Abstract
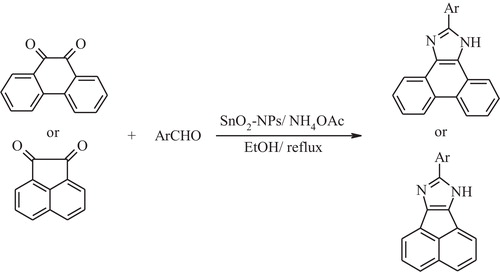
In this work, direct three component condensation of 9,10-phenanthraquinone or acenaphthenequinone with aryl aldehyde and ammonium acetate to generate highly substituted imidazole derivatives was performed over tin oxide nanoparticles. The resulting reactions were conducted at high efficiency in ethanol under reflux conditions.
1 Introduction
Nano-sized particles increase the exposed surface area of active catalyst components, thereby dramatically enhancing the contact between reactants and the catalyst. During the past decade, SnO2 has been widely used in solid-state gas sensors [Citation1], transparent conducting electrodes [Citation2], rechargeable Li batteries [Citation3], optical electronic devices [Citation4] and solar cells [Citation5]. SnO2 nano structures are considered one of the most important oxide nano structures due to their unique properties and potential applications [Citation6,Citation7]. Imidazoles are an important class of heterocycles and include many substances of both biological and chemical interest. Insertion of the imidazole nucleus is an important synthetic strategy in drug discovery. In the past few years, imidazole derivatives have occupied a unique place in the field of medicinal chemistry. They have wide range of biological activities and are well known analgesics, anti-inflammatory, antiparasitic, anthelmintic, platelet aggregation inhibitors and antiepileptic agents [Citation8–Citation13]. Numerous methods have been reported on for the synthesis of highly substituted imidazoles using various catalytic systems such as l-proline [Citation14], ZrCl4 [Citation15], InCl3·3H2O [Citation16], HClO4-SiO2 [Citation17], BF3-SiO2 [Citation18], heteropolyacids [Citation19], Yb(OTf)3 [Citation20], molecular iodine [Citation21], silica sulfuric acid [Citation22], cerium ammonium nitrate (CAN) [Citation23], ionic liquids [Citation24], NiCl2·6H2O/Al2O3 [Citation25] and a Schiff base transition metal complex [Citation26]. However, some of these methods suffer from at least one of the following disadvantages: strongly acidic waste, high reagent toxicity, tedious work-up procedures, unsatisfactory yields, harsh reaction conditions, moisture sensitive catalysts and non-recyclable reagents. Therefore, the development of a simple, efficient, and versatile approach for the preparation of highly substituted imidazoles is still an active area of research requiring further improvements towards milder reaction conditions and higher product yields.
In this investigation, the goal was to synthesize highly substituted imidazoles by reacting 9,10-phenanthraquinone or acenaphthenequinone with aryl aldehyde, and ammonium acetate in the presence of SnO2 nanoparticles to prepare a new series of highly substituted imidazole derivatives (). The structures of the compounds were established on the basis of the analytical spectral data.
2 Experimental
Tin oxide nanoparticles (SnO2-NPs) with an average size of 20–50 nm were purchased from the Tecnan Spanish Company. The melting points of these materials were recorded using an electro-thermal melting point apparatus. The NMR spectra were recorded in CDCl3 using tetramethylsilane as an internal standard on a Bruker Avance DRX 400 MHz spectrometer. The FT-IR spectra were recorded using an SP-1100, P-UV-Com instrument. The different products were identified using the FT-IR, 1H NMR and EI-MS spectra.
2.1 General procedure for the preparation of highly substituted imidazole derivatives
A mixture of aldehyde (1 mmol), 9,10-phenanthraquinone (1 mmol) or acenaphthenequinone (1 mmol), ammonium acetate (2 mmol) and nano-SnO2 (0.25 mmol) was dissolved in EtOH (5 mL) and heated using reflux for a stipulated time. The reaction progress was monitored using TLC. After the completion of the reaction, the corresponding product was obtained through simple filtering and was recrystallized from ethanol to yield highly pure imidazole derivatives.
The spectral data of the newly products are:
2.1.1 8-Phenyl-7H-acenaphtho[1,2-d]imidazole (entry 7)
m.p. = 306–308 °C;FT-IR (KBr): 3433 (N–H), 3051(C–H), 1612 (C=N), 1585(N–H bending), 1533¡1467 (C=C), 1276 (C–N), 821(C–H out of plane bending)cm−1; 1H NMR(400 MHz, CDCl3): δ: 8.88 (dd, 1H, J = 7.6, J = 1.2 Hz), 8.77 (dd, 1H, J = 7.6, J = 1.2 Hz), 8.43 (d, 1H, J = 6.8 Hz), 8.33 (dd, 1H, J = 8, J = 0.8 Hz), 8.04–8.10 (m, 2H),7.83–7.89 (m, 3H),7.79 (s, 1H),7.63–7.70 (m, 2H) ppm; EI-MS (m/z): 268 (M+).
2.1.2 3-(7H-acenaphtho[1,2-d]imidazol-8-yl)phenol (entry 8)
m.p. = 333–335 °C;FT-IR (KBr): 3433 (N–H and O–H), 3050 (C–H), 1618 (C=N), 1550 (N–H bending), 1500,1470 (C=C), 1276 (C–N stretch(, 1250 (C–O), 770 (C–H out of plane bending) cm−1; 1H NMR(400 MHz, CDCl3): δ: 8.88 (dd, 1H, J = 7.6, J = 1.2 Hz), 8.77 (dd, 1H, J = 7.6, J = 1.2 Hz), 8.43 (d, 1H J = 6.8 Hz), 8.31–8.33(m, 1H), 8.07–8.10 (m, 2H), 7.87 (s, 1H),7.78–7.86 (m, 3H), 7.67–7.70 (m, 1H), 7.63–7.66 (m, 1H) ppm; EI-MS (m/z): 284 (M+).
2.1.3 2-(7H-acenaphtho[1,2-d]imidazol-8-yl)phenol (entry 9)
m.p. = 294–296 °C;FT-IR (KBr): 3440 (N–H and O–H), 3053 (C–H), 1600 (C=N), 1537 (N–H bending), 1467,1433 (C=C), 1282 (C–N(,1224 (C–O), 773 (C–H out of plane bending)cm−1; 1H NMR(400 MHz, CDCl3):δ: 8.88 (dd, 1H, J = 7.2, J = 1.2 Hz), 8.77 (dd, 1H, J = 7.2, J = 1.2 Hz), 8.43 (d, 1H, J = 6.8 Hz), 8.31–8.33(m, 1H), 8.07–8.11 (m, 2H),7.84–7.89 (m, 2H),7.78 (s, 1H),7.63–7.70 (m, 2H) ppm; EI-MS (m/z): 284 (M+).
2.1.4 8-(2-Chlorophenyl)-7H-acenaphtho[1,2-d]imidazole (entry 10)
m.p. = 326–328 °C;FT-IR (KBr): 3400 (N–H), 3100 (C–H), 1600 (C=N), 1575 (N–H bending), 1535¡1460 (C=C), 1277(C–N), 1094(C–Cl), 821 (C–H out of plane bending)cm−1; 1H NMR(400 MHz,CDCl3): δ: 8.88 (dd, 1H, J = 7.2, J = 1.2Hz), 8.77 (dd, 1H, J = 7.2, J = 1.2 Hz), 8.43 (d, 1H, J = 6.4 Hz), 8.33 (dd, 1H, J = 8, J = 1.2 Hz), 8.08–8.10 (m, 2H),7.81–7.89 (m, 2H),7.79 (s, 1H),7.63–7.70 (m, 2H) ppm; EI-MS (m/z): 302(M+).
2.1.5 8-(4-Fluorophenyl)-7H-acenaphtho[1,2-d]imidazole (entry 11)
m.p. = 302–304 °C;FT-IR (KBr): 3430 (N–H), 3100 (C–H), 1600 (C=N), 1575 (N–H bending), 1535,1460 (C=C), 1263 (C–F), 1277 (C–N), 780 (C–H out of plane bending)cm−1; 1H NMR(400 MHz, CDC3): δ: 8.88 (dd, 1H, J = 7.2, J = 1.2 Hz), 8.77(dd, 1H, J = 7.2, J = 1.2 Hz), 8.43 (d, 1H, J = 6.4 Hz), 8.33 (dd, 1H, J = 8, J = 1.2 Hz), 8.08–8.10 (m, 2H),7.81–7.89 (m, 2H),7.79 (s, 1H),7.63–7.70 (m, 2H) ppm; EI-MS (m/z): 286(M+).
2.1.6 7-(4-Bromophenyl)-8-phenyl-7H-acenaphtho[1,2-d]imidazole (entry 12)
m.p. = 264–266 °C;FT-IR (KBr): 3410 (N–H), 3100 (C–H), 1612 (C=N), 1585 (N–H bending), 1533¡1467 (C=C), 1276 (C–N), 1068(C–Br), 775 (C–H out of plane bending)cm−1; 1H NMR(400 MHz, CDCl3): δ: 8.88 (d, 1H, J = 7.6 Hz), 8.77 (d, 1H, J = 8 Hz), 8.43 (d, 1H, J = 6.8 Hz), 8.35 (s, 1H), 8.31 (d, 1H, J = 7 Hz),8.15 (d, 1H, J = 7 Hz), 8.08–8.10 (m, 2H),7.90–7.83 (m, 4H),7.79–7.81 (m, 1H) 7.64–7.70 (m, 2H) ppm; EI-MS (m/z): 423(M+).
3 Results and discussion
To optimize the reaction conditions and obtain the best catalytic activity, a reaction of benzaldehyde (1 mmol), acenaphthenequinone (1 mmol) and ammonium acetate (2 mmol) was used as a model reaction and was conducted using different catalysts and solvents. Initially, various catalysts were tested, and the representative results are listed in . The tin oxide nanoparticles returned better results than other catalysts listed in in terms of the yield and reaction rate.
Table 1 Comparison of the catalytic activity of tin oxide nanoparticles with other catalysts.Table Footnotea
After the selection of the catalyst, the model reaction was carried out using several solvents, such as EtOAc, CH2Cl2, EtOH, THF, DMF, and under solvent-free conditions to investigate the efficiency of the catalyst. In this study, it was found that ethanol under reflux conditions is most efficient, with respect to reaction time and yield of the desired product ().
Table 2 Investigation of affects the solvent during synthesis of the model reaction.Table Footnotea
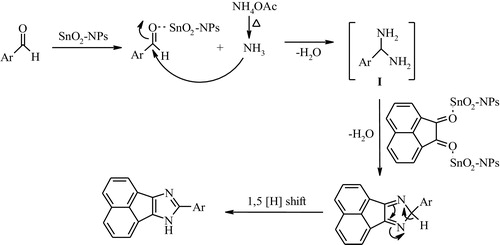
A variety of highly substituted imidazole derivatives were prepared from 9,10-phenanthraquinone or acenaphthenequinone, aldehydes and ammonium acetate in the presence of tin oxide nanoparticles in ethanol under reflux conditions, which resulted in excellent yields (, entries 1–11). The substituted aldehydes bearing electron-withdrawing or electron-donating groups were treated with ammonium acetate in combination with 9,10-phenanthraquinone or acenaphthenequinone under the same experimental conditions and corresponding to the desired products to isolate the effects that resulted in good to excellent yields. Additionally, the reaction of acenaphthenequinone, benzaldehyde, ammonium acetate and 4-bromoaniline in the presence of SnO2 nanoparticles resulted in the creation of tetra substituted imidazole and exhibited good yields under similar conditions (, entry12). It is worth mentioning that the corresponding highly substituted imidazoles were isolated by crystallization from a crude filtrate. As seen in , we assumed that theSnO2 nanoparticles active the carbonyl group of aldehydes and facilitate the formation of a diamine intermediate. Next, the condensation of the diamine intermediate with acenaphthenequinone, intramolecular cyclization and subsequently [Citation1,Citation5] sigmatropic proton shifts to generate the corresponding 8-aryl-7H-acenaphtho[1,2-d]imidazole derivatives. In the case of 9,10-phenanthraquinone, the reaction proceeds in the same fashion as above [Citation27].
Table 3 Synthesis of highly substituted imidazole derivatives using nano-SnO2.Table Footnotea
It is noteworthy to highlight that the catalyst could be recovered and reused without a significant loss of activity. The recovered catalysts were dried and weighed. Afterwards, the required amounts of fresh benzaldehyde, acenaphthenequinone and ammonium acetate were added according to the catalyst. The results showed that the catalyst can be reused six consecutive times, with only a slight loss in activity ().
Table 4 Recycling of nano-SnO2 for the synthesis of 8-phenyl-7H-acenaphtho[1,2-d]imidazole.
4 Conclusions
In this work, we developed an efficient and one-pot procedure for the synthesis of highly substituted new imidazole derivatives by the multicomponent reaction of 9,10-phenanthraquinone or acenaphthenequinone, aldehyde and ammonium acetate using tin oxide nanoparticles (SnO2-NPs) as a catalyst in ethanol under reflux conditions.
Acknowledgments
We are grateful to Islamic Azad University of Rasht Branch for their financial assistance in this work.
Notes
Peer review under responsibility of Taibah University.
References
- M.H.Shahrokh AbadiM.N.HamidonA.H.ShaariN.AbdullahR.WagiranSnO2/Pt thin film laser ablated gas sensor arraySensors11201177247735
- K.L.ChopraS.MajorD.K.PandyaTransparent conductors—a status reviewThin Solid Films1021983146
- Z.PengZ.ShiM.LiuMesoporous Sn–TiO2 composite electrodes for lithium batteriesChem. Commun.200021252126
- A.AokiH.SasakuraTin oxide thin film transistorsJpn. J. Appl. Phys.91970582
- J.A.AyllonM.L.CantuApplication of MEH-PPV/SnO2 bilayer as hybrid solar cellAppl. Phys. A2008249255
- B.ChengJ.M.RussellW.ShiL.ZhangE.T.SamulskiLarge-scale, solution-phase growth of single-crystalline SnO2 nanorodsJ. Am. Chem. Soc.126200459725973
- F.Paraguay-DelgadoW.Antunez-FloresM.Miki-YoshidaA.Aguilar-ElguezabalP.SantiagoR.DiazJ.A.AscencioStructural analysis and growing mechanisms for long SnO2 nanorods synthesized by spray pyrolysisNanotechnology162005688694
- U.UcucuN.G.KaraburunI.IsikdagSynthesis and analgesic activity of some 1-benzyl-2-substituted-4, 5-diphenyl-1H-imidazole derivativesFarmaco562001285290
- A.YesiladaS.KoyunogluN.SaygiliE.KupeliE.YesiladaE.BedirI.KhanSynthesis, anti-inflammatory and analgesic activity screening of some new 4-(3H)-quinazolinone derivativesArch. Pharm. Pharm. Med. Chem.337200496104
- L.QuattaraM.DebaertR.CavierSynthesis and antiparasitic activity of new nitro-5-imidazole derivativesFarmaco Sci.421987449456
- S.DuttaG.MariappanS.RoyM.VermaAnthelmintic activity of some 2-substituted 4,5-diphenyl imidazoleIndian Drugs4620095053
- A.K.SenguptaT.BhattacharyaSynthesis and antimicrobial activity of some substituted 2-phenyl-3-arylquinazol-4-onesJ. Indian Chem. Soc.601983373376
- L.NavidpourH.ShadniaH.ShafaroodiM.AminiA.R.DehpourA.ShafieeDesign, synthesis, and biological evaluation of substituted 2-alkylthio-1,5-diarylimidazoles as selective COX-2 inhibitorsBioorg. Med. Chem.15200719761982
- S.SamaiG.C.NandiP.SinghM.S.Singhl-Proline: an efficient catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazolesTetrahedron6520091015510161
- G.V.M.SharmaY.JyothiP.S.LakshmiEfficient room temperature synthesis of tri and tetrasubstituted imidazoles catalyzed by ZrCl4Synth. Commun.36200629913000
- S.D.SharmaP.HazarikaD.KonwarAn efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by InCl3·3H2OTetrahedron Lett.49200822162220
- S.KantevariS.V.N.VuppalapatiD.O.BiradarL.Nagarapuhighly efficient, one-pot, solvent-free synthesis of tetrasubstituted imidazoles using HClO4–SiO2 as novel heterogeneous catalystJ. Mol. Catal. A: Chem.2662007109113
- B.SadeghiB.B.F.MirjaliliM.M.HashemiBF3·SiO2: an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazolesTetrahedron Lett.49200825752577
- M.M.HeraviF.DerikvandF.F.BamoharramHighly efficient, four-component one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacids as green and reusable catalystsJ. Mol. Catal. A: Chem.2632007112114
- L.M.WangY.H.WangH.TianYtterbium triflate as an efficient catalyst for one-pot synthesis of substituted imidazoles through three-component condensation of benzil, aldehydes and ammonium acetateJ. Fluor. Chem.127200615701573
- H.BehmadiM.RoshaniS.M.SaadatiSynthesis of phenanthrimidazole from 9,10-phenanthraquinone and aldehydes by molecular iodine as catalystChin. Chem. Lett.20200958
- A.ShaabaniA.RahmatiSilica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstituted imidazolesJ. Mol. Catal. A: Chem.2492006246248
- J.N.SangshettiN.D.KokareS.A.KotharkaraD.B.ShindeCeric ammonium nitrate catalysed three component one-pot efficient synthesis of 2,4,5-triaryl-1H-imidazoleJ. Chem. Sci.52008463467
- A.HasaninejadA.ZareM.ShekouhyJ.Ameiri RadCatalyst-free one-pot four component synthesis of polysubstituted imidazoles in neutral ionic liquid 1-butyl-3-methylimidazolium bromideJ. Comb. Chem.122010844849
- M.M.HeraviK.BakhtiariH.A.OskooieS.TaheriSynthesis of 2,4,5-triaryl-imidazoles catalyzed by NiCl2·6H2O under heterogeneous systemJ. Mol. Catal. A: Chem.2632007279281
- S.DamavandiSchiffbase transition metal complex catalyzed one-pot synthesis of 2-aryl-1H-phenanthro[9,10-d]imidazolesSynth. React. Inorg. Metal-Organ. Nano-Met. Chem.41201112741277
- J.SafariS.Gandomi-RavandiZ.AkbariSonochemical synthesis of 1,2,4,5-tetrasubstituted imidazoles using nanocrystalline MgAl2O4 as an effective catalystJ. Adv. Res.42013509514