method was validated by evaluation of different parameters like specificity, accuracy, precision, system suitability, linearity, LOD and LOQ according to ICH guidelines. The method has been successfully applied for the analysis of drugs in pharmaceutical dosages forms.
1 Introduction
Deferasirox [4-[(3Z, 5E)-3,5-bis(6-oxo-1-cyclohexa-2,4-dienylidene)-1,2,4-triazolidin-1-yl] benzoic acid [Citation1] is an orally active chelator that is highly selective for iron (III). It is a tridentate ligand that binds iron with high affinity in a 2:1 ratio [Citation2]. It is listed in the Merck index 14th edition [Citation3]. Deferasirox (EXJADE) has been investigated in adult and pediatric patients (aged 2 years and older) with chronic iron overload due to blood transfusions. Deferasirox is used to remove excess iron from the body after a person has too many blood transfusions. The underlying conditions requiring transfusion included beta-thalassaemia, sickle cell disease and other congenital and acquired anaemias (myelodysplastic syndromes, Diamond-Blackfan syndrome, aplastic anemia) and in patients with non-transfusion-dependent thalassemia syndromes. In order to develop a new precise method there is an immense need for developing a rapid, sensitive and validated analytical method for day-to-day analysis, in routine analysis in quality control laboratories of the drug and which can be even applicable for pharmaceutical dosage forms [Citation4]. Few LC methods for determination of deferasirox were reported in the literature review [Citation5–Citation8] and some reports mainly included the determination of deferasirox in biological fluids [Citation9,Citation10], Terbium-sensitized fluorescence method for determination of deferasirox in biological fluids and tablet formulation [Citation11]. Deferasirox promotes excretion of iron, primarily in the feces. Two molecules of Deferasirox combine with iron in the blood and then removed from the body by the kidneys [Citation12,Citation13].
Salicylamide (SAL), 2-hydroxybenzamide is a salicylic acid derivative that has analgesic, anti-inflammatory and antipyretic actions. It is used for pain, fever and inflammatory disorders such as osteoarthritis and rheumatoid arthritis [Citation14]. The USP [Citation15] recommended non-aqueous titration method for the determination of SAL in raw material using sodium methoxide as a titrant and thymol blue as indicator. Several analytical methods are described for the determination of SAL, whether in dosage forms or in biological fluids. Such methods include; spectrophotometry [Citation16], spectrofluorimetry [Citation17,Citation18], HPTLC [Citation19], HPLC [Citation20].
Salicylic acid (SA) is keratolytic in nature and chemically 2-hydroxy benzoic acid [Citation21]. Synthetic SA may contain impurities such as 4-hydroxybenzoic acid, 4-hydroxyisophthalic acid and phenol. RP-HPLC methods [Citation22,Citation23] for the assay in dermopharmaceutical forms [Citation24] and in an ointment product [Citation25] have also been reported ().
2 Experimental
2.1 Chemical and reagents
Deferasirox bulk drug obtained as gift sample from Cadila Pharma, Ahmedabad. Salicylic acid, salicylamide and Orthophosphoric acid were obtained from SD Fine-Chem Limited. Acetonitrile (fisher Qualigens, HPLC grade) were obtained from Thermo Fisher Scientific India Pvt. Ltd. and potassium dihydrogen phosphate was obtained from (Merck Specialties Private Limited).
2.2 Equipments
2.3 Preparation of buffer
Dissolved 1.36 g of potassium dihydrogen phosphate in 1000 mL high purity demonized Milli-Q water (Millipore, Milli-Q, Bedford, MA, USA, purification system) and pH was adjusted 3.2 with ortho-phosphoric acid and filtered through 0.22 μ size nylon filter under vacuum.
2.4 Preparation of mobile phase
The mobile phase was prepared by mixing 400 mL phosphate buffer pH 3.2 and 600 mL of acetonitrile (HPLC grade). The mixture was sonicated in Expo-Hi Tech sonicator for 5 min.
2.5 Preparation of diluent
Diluent for deferasirox is 99.5 mL acetonitrile and 0.5 mL water.
2.6 Preparation of standard and sample solution
The standard stock solutions were prepared by accurately weighing 100 mg of each salicylamide, salicylic acid and deferasirox in 100 mL volumetric flask (1000 μg/mL) in acetonitrile. Sample solutions were prepared by appropriate dilution of the standard solutions with the diluent.
3 Result and discussion
3.1 Method development and optimization
To develop a precise, accurate and suitable RP-HPLC method for the simultaneous estimation of salicylamide, salicylic acid and deferasirox different mobile phases, solvent–buffer ratios and pH were tried to proposed final chromatographic conditions. Deferasirox is soluble in methanol and acetonitrile–water mixture. The peak shapes, resolution and symmetry of salicylamide, salicylic acid and deferasirox were good with above gradient elution at a 1.0 mL/min flow rate. The method developed was unique in determining the impurities even at low levels than that of specifications. The developed method was successfully applied to estimate the amount of deferasirox.
3.2 Optimized conditions
3.3 Dosage form analysis
The proposed method was successfully applied to the simultaneous determination of salicylamide, salicylic acid and deferasirox in laboratory prepared mixtures and in their combined bulk dosages forms. The average percent recoveries of different concentrations were based on the average of three replicate determinations ().
Table 3 Accuracy data (three concentrations of 50%, 100% and 150% levels).
4 Validation of method
The optimized RP-HPLC assay method was validated for specificity, linearity, accuracy, precision (repeatability and intermediate precision), recovery and system suitability according to International Conference on Harmonization (ICH) guidelines for the validation of bioanalytical method [Citation26] and the US Food and Drug Administration (FDA) [Citation27].
4.1 Specificity
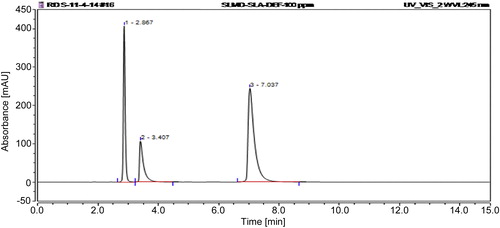
Specificity of method can be absence of any interference at retention times of samples. The specificity of the method was demonstrated by injection of standard solution of salicylamide, salicylic acid and deferasirox at concentration of 100 μg/mL. The elution peaks of salicylamide, salicylic acid and deferasirox presented in representative chromatograms shown in . The representative chromatogram for simultaneous determination of the studied drugs in API pharmaceutical dosages forms ().
4.2 Linearity
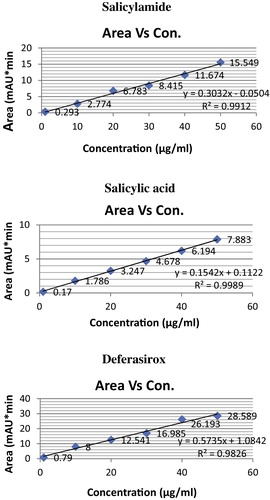
The linearity of salicylamide, salicylic acid and deferasirox were studied by preparing standard solution at five different concentrations ranging from 1.0 to 50.0 μg/mL. Each concentration was injected in a five replicates and mean value of peak area was taken for calibration curve.
4.2.1 Construction of the calibration curves
Working solutions containing (1.00–500.00) μg/mL were prepared by serial dilution of standard solution with the acetonitrile. In all cases, 10 μL aliquots were injected (triplicate) and eluted with the mobile phase under the following chromatographic conditions. The average peak area ratio of each drug and the internal standard were plotted versus the final concentration of the drug in μg/mL to get the calibration graph. Alternatively, the corresponding regression equation was derived ( and ).
Table 1 Summary of linearity data.
4.3 Precision
The precision of the assay was studied with respect to both intra-day (Repeatability) and Inter-day (Intermediated) precisions. Repeatability was calculated from five replicate injections of three different concentrations of salicylamide, salicylic acid and deferasirox in the same equipment on the same day. Inter day precision was checked with the same concentrations as intra-day assay and the determination of each compound was repeated day by day during 3 days. The method was found to be precise with RSD values within for intra-day and inter day assay. Evaluation of the intra-day and inter-day precision for the determination of salicylamide, salicylic acid and deferasirox by the proposed HPLC method according to ICH guidelines ().
Table 2 Intra-day and inter-day precision data.
4.4 Limit of detection (LOD) and limit of quantification (LOQ)
The limit of detection (LOD) is defined as the lowest concentration of an analyte that can reliably be differentiated from background levels. The standard solutions of the compounds for LOD were prepared by diluting them sequentially. Limit of quantification (LOQ) of an individual analytical procedure is the lowest amount of analyte that can be quantitatively determined with suitable precision and accuracy. LOD and LOQ were determined calculating the signal-to-noise ratio of each compound by injecting a series of solution until the S/N ratio 3 for LOD and 10 for LOQ. where S is the standard deviation of y-intercepts of regression.
4.5 Accuracy
Accuracy of the assay method was calculated for salicylamide, salicylic acid and deferasirox by recovery studies at three concentrations of 50%, 100% and 150% levels by standard addition method. The mean % recoveries for salicylamide, salicylic acid and deferasirox were found to be 99.74%, 99.52% and 99.40% respectively ().
4.6 System suitability
System suitability was performed by using 100 μg/mL of salicylamide, salicylic acid and deferasirox by making six replicate injections. Chromatographic parameters calculated from experimental data, such as Number of theoretical plates, % RSD of peak area and resolution factors (Rs) are given in . The system was deemed to be suitable for use if the capacity factors were in the range of 2–20 (2 < K′ < 20), lower than 2 for tailing factor, more than 2 for resolution (Rs), greater than 2000 number of theoretical plates (N), resolution between salicylamide, salicylic acid and deferasirox of at least two and less than 2% relative standard deviation (% RSD) for peak area.
Table 4 System suitability parameters.
5 Conclusion
An accurate, sensitive, and precise RP-HPLC method with PDA detection was developed and validated for quality control analysis of salicylamide, salicylic acid and deferasirox in their bulk API dosages forms. The proposed method is very rapid, where the total analytical run time for three APIs and the internal standard is less than 10 min. The method can also be readily adapted to routine quality control analysis.
Acknowledgements
The authors are thankful to the Department of Chemistry (DST-FIST Sponsored), Mahatma Gandhi Campus, Maharajakrishnakumarsinhji Bhavnagar University, Bhavnagar for providing research and library facilities. The authors are also thankful to Cadila Pharmaceutical Company Ahmadabad for providing API drug as gift sample.
Notes
Peer review under responsibility of Taibah University.
References
- http://www.chemblink.com
- http://www.druglib.com/druginfo/exjade/description_pharmacology
- The Merck Index14th ed.2006Merck and Co., Inc.White House Station (USA)483
- The International Conference on Harmonization Q2 (A), Validation of Analytical Procedures Text and Methodology, 6th November 19961996
- K.AnandakumarR.ChinthalaV.SubhashM.JayamariappanDevelopment and validation of newer analytical methods for the estimation of deferasirox in bulk and in tablet dosage form by UV spectroscopy and RP-HPLCJ. Pharm. Res.4201129983000
- K.RavikiranK.V.SurendranathP.RadhakrishnanandJ.SatishP.V.V.SatyanarayanaA stability indicating LC method for deferasirox in bulk drugs and pharmaceutical dosage formsChromatographia722010441446
- V.K.ChakravarthyD.GowrisankarLC determination of deferasirox in pharmaceutical formulationJ. Glob. Trends Pharmaceut. Sci.120103745
- V.SambasivaraoR.S.PhaniR.SeetharamanK.S.LakshmiRP-HPLC method development for analysis of deferasirox in formulationIJPI's J. Anal. Chem.120113135
- L.P.YangS.J.KeamG.M.KeatingDeferasirox: a review of its use in the management of transfusional chronic iron overloadDrugs67200722112230
- J.KarnonK.TolleyJ.OyeeK.JewittD.OssaR.AkehurstCost-utility analysis of Deferasirox compared to standard therapy with Desferrioxamine for patients requiring iron chelation therapy in the United KingdomCurr. Med. Res. Opin.24200816091621
- J.L.ManzooriA.JouybanM.AmjadiV.Panahi-AzarJ.Tamizi Eand Vaez-GharamalekiTerbium-sensitized fluorescence method for the determination of deferasirox in biological fluids and tablet formulationJ. Biol. Chem. Lumin. Lumin.262011244250
- http://www.drugs.com
- http://www.drugbank.ca/drugs/DB01609
- S.C.SweetmanMartindale: The Complete Drug Reference37th ed.2009Pharmaceutical PressLondon
- The United States Pharmacopoeia 30th and the National Formulary 25th, Rockville, MD, USA2007
- A.R.ZareiA.AfkhamiN.SarlakSimultaneous spectrophotometric determination of paracetamol and salicylamide in human serum and pharmaceutical formulations by a different kinetic methodJ. AOAC Int.88200516951701
- J.A.Murillo PulgarinA.Alanon MolinaI.Sanchez-Ferrer RoblesSimultaneous determination of salicylic acid and salicylamide in biological fluidsSpectrochim. Acta A: Mol. Biomol. Spectrosc.792011909914
- M.Sharaf El-DinM.EidA.M.Zeid El-DinDevelopment and validation of RP-HPLC method for simultaneous determination of ascorbic acid and salicylamide in their binary mixtures: application to combined tabletsJ. Chromatogr. Sep. Tech.3201210.4172/2157-7064.1000137
- C.SullivanJ.ShermaDetermination of salicylamide in pharmaceutical tablets by high-performance thin-layer chromatography with ultraviolet absorption densitometryActa Chromatogr.16200615371543
- J.V.AukunuruU.B.KompellaG.V.BetageriSimultaneous high performance liquid chromatographic analysis of acetaminophen, salicylamide, phenyltoloxamine and related productsJ. Liq. Chromatogr. Relat. Technol.232000565578
- The Indian Pharmacopeia, New Delhi2010The Controller of Publication III, P. No. 2089–90
- E.MikamiT.GotoT.OhnoH.MatsumotoM.NishidaSimultaneous analysis of dehydroacetic acid, benzoic acid, sorbic acid and salicylic acid in cosmetic products by solid-phase extraction and high-performance liquid chromatographyJ. Pharm. Biomed. Anal.282002261267
- S.H.HassonvilleP.ChiapJ.F.LiegeoisB.EvrardL.DelattreJ.CrommenG.PielP.HubertDevelopment and validation of a high-performance liquid chromatographic method for the determination of cyproterone acetate in human skinJ. Pharm. Biomed. Anal.362004133143
- R.D.MariniA.PantellaM.A.BimazubeteP.ChiapP.H.HubertJ.CrommenOptimisation and validation of a generic liquid chromatographic method for the simultaneous determination of six corticosteroids and salicylic acid in dermopharmaceutical formsChromatographia552002263269
- E.R.Kedor-HackmannE.A.GianottoM.I.SantoroDetermination of beta methasone dipropionate and salicylic acid in pharmaceutical preparations by high-performance liquid chromatographyDrug Dev. Ind. Pharm.241998553555
- ICH Guideline Q2AText on validation of analytical proceduresInternational Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use1994 http://www.ich.org (November 2012)
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER)Guidance for Industry, Bioanalytical Method Validation2001 http://www.fda.gov (January 2013)