Abstract
In addition to several important traditional medicine applications of Solanum incanum, the plant is a rich source of important cytotoxic glycoalkaloids, such as solamargine and solasonine. Because S. incanum is a potential source of compounds for steroid synthesis, it is worthwhile to study the content of these important glycoalkaloids during plant developmental stages. Therefore, an attempt has been made to quantitatively estimate solasonine and solamargine content using an optimized isolation process and a newly developed and validated HPTLC method using different parts of S. incanum plants at different developmental stages and comparing changes in the whole-plant GAs profile during the growth and development of S. incanum plants in Oman. Solamargine and solasonine produced well-separated compact bands with Rf values of 0.26 and 0.14, respectively, on silica gel HPTLC plates using chloroform:methanol:5% ammonia (7:3:0.5, v/v/v) after visualization using anisaldehyde sulphuric acid reagent. The chromatograms were scanned at 530 nm wavelength and the simultaneous method was linear (r2 ≥ 0.9962) in concentrations ranging from 50 to 2000 ng/spot for both of the drugs. The validated method was applied to analyse solamargine and solasonine in small, young, immature and mature leaves as well as stem and root parts up to the 40th week of plants’ growth, and showed a rich concentration of glycoalkaloids with large variations at different stages of plant development. Hence, this study highlights the importance of developmental stages of particular organs and the overall age of the plant when harvesting for these GAs from S. incanum plants.
1 Introduction
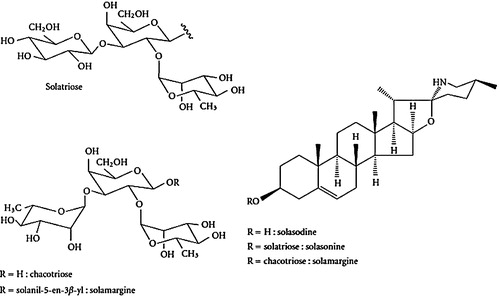
The genus Solanum (Family: Solanaceae), with more than 1700 species, is widespread in the temperate and tropical regions of the world [Citation1,Citation2]. It is characterized by the presence of steroidal glycoalkaloids (SGAs), which are of great interest from both ecological and human health perspectives [Citation3–Citation6]. Approximately 20 Solanum species have been recorded in Arabia. In Oman, there are seven known species of Solanum, including S. cordatum, S. incanum, S. melongena, S. nigrum, S. surattense, S. tuberosum, and S. villosum [Citation7]. Solanum incanum, commonly known as thorn apple, is an important medicinal plant. In Oman, the leaves, fruits (berries) and roots of S. incanum are used as a traditional medicine to treat bruised fingers, dyspepsia, earache, and haemorrhoids [Citation8]. Glycoalkaloids consist of two structural components, which account for their amphiphilic nature. The aglycone unit consists of a hydrophobic 27-carbon skeleton of cholestane with nitrogen incorporated into the F ring. The second unit is a hydrophilic carbohydrate side chain attached to the 3-OH position [Citation5]. Generally, the glycoalkaloids are referred to as α-compounds; cleavage (by acid or enzymatic hydrolysis) of the individual sugars of the glycoside leads to β-, γ-, or δ-compounds, depending on the number of sugars in the side chain [Citation9]. The majority of plants utilize glycoalkaloids as the main aglycone is solasodine in the form of water soluble triglycosidessolasonine (SN) and solamargine (SM), which occurs in approximately 200 species of Solanum plants [Citation10,Citation11]. These two compounds bear the same aglycone, solasodine, and differ from each other only in the nature of the trioses involved, namely, solatriose for solasonine and chacotriose for solamargine () [Citation5,Citation9]. Solamargine and solasonine are economically beneficial because their chemical structures are very similar to steroidal hormones. Therefore, they have been proposed for their use as important sources in the production of medicines, such as contraceptives and steroidal anti-inflammatory drugs [Citation2,Citation12]. These glycoalkaloids have been studied for their antidiabetic, antifungal, antiparacetic, antibiotic, antimicrobial, antiviral and, most importantly, anticancer properties [Citation12,Citation13].
Biological investigations of solamargine and solasonine showed significant cytotoxicity against several human cancer cell lines and skin tumours [Citation14]. Because S. incanum is a potential source of compounds required for steroid synthesis, it is worthwhile to study the content of these important glycoalkaloids during plant developmental stages.
Steroidal glycoalkaloids (SGAs) lack chromatographic groups that can be analysed in the common operating range of UV spectrophotometry and absorb only at the low wavelength end of the UV spectrum. This means that they have low UV sensitivity and can be detected and identified by UV diode array detection (DAD) only when present in relatively high amounts (5–10 ng/injection) [Citation15–Citation17]. The absence of chromophores in SGAs makes their detection a major challenge in the assay of a biological sample [Citation18]. A useful analysis method consists of three major steps: extraction of the alkaloids with aqueous or non-aqueous solvents, pre-purification or removal of interfering substances (impurities), and chromatographic procedures [Citation4,Citation10]. Different methodologies have been proposed to analyse Solanaceae glycoalkaloids in foodstuffs and plant materials, including colorimetric, chromatographic, or immunoassay methods [Citation9,Citation19]. The chromatographic methods, such as high-performance thin-layer chromatography (HPTLC), high-performance liquid chromatography (HPLC), and gas chromatography (GC), can be used to determine the levels of solasodine and its glycosides from their elaborating plant [Citation3,Citation10,Citation11,Citation15–Citation17,Citation19,Citation20]. Among the various analytical techniques, high-performance thin-layer chromatography (HPTLC), in particular, appears to be suitable for phytomolecules of varying natures and provides a rational approach in the authentication and quality assessment of crude medicinal herbs and their formulations [Citation18]. To the authors’ knowledge, there are very few published studies reporting quantitative data for α-solamargine and α-solasonine levels in S. incanum plants in different parts and developmental stages. Therefore, an attempt has been made to quantitatively estimate solasonine and solamargine contents using an optimized isolation process and a newly developed and validated HPTLC method for different parts of S. incanum plants at different developmental stages. We also compared the changes in the whole-plant GAs profile during the growth and development of S. incanum plants in Oman.
2 Materials and methods
2.1 Plant material
S. incanum L. seeds were obtained from original plants grown in Al Jabal Al Akhadar, Oman. The seeds were grown in the Green House, Sultan Qaboos University. Germination (on damp filter paper in Petri dishes at 21–25 °C, 14 h light, 70% RH) occurred within 3 weeks, and one month seedlings were transplanted into 15-cm-diameter pots containing acid-washed sand soil:clay soil:compost (1:1:1) and were grown in the University's green house at 21–25 °C, 14 h light, 70% RH. The plants were irrigated three times a week. Sampling commenced 12 weeks after germination. Five plants were randomly selected for analysis every 4 weeks for 40 weeks.
Each plant was divided into six different parts, according to a modification of the scheme proposed by Eltayeb et al. [Citation7]: ‘Small’ leaves, <3.4 cm in length; ‘Young’ leaves, 3.4–7.4 cm in length; ‘Immature’ leaves, 7.4–11.00 cm in length; ‘Mature’ leaves, >11.00 cm in length; Stem, the entire stem was used; Root, the entire root was used. After measuring, plant material was weighed fresh, dried in an oven at 60 °C to a constant weight (ca 3 days) and reweighed.
2.2 Chemicals and reference compounds
Solamargine and solasonine were obtained from Glycomix (Whiteknights Road, United Kingdom) and were used as reference compounds. Distilled water was deionized before use. HPLC-grade methanol and chloroform were obtained from Sigma–Aldrich (Germany). Ammonia solution was obtained from Merck (Darmstadt, Germany).
2.3 HPTLC instrumentation and conditions
The samples were spotted in the form of bands (4-mm width), with a CAMAG microlitre syringe on precoated HPTLC silica gel glass plates (60F-254; 20 cm × 10 cm, Merck KGaA, Germany) using a CAMAG Linomat V (Muttenz, Switzerland) and were controlled by WinCATS software (CAMAG). A constant application rate of 120 nL/s was employed and the space between two bands was 6.2 mm. The slit dimension was maintained at 5.00 mm × 0.30 mm, and a 20 mm/s scanning speed was employed. The mobile phase of the GA consisted of chloroform:methanol:5% ammonia, (7:3:0.5, v/v/v). Linear ascending development was carried out in a 20 cm × 10 cm twin trough glass chamber, saturated with the mobile phase. The optimized chamber saturation time for the mobile phase was 15 min at room temperature. The length of chromatogram run was 90 mm. Subsequent to development, HPTLC plates were dried in an oven at 60 °C for 5 min. Densitometric scanning was performed on a CAMAG TLC scanner IV (absorbance mode 530 nm) with WinCATS software after spraying the developed plate with anisaldehyde sulphuric acid reagent and heating it on a hot plate at 110 °C for 5 min.
2.4 Sample preparation
A simple method was used for the extraction in which 0.5 g of dried and powdered S. incanum plants (leaves, stems, and roots) were extracted using 30 mL of methanol:chloroform (2:1) by sonication at 40 °C for 45 min. The mixture was filtered using Whatman No. 1 filter paper and washed with methanol. The obtained filtrate was evaporated to dryness in a water bath at 40 °C. Dried extracts were further dissolved in methanol and filtered using a 0.2-μm syringe filter. The final volume was brought to 5 mL with methanol and stored at 4 °C prior to application to HPTLC plates for quantification.
2.5 Solamargine and solasonine standards
One mg/mL (1000 μg/mL) solutions of the solamargine and solasonine standards were prepared in methanol. A mixture of the glycoalkaloids, solamargine and solasonine was prepared by mixing 0.5 mL of each compound so that the concentration of each compound in the mixture was 0.5 mg/mL (500 μg/mL). These solutions were used for application on HPTLC plates to prepare the standard plot.
2.6 Calibration curve of solamargine and solasonine
Different volumes of the GA mixture standard solution (0.1, 0.2, 0.4, 0.5, 1.0, 2.0, 4.0, 5.0, and 10.0 μL of 500 μg/mL) were spotted in triplicate on the HPTLC plate to obtain 50, 100, 200, 250, 500, 1000, 2000, 2500, and 5000 ng/spot of each GA, respectively. The data for the peak area vs. solamargine and solasonine concentrations were treated with the linear-least square regression, and the regression equation obtained from the standard curve was used to estimate both GAs in different samples.
2.7 Quantification of solamargine and solasonine in different samples
The method was applied to analyse solamargine and solasonine content in small, young, immature and mature leaves along with stems and roots of S. incanum plants at different stages of plant growth (at intervals of 4 weeks up to 40 weeks). Samples of 10 μL each were applied in quadruplicate on TLC plates. The GA yield was quantified using the regression equation from the calibration curve.
2.8 Method validation
The developed method was validated as per the ICH guidelines [Citation20] similar to the other chromatographic HPTLC methods reported by laboratory [Citation21–Citation23], which are in use for the quality control of herbal drugs.
2.8.1 Accuracy as recovery
The accuracy of the present method was assessed in samples through recovery studies, in which pre-analysed samples were spiked with an extra 50%, 100% and 150% of the GA mixture and analysed by the proposed method. The experiment was conducted six times, the average recovered GAs content was quantified using the regression equation, and the % recovery was calculated accordingly.
2.8.2 Precision
Inter-day and intra-day precision analyses were performed by spotting three different concentrations of the GA stock solutions (50, 100 and 200 ng/spot for solamargine and 100, 200 and 500 ng/spot for solasonine) in the same day and in three different days, respectively. Inter-analyst precision was carried out by repeating same procedure with a different analyst. Intermediate precisions were determined in terms of % RSD of the area.
2.8.3 Specificity
The specificity of the method was assessed by comparing the Rf and absorption spectra of GAs in sample and standard tracks. The peak purity of GAs was assessed by comparing the spectra at three levels, i.e., peak start, peak apex, and peak end position of the band.
2.8.4 Robustness
The robustness of the proposed method was determined at three concentrations (50, 100 and 200 ng/spot for solamargine and 100, 200 and 500 ng/spot for solasonine) in two different ways, i.e., by changing the composition of mobile phase and changing the detecting wavelength. The % RSD of the peak area was calculated to assess the robustness of the method.
2.8.5 Sensitivity
The sensitivity of the method was determined as the limits of detection (LOD) and quantification (LOQ). Decreasing amounts of the GAs standard solution were applied to a plate and the chromatographic–densitometric analysis was performed as described above. The concentration of sample giving a signal to noise ratio of three was fixed as the LOD, whereas the concentration of the sample giving a signal to noise ratio of 10 was fixed as LOQ.
2.9 Statistical analysis
The computer programme SPSS was used for statistical analysis using two-way ANOVA.
3 Results and discussion
3.1 Optimization of the mobile phase
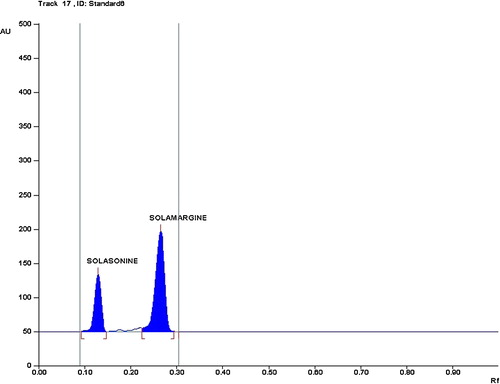
The composition of the mobile phase was optimized by testing different solvent compositions of varying polarities. From the different tested compositions of the mobile phase, the desired resolution of the compounds, together with symmetrical and reproducible peaks, was achieved using CHCl3:MeOH:5% NH3 (7:3:0.5, v/v/v) as the mobile phase. Well-separated and compact bands of solasonine and solamargine were visualized using anisaldehyde sulphuric acid as the spraying reagent at Rf 0.13 and 0.27, respectively. Chromatograms were scanned at 530 nm ( and ).
3.2 Calibration curve for GAs
The linear regression data for the calibration plot are indicative of a good linear relationship between the peak area and a wide range of concentrations. The linear regression calibration curves plotted the peak area against the concentration and were linear from 50 to 2000 ng/mL for both solamargine and solasonine with good linear relationships of 0.9987 and 0.9962, respectively ().
Table 1 Calibration data of solamargine and solasonine.
3.3 Validation of the method
3.3.1 Accuracy
The accuracy of the method was analysed by the standard addition method at the following levels (0, 50, 100, and 150), which showed good recovery within the range of 96.7–102.2% for solamargine and 97.1–100.7% for solasonine ().
Table 2 Accuracy of the method (n = 6).
3.3.2 Precision
Intermediate precisions were determined and reported in terms of % RSD. Intermediate precision includes data from inter-day, intra-day and inter-analyst precision measurements. The satisfactory result of the precision measurements indicates that the method can be adopted in any lab and by any qualified person for the routine analysis of GAs. The results of the analysis are depicted in .
Table 3 Precision of the method (n = 6).
3.3.3 Specificity
The spectra of GAs in both the standard and sample tracks were highly correlated and confirm that the scanning mode is effective and sensitive.
3.3.4 Robustness
The robustness of the method was assessed by introducing small changes in the composition of the mobile phase and detection wavelength, and the effect on the desired result was reported as % RSD. We tested mobile phases with compositions of chloroform:methanol:5% ammonia (7:3:1 v/v/v) and (7:2:0.50 v/v/v) and a detection wavelength of 530 ± 2 nm to identify any variation. The results of the robustness measurements are shown in .
Table 4 Robustness of the method by changing mobile phase compositions and the detection wavelength.
3.3.5 Sensitivity
The limit of detection (LOD) and limit of quantification (LOQ) represent the concentration of the analyte that would yield signal to noise ratio of 3 and 10, respectively. For the proposed HPTLC method, the LOD and LOQ of solamargine and solasonine were 20 and 50 ng/spot, respectively.
3.4 Quantitative evaluation of GAs in S. incanum samples
Solamargine and solasonine concentrations in different samples were analysed using the regression equation and the area values obtained from WinCATS software. The identity of the GAs bands from the sample solution was confirmed by comparing its Rf and spectra with that of the standard GAs. The Rf values for solamargine and solasonine were 0.26 ± 0.02 and 0.14 ± 0.02, respectively. The method developed here was sufficient for good separation of both of the compounds from the impurities. The spectral analysis of the sample peak confirmed the absence of any possible impurities in both compounds. The results from GAs determination in samples proved that the method is suitable for use in drug quality control.
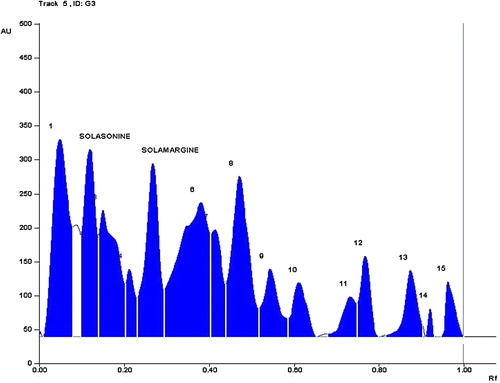
The method was applied to analyse solasonine and solamargine content in small, young, immature and mature leaves along with stem and root samples from S. incanum at intervals of 4 weeks up to 40 weeks. The mean values of GAs in the samples are shown in and .
Fig. 4 Content of solamargine expressed in mean ± S.D. (mg/g dry weight basis) as analysed by HPTLC for different samples of S. incanum plants grown in Oman for different time intervals (n = 4).
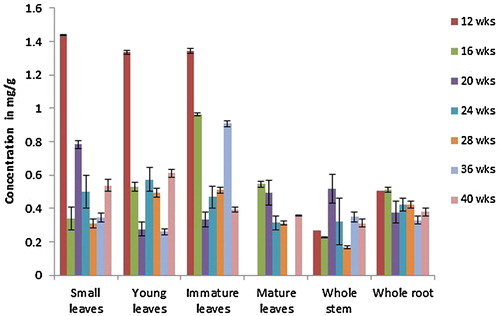
Fig. 5 Content of solasonine expressed in mean ± S.D. (mg/g dry weight basis) as analysed by HPTLC for different samples of S. incanum plants grown in Oman for different time intervals (n = 4).
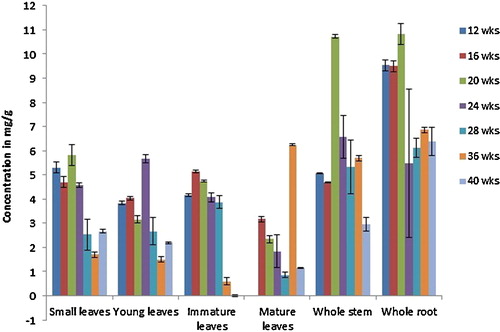
S. incanum plants did not produce older leaves (mature leaves) before 12 weeks. Although the plants started to flower by 28 weeks, no fruits were available for analysis within the duration of the experiment. All of the organs tested contained solamargine at all stages of development, with the highest concentrations in leaves at the early stages of growth. Leaves showed a higher level of solamargine compared to other samples, except at the 20th week. Within the four types of leaves investigated in this study, immature leaves displayed the highest solamargine concentrations. As leaves developed, the levels declined and the concentrations in mature leaves were only approximately 57% of those in immature leaves. The solamargine content of small leaves was not significantly different from that of young leaves. However, there was a significant difference between these two types of leaves and immature and mature leaves.
Overall, the lowest solamargine concentrations in the tested organs were found in the stem, whereas levels in the root were higher and nearly double that in the stem at some stages (12 and 16 weeks). On average, the solamargine levels in stem samples were approximately half of that in the leaf samples (immature, small and young leaves) in any particular week. However, stem samples collected at the 20th week of plant growth showed the highest content of solamargine among the stem samples. Moreover, the levels of solamargine in the roots were significantly less than the small, young and immature leaves, but not significantly different from that of mature leaves. If we compared the solamargine levels between the seven different stages of plant growth, we found that the highest concentration was found at the early stages of the 12-week stage followed by the 18-week stage and declined later as the plant matures. However, the solamargine levels did not change significantly from the 20-week stage and upwards as analysed by Tukey's test.
Similarly, all of the organs studied contained solasonine at all stages of development. In general, the level of solasonine is much higher (approximately ninefold) than solamargine in the S. incanum plants tested in this study. The highest levels were found in root samples followed by stem samples, unlike solamargine, which had the highest content in leaf samples. The lowest solasonine concentrations were found in mature leaves. The highest total solasonine concentration was found at the early stages of development, up to week 20, but the differences in concentrations between the early stages were not significant. Solasonine content was found to decrease after the 20th week (highest) and during further growth of the plant. The decrease was significant at the 24-week stage, and the concentrations continued to decrease after this time point, but not in a significant manner.
As the leaves developed, solasonine levels declined and the concentration in mature leaves was only approximately 50% of that in small, young, and immature leaves. Solasonine content in leaf samples remained constant up to the 24th week of the plant's growth and decreased thereafter. The content was found to be highest in small leaves and immature leaves (with no significant difference between them) followed by young leaves and least in the samples from mature leaves.
Among the stem samples, solasonine levels were highest at the twentieth week of the plant's growth and decreased thereafter. Analysis of solasonine in root samples showed an increasing pattern up to the 20th week. After this period the solasonine contents decreased in both the roots and stems, and this decrease marked a higher percentage of decrease in total solasonine content at this stage and subsequent stages.
The study revealed that the highest solamargine contents were found in young and immature leaves of S. incanum at different stages of plant growth, suggesting that these organs are the major sites of GA synthesis in this species. In contrast, stems and roots would appear to be less active areas of solamargine biosynthesis and/or accumulation. On the other hand, solasonine content was highest in the entire root, and this is the richest or major glycoalkaloid containing organ in S. incanum. Our study found that the glycoalkaloid contents in the different parts of S. incanum are higher than what was reported in the aubergine species. When three aubergine species and their accessions were examined for their glycoalkaloid content, the common aubergine S. melongena and three accessions had 1.1–2.0 mg/100 g, S. aethiopicum had 1.95 mg/100 g, and the variants possessed 1.1–5.4 mg/100 g. S. macrocarpan, another cultivated species, contained a significantly higher glycoalkaloids content of 221 mg/100 g, and the variants of this species contained 140–148 mg/100 g [Citation9]. α-Solasonine and α-solamargine are the principal glycoalkaloids of the aubergine species; the total glycoalkaloid content in the aubergine species is far less than in the other the Solanum species [Citation5]. Shanker et al. [Citation18] used methanol for the ultrasonic extraction of the aerial part of S. xanthocarpum and showed that the solamargine and solasonine contents in % w/w were 0.257 ± 0.012 and 0.102 ± 0.010, respectively.
4 Conclusion
In this study, we found that the solamargine content of S. incanum leaves was approximately half that found in S. xanthocarpum, whereas the solasonine content was six times higher in the leaves than in the aerial parts of S. xanthocarpum. Our findings highlight the importance of the developmental stages of a particular organ and the overall age of the plant when harvesting S. incanum plants for these GAs.
Acknowledgements
We are grateful to the Department of Biology, College of Science, Sultan Qaboos University for providing financial support as well as laboratory space and facilities to complete this work. Ms. Sana Al Sinani acknowledges receipt of study leave from the Ministry of Education, Sultanate of Oman.
Notes
Peer review under responsibility of Taibah University.
References
- M.A.BhatS.AhmadJ.AslamB.MujibMahmooduzzfarSalinity stress enhances production of solasodine in Solanum nigrum LChem. Pharm. Bull.5620081721
- A.MauryaN.ManikaR.K.VermaS.C.SinghS.K.SrivastavaSimple and reliable methods for the determination of three steroidal glycosides in the eight species of solanum by reversed-phase HPLC coupled with diode array detectionPhytochem. Anal.2420138792
- A.S.CarmanS.S.KuanG.M.WareO.J.FrancisG.P.KirschenheuterRapid high-performance liquid chromatographic determination of the potato glycoalkaloids alpha-solanine and alpha-chaconineJ. Agric. Food. Chem. Am. Chem. Soc.1986279282
- M.FriedmanL.DaoDistribution of glycoalkaloids in potato plants and commercial potato productsJ. Agric. Food. Chem.401992419423
- S.E.MilnerN.P.BruntonP.W.JonesN.M.O’ BrienS.G.CollinsA.R.MaguireBioactivities of glycoalkaloids and their aglycones from Solanum SpeciesJ. Agric. Food Chem.59201134543484
- M.J.ManaseA.C.Mitaine-OfferD.PertuitT.MiyamotoC.TanakaS.DelemasureP.DutartreJ.F.MirjoletO.DuchampM.A.Lacaille-DuboisSolanum incanum and S. heteracanthum as sources of biologically active steroid glycosides: confirmation of their synonymyFitoterapia83201211151119
- E.A.EltayebA.S.Al-AnsariJ.G.RoddickChanges in the steroidal alkaloid solasodine during development of Solanum nigrum and Solanum incanumPhytochemistry461997489494
- S.GhazanfarA.Al-Al-SabahiMedicinal plants of Northern and Central Oman (Arabia)Econ. Bot.4719938998
- C.Sánchez-Mata MaW.E.YokoyamaY.J.HongJ.Prohensα-Solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (Solanum aethiopicum L.) eggplantsJ. Agric. Food Chem.58201055025508
- L.DinanJ.HarmathaR.LafontChromatographic procedures for the isolation of plant steroidsJ Chromatogr. A9352001105123
- P.TrivediK.PundarikakshuduNovel TLC densitometric method for quantification of solasodine in various solanum species, market samples and formulationsChromatographia652007239243
- R.F.J.TiossiM.A.MirandaJ.P.B.D.SousaF.S.G.PracaM.V.L.B.BentleyJ.D.McchesneyJ.K.BastosA validated reverse phase HPLC analytical method for quantitation of glycoalkaloids in Solanum lycocarpum and its extractsJ. Anal. Methods Chem.2012201218
- M.H.LeeJ.J.ChengC.Y.LinY.J.ChenM.K.LuPrecursor-feeding strategy for the production of solanine, solanidine and solasodine by a cell culture of Solanum lyratumProcess Biochem.422007899903
- A.MauryaS.GuptaS.NegiS.K.SrivastavapH-Zone-refining centrifugal partition chromatography for preparative isolation and purification of steroidal glycoalkaloids from Solanum xanthocarpumJ. Sep. Sci.32200931263132
- P.G.CrabbeC.FryerRapid quantitative analysis of solasodine, solasodine glycosides and solasodiene by high-pressure liquid chromatographyJ. Chromatogr.A187198087100
- P.KuronenT.VäänänenE.PehuReversed-phase liquid chromatographic separation and simultaneous profiling of steroidal glycoalkaloids and their aglyconesJ. Chromatogr. A86319992535
- S.CherkaouiK.BekkoucheP.ChristenJ.L.VeutheyNon-aqueous capillary electrophoresis with diode array and electrospray mass spectrometric detection for the analysis of selected steroidal alkaloids in plant extractsJ. Chromatogr. A9222001321328
- K.ShankerS.GuptaP.SrivastavaS.K.SrivastavaS.C.SinghM.M.GuptaSimultaneous determination of three steroidal glycoalkaloids in Solanum xanthocarpum by high performance thin layer chromatographyJ. Pharm. Biomed. Anal.542011497502
- R.C.EanesN.TekO.KirsoyA.FraryS.DoganlarA.E.AlmeidaDevelopment of practical HPLC methods for the separation and determination of eggplant steroidal glycoalkaloids and their aglyconesJ. Liq. Chrom. & R. T.3120089841000
- S.K.BranchGuidelines from the International Conference on Harmonisation (ICH)J. Pharm. Biomed. Anal.382005798805
- Y.T.KamalS.M.MusthabaM.SinghR.ParveenS.AhmadS.BabootaI.AliK.M.SiddiquiS.M.A.ZaidiDevelopment and validation of HPLC method for simultaneous estimation of piperine and guggulsterones in compound Unani formulation (tablets) and a nanoreservoir systemBiomed. Chromatogr.26201211831190
- Y.T.KamalM.SinghE.T.TamboliR.ParveenS.M.A.ZaidiS.AhmadRapid RP-HPLC method for the quantification of glabridin in crude drug and in polyherbal formulationJ. Chromatogr. Sci.2012
- M.SinghY.T.KamalR.ParveenS.AhmadDevelopment and validation of a stability-indicating HPTLC method for analysis of arjunolic acid in a herbal formulationJ. Planar Chromatogr.242011172175