Abstract
A simple procedure has been developed for the synthesis of ZnO nanorods (ZnO NRs). The newly synthesized crystals were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). Moreover, the catalytic activity was examined in the one-pot, three-component synthesis of 2,4,5-triaryl-1H-imidazoles in water under reflux conditions. The results confirmed ZnO NRs to be mild, benign and effective catalysts for the preparation of tri-substituted imidazoles in high yield via an operationally simple and environmentally friendly procedure. Furthermore, the catalyst can be conveniently recovered and reused for at least three runs without any activity loss.
1 Introduction
Substituted imidazoles exhibit a wide-range of biological activities, including anti-inflammatory [Citation1], fungicidal [Citation2] and anti-tumour [Citation3]. Some derivatives of multi-substituted imidazoles inhibit p38 MAP kinase [Citation4], interleukin-1 (IL-1) biosynthesis [Citation5] and selective glucagon receptor antagonists [Citation6]. Recent investigations in green and organometallic chemistry have extended the application of imidazoles as ionic liquids [Citation7] and N-heterocyclic carbenes [Citation8,Citation9]. Some marketed medicines, such as omeprazole [Citation10], consist of modified imidazole cores in their structures. In addition to being biologically active, the imidazole core acts as a main part in some advanced materials, such as fluorescence labelling agents [Citation11], biological imaging agents [Citation12], and chromophores for non-linear optic systems [Citation13]. Due to their vast range of pharmacological, biological and industrial activities, the development of protocols or the synthesis of imidazoles is an interesting target for chemists.
A number of methods have been developed for the synthesis of 2,4,5-triarylimidazoles. The reported procedures involve the multi-component cyclo-condensation of 1,2-diketones (benzoins or benzils) using an aldehyde or an ammonium salt as the nitrogen source (in most cases ammonium acetate) in the presence of a strong protic acid (such as H3PO4 [Citation14] and H2SO4 [Citation15]), ionic liquids (such as 1-ethyl-3-methylimidazole acetate ([EMIM]OAc) [Citation16] and diethyl ammonium hydrogen phosphate ([(Et2)NH2]H2PO4) [Citation17]), ytterbium trifluoromethanesulfonate (Yb(OTf)3) [Citation18], NiCl2·6H2O supported onto acidic alumina [Citation19], silica sulphuric acid (SSA) [Citation20], ceric (IV) ammonium nitrate (CAN) [Citation21], l-systeine [Citation22], BF3/SiO2 [Citation23], l-proline [Citation24], nano-silica phosphoric acid (nano-SPA) [Citation25], N-bromosuccinimide (NBS) [Citation26], silica-supported SbCl3 [Citation27] and nanocrystalline MgAlO4 [Citation28].
However, despite their potential utility, some of the reported methods suffer from the use of relatively expensive moisture-sensitive reagents, high temperatures, long reaction times, tedious work-up procedures using toxic reagents or solvents, harsh reaction conditions, and large catalytic loadings, leading to the generation of a large amount of waste. Moreover, the synthesis of these N-bearing heterocyclic compounds has been carried out in polar solvents, such as methanol, acetic acid, DMF and DMSO, which leads to complex isolation procedures. Hence, the development of clean, high-yield, and environmentally benign approaches for imidazole synthesis is still desirable and in demand.
Recently, organic syntheses involving multi-component reactions (MCRs) have received considerable attention. In this strategy, the target products are often obtained in a one-pot process in which three or more starting materials with different functionalities are incorporated together with high atom economy and high selectivity. This single-step ideal route minimizes the tedious work-up procedures and environmentally hazardous wastes. Many complex and important organic molecules have been prepared from this simple and powerful protocol [Citation29].
Different nano-metal oxides have been of great interest in recent years due to their catalytic, thermal, mechanical, optical and biomedical applications [Citation30–Citation32]. Various ZnO nanostructures, such as nanoparticles, nanorods, nanowires, nanobelts, nanotubes, nanobridges and nanonails, nanowalls, nanohelixes and polyhedral cages, have been synthesized and characterized in recent years [Citation33–Citation36]. Nano-zinc oxides showed significant applications in various areas, such as optoelectronics [Citation36,Citation37], ferromagnetism [Citation38], piezoelectric transducers [Citation39], surface acoustic wave devices [Citation40] and gas sensors [Citation41]. They also showed antibacterial [Citation42] and antioxidant properties [Citation43].
It is noteworthy that among one-dimensional nanostructures, ZnO nanorods and nanowires have been widely studied due to their ease of formation and device applications [Citation44]. These forms exhibit unique and adjustable properties due to their large surface area-to-volume ratios. Several methods, including template-assisted sol–gel [Citation45], laser-induced [Citation46], hydrothermal [Citation47], solvothermal [Citation48], precipitation [Citation49] and chemical vapour deposition (CVD) [Citation50] techniques, have been developed for the production ZnO nanorods and nanowires.
The growth conditions affect the characteristics and properties of the ZnO NRs. For this reason, various studies have reported the controlled growth of ZnO nanostructures to promote their widespread applications. Recently, ZnO nanorods were prepared in the presence of different capping agents or surfactants, which were used to control the particle size and morphology [Citation42]. However, although various methods have been proposed for the synthesis of ZnO nanostructures, most of these techniques are expensive and complicated and require advanced instruments or high temperatures.
In the last decade various morphologies of nano-ZnO have been reported as catalysts in different organic reactions, such as the synthesis of β-phosphonomalonates [Citation51], knoevenagel condensation [Citation52] and the syntheses of tetrazoles [Citation53], coumarines [Citation54], ferrocenylphosphonates [Citation55], 3-indolyl-3-hydroxy oxindoles [Citation56], dihydropyranochromenes [Citation57], 1H-pyrazolo phthalazinediones [Citation58], 1,4-dihydropyridine [Citation59], imidazo[1,2-a]azines [Citation60] and Pyranopyrazoles [Citation61].
In this study, a simple and inexpensive precipitation method was used for the synthesis of ZnO nanorods by employing polyethylene glycol (PEG 2000) as a capping agent. The activity of the synthesized ZnO NRs as an inexpensive and easy-to-handle catalyst for the synthesis of 2,4,5-triarylimidazoles from the one-pot, three-component cyclo-condensation of benzils, aryl aldehydes and ammonium acetate in water under reflux conditions has been examined ().
2 Experimental
2.1 Chemicals and apparatus
All of the chemicals and solvents were purchased from Merck. Melting points were determined by the capillary tube method using a Stuart Scientific apparatus and are uncorrected. Fourier transform infrared (FT-IR) spectra (KBr discs, 500–4000 cm−1) were recorded using a Bruker FT-IR model Tensor 27 spectrometer. 1H NMR spectra were recorded in CDCl3 on a Brucker 400 MHz spectrometer. X-ray diffraction patterns (XRD) were recorded by a Philips X-ray diffractometer (Cu Kα1 radiation, cobalt anode, wavelength of 1.7889 Å, 40 kV, 40 mA). Scanning electron microscopy (SEM, model EM 3200, kyky) was used to characterize the nanostructures. Thin layer chromatography (TLC) was performed on commercial aluminium-backed plates of silica gel (60 F254) to monitor reaction progress.
All of the products were characterized by comparing their spectroscopic data (FT-IR and 1H NMR) with those of authentic samples in the literature. The yields refer to isolated products.
2.2 Synthesis of ZnO nanorods
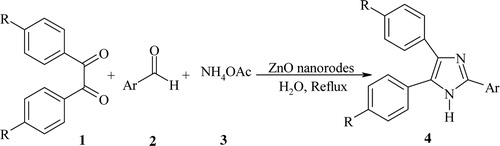
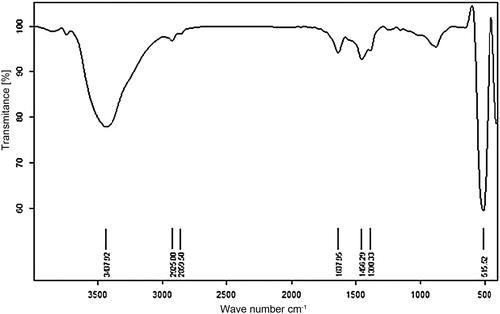
ZnO NRs were synthesized according to the previously reported method [Citation55], with some modification. Zn(OAc)2·2H2O (1 g) and PEG 2000 (0.3 g) in 140 mL of doubly distilled water at laboratory temperature were dissolved to obtain a transparent solution. Then, 1.5 mL of NH3 (25%) was added dropwise to the aforementioned mixture and refluxed at 80 °C for 6 h. The reaction was terminated by cooling to room temperature followed by centrifugation and washing the precipitate. In the next step, H2O (90 mL) was added to the mixture and refluxed for 9 h. This solution was then cooled, centrifuged and washed with deionized water and absolute ethanol. The ZnO NRs were dried at 100 °C in an oven. The obtained ZnO NRs were characterized by FT-IR (), XRD () and SEM ().
2.3 General procedure for the synthesis of 2,4,5-triarylimidazoles
ZnO NRs (20 mol%) were added to a mixture of benzils (1 mmol), arylaldehydes (1 mmol) and NH4OAc (4 mmol) in H2O (5 mL). The resulting mixture was refluxed in a round-bottom flask equipped with a condenser and magnetic stirrer until the completion of the reaction. The mixture was then cooled and filtered. The solid residue was washed with EtOAc (2× 5 mL). After filtrate extraction, the organic layer was concentrated by vaporizing the solvent under reduced pressure. The resulting mixture was then purified by thin-layer chromatography (eluent, 9:1 petroleum ether:EtOAc) to produce pure 2,4,5-triarylimidazoles.
2.3.1 2-(3-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (4h)
White powder, IR (KBr, υ cm−1): 3430 (NH), 1592 (C=C), 1515 (C=N); 1H NMR (CDCl3, 400 MHz): δ 12.7 (s, IH, NH), 7.8–6.78 (m, 14H, Ar–H), 3,82 (s, 3H, OCH3).
2.3.2 2-(4-Chlorophenyl)-4,5-bis(4-chlorophenyl)-1H-imidazole (4r)
White powder, IR (KBr, υ cm−1): 3435 (NH), 1609 (C=C), 1517 (C=N); 1H NMR (CDCl3, 400 MHz): δ 12.8 (s, IH, NH), 8.07 (d, 2H, J = 8.4 Hz, Ar–H), 7.56–7.51 (m, 6H, Ar–H), 7.3 (t, 2H, J = 8.8 Hz, Ar–H), 7.15 (t, 2H, J = 8.4 Hz, Ar–H).
2.3.3 2-p-Tolyl-4,5-bis(4-methoxyphenyl)-1H-imidazole (4x)
Pale yellow powder, IR (KBr, υ cm−1): 3409 (NH), 1623 (C=C), 1526 (C=N); 1H NMR (CDCl3, 400 MHz): δ 12.75 (s, IH, NH), 7.93–8.05 (m, 5H, Ar–H), 7.73 (d, 2H, J = 8.4 Hz, Ar–H), 7.28–7.44 (m, 5H, Ar–H), 4.02 (s, 6H, OCH3), 2.37 (s, 3H, CH3).
3 Results
3.1 Characterization of ZnO NRs
The FT-IR spectrum () of the ZnO NRs shows an absorption band of approximately 515 cm−1 corresponding to Zn–O stretching vibration modes, which is the typical characteristic band of pure ZnO. The weak peak at 1394 cm−1 may be due to the symmetric stretching of carboxylate groups, probably from the small amount of residual zinc acetate remaining from the synthesis. The broad absorption peaks at approximately 3437 and 1456 cm−1 are attributed to vibrations of OH groups corresponding to the absorption of water molecules on the ZnO surface. The peaks at approximately 2925 and 2856 cm−1 are due to the C–H stretching of acetate [Citation62,Citation63].
The structures of the ZnO nanocrystals were studied by XRD pattern. shows the X-ray diffraction pattern of obtained ZnO nanorods. Peaks are observed at angles (2θ) of 37.03, 40.175, 42.335, 55.8, 66.655, 74.535, 78.96, 80.89 and 82.315; after converting to Cu values by multiplying by 0.86, these angles confirm the 1 0 0, 0 0 2, 1 0 1, 1 0 2, 1 1 0, 1 0 3, 2 0 0, 1 1 2 and 2 0 1 crystal planes, respectively. The XRD pattern is compared with the reference pattern (JCPDS No. 36-1451 for ZnO) corresponding to wurtzite-type ZnO.
shows the SEM image of the ZnO nanorods prepared using PEG 2000 as a capping agent. The average diameter of these nanorods is in the range 35–70 nm. It should be noted that the average size of most of the synthesized nanorods estimated from SEM images is approximately 50 nm [Citation55].
3.2 Synthesis of tri-substituted imidazoles
Initially, the condensation of benzil (1 mmol), 4-chlorobenzaldehyde (1 mmol) and ammonium acetate (4 mmol) was chosen as a model to identify the optimized reaction conditions (). The corresponding 2-(4-chlorophenyl)-4,5-diphenyl-1H-imidazole was obtained in an 83% yield after 1.45 h in the presence of 20 mol% of the nano-catalyst in H2O under reflux (, entry 7). Performing the reaction under solvent-free conditions both at room temperature and in the presence of conventional heating did not produce satisfactory results (entries 4 and 5). The use of CH3CN, C2H5OH and H2O/C2H5OH (1/1, v/v) as the solvent yielded worse results compared to the use of water under the same conditions (entries 1, 2, 3 and 6). Decreasing the amount of NH4OAc to 2 mmol decreased the yield (entry 11), and the amount of catalyst used was not suitable in entries 8 and 9. Using ammonium carbonate as a source of ammonia did not produce satisfactory results (entry 10). The optimal conditions were determined to be the utilization of benzil, aldehydes and NH4OAc (molar ratio of 1/1/4) in the presence of ZnO NRs (20 mol%) in H2O under reflux (entry 7).
Table 1 Screening for optimal conditions for the condensation of benzil (1 mmol), 4-chlorobenzaldehyde (1 mmol) and NH4OAc (4 mmol) in the presence ZnO NRs.
In the next step, the general applicability of the ZnO NRs as catalysts for the synthesis of various derivatives of 2,4,5-triaryl-1H-imidazoles was investigated (). Benzaldehyde and its activated derivatives as well as deactivated aromatic aldehydes successfully produced the corresponding tri-substituted imidazoles 4a–m. It should be noted that electron-deficient substituents yielded better results than their corresponding electron-donating substituents. The reaction times of the 3- and 2-substituted arylaldehydes (entries 2 and 7) are slightly longer than those of their 4- substituted analogous (entries 6 and 9). This might be due to indirect inductive and/or resonance effects on the reaction centre. Steric hindrance in 2-substituted derivatives is another potential cause of this sluggish response. Furfural and 1-naphthaldehyde also reacted successfully (entries 14 and 15). The specialty of the catalyst was also observed for the reactions of unsaturated aldehydes, such as 3-phenyl-2-propenal. In this case, no by-products were obtained (entry 16). In addition, substituted benzils successfully underwent three-component condensation with various aldehydes (, entries 17–25).
Table 2 Syntheses of 2,4,5-triaryl-1H-imidazoles by one-pot multi-component reactions of benzils (1 mmol), different aldehydes (1 mmol) and NH4OAc (4 mmol) in the presence of ZnO NRs (20 mol%) in H2O (5 mL) under reflux.
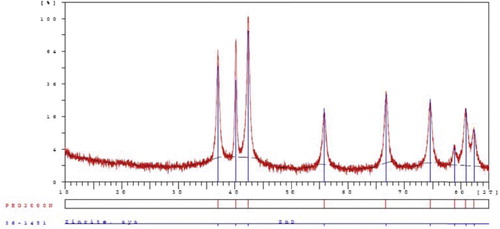
In the next step, the reusability of the recovered catalyst was studied as another efficient and important aspect of this protocol. For this reason, the ZnO nanorods were recovered from the reaction mixture of 2-(4-chlorophenyl)-4,5-diphenyl-1H-imidazole (4b) by filtration and further washing the solid residue with EtOAc (2× 10 mL). Drying overnight at room temperature and heating at 70 °C for 2 h gave the recovered catalyst. The obtained nanocatalyst was reused and recycled for four runs with almost no decrease in activity. shows that the recovered nano-ZnO catalyzed the one-pot, three-component reaction to give tri-substituted imidazole 4b with yields of 83, 83, 81 and 80% for four consecutive separations and reuses.
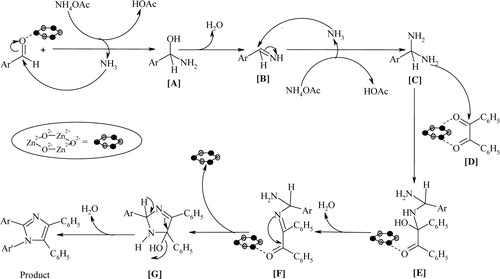
Finally, the proposed mechanism for the synthesis of 2,4,5-trisubstitiuted imidazoles is outlined in . The catalyst activates the aldehyde carbonyl group to form hydroxylamine intermediate [A], which is dehydrated to imine [B]. The nucleophilic attack of another NH3 yields the diamine intermediate [C]. The consequent nucleophilic attack of [C] on activated benzil [D] gives [E], which releases water to form [F]. The intramolecular nucleophilic attack on [F] leads to the cyclisation of [G]. From this stage, the nanocatalyst can be utilized for another cycle. Subsequently, the dehydration of [G] gives the tri-substituted imidazole product.
4 Conclusions
In summary, a modified route for the synthesis of ZnO nanorods has been introduced. The synthesized nanorods are an effective, eco-friendly and reusable Lewis acid catalyst that catalyses the one-pot, three-component reaction of benzils with aldehydes and ammonium acetate to form 2,4,5-triaryl-1H-imidazoles in high yields. The simple operation and easy work-up of this reaction procedure along with the reusability and efficiency of the synthesized nanocatalysts are the highlights of this work. The authors expect this protocol to find extensive applications in the fields of heterogeneous nanocatalysts, drug preparation development and combinatorial organic synthesis.
Acknowledgment
The authors thank Alzahra University for partial financial support.
Notes
Peer review under responsibility of Taibah University.
References
- J.G.LombardinoE.H.WisemanPreparation and antiinflammatory activity of some nonacidic trisubstituted imidazolesJ. Med. Chem.17197411821188
- A.F.PozherskiiA.T.SoldatenkovA.Y.KatritzkyHeterocycles in Life and Society1997WileyNew York179180
- L.WangK.W.WoodsQ.LiK.J.BarrR.W.McCroskeyS.M.HannickL.GherkeR.B.CredoY.H.HuiK.MarshR.WarnerJ.Y.LeeN.Z.MozngD.FrostS.H.RosenbergH.L.ShamPotent, orally active heterocycle-based combretastatin A-4 analogues: synthesis, structure–activity relationship, pharmaco kinetics, and in vivo antitumor activity evaluationJ. Med. Chem.45200216971711
- J.C.LeeJ.T.LaydonP.C.McDonnellT.F.GallagherS.KumarD.GreenD.McNultyM.J.BlumenthalJ.R.KeysS.W.L.VatterJ.E.StricklerM.M.McLaughlinI.R.SiemensS.M.FisherG.P.LiviJ.R.WhiteJ.L.AdamsP.R.YoungA protein kinase involved in the regulation of inflammatory cytokine biosynthesisNature3721994739745
- T.F.GallagherS.M.Fier-ThompsonR.S.GarigipatiM.E.SorensonJ.M.SmietanaD.LeeP.E.BenderJ.C.LeeJ.T.LaydonD.GriswoldM.C.Chabot-FletcherJ.J.BretonJ.L.Adams2,4,5-Triarylimidazole inhibitors of IL-1 biosynthesisBioorg. Med. Chem. Lett.5199511711176
- L.L.ChangK.L.SidlerM.A.CascieriS.de LaszloG.KochB.LiM.MacCossN.MantloS.O’KeefeM.PangA.RolandoW.K.HagmannSubstituted imidazoles as glucagon receptor antagonistsBioorg. Med. Chem. Lett.11200125492553
- P.WasserscheidW.KeimIonic liquids – new solutions for transition metal catalysisAngew. Chem. Int. Ed. Eng.39200037723789
- W.A.HermannC.KocherN-heterocyclic carbenesAngew. Chem. Int. Ed. Eng.36199721622187
- C.Z.ZhangJ.HuangM.L.TrudellS.P.NolanPalladium-imidazol-2-ylidene complexes as catalysts for facile and efficient Suzuki cross-coupling reactions of aryl chlorides with arylboronic acidsJ. Org. Chem.64199938043805
- P.LindbergP.NordbergT.AlmingerA.BrandstormB.WallmarkThe mechanism of action of the antisecretory agent omeprazoleJ. Med. Chem.29198613271329
- H.-J.ZhuJ.-S.WangK.S.PatrickJ.L.DonovanC.L.DeVaneJ.S.J.MarkowitzA novel HPLC fluorescence method for the quantification of methylphenidate in human plasmaJ. Chromatogr. B: Anal. Technol. Biomed. Life Sci.85820079197
- Y.-F.SunW.HuangC.-G.LuY.-P.CuiThe synthesis, two-photon absorption and blue up conversion fluorescence of novel, nitrogen-containing heterocyclic chromophoresDyes Pigments8120091017
- M.StaehelinD.M.BurlandM.EbertR.D.MillerB.A.SmithR.J.TwiegW.VolksenC.A.WalshRe-evaluation of the thermal stability of optically nonlinear polymeric guest-host systemsAppl. Phys. Lett.61199216261628
- J.LiuJ.ChenJ.ZhaoY.ZhaoL.LiH.ZhangA modified procedure for the synthesis of 1-arylimidazolesSynthesis200326612666
- H.WeinmannM.HarreK.KoeingE.MertenU.TilestamEfficient and environmentally friendly synthesis of 2-amino-imidazoleTetrahedron Lett.432002593595
- H.ZangQ.SuY.MoB.-W.ChengS.JunIonic liquid [EMIM]OAc under ultrasonic irradiation towards the first synthesis of trisubstituted imidazolesUltrason. Sonochem.172010749751
- A.A.MarzoukV.M.AbbasovA.H.TalybovSh.Kamel MohamedSynthesis of 2,4,5-triphenyl imidazole derivatives using diethyl ammonium hydrogen phosphate as green, fast and reusable catalystWorld J. Org. Chem.12013610
- L.-M.WangY.-H.WangH.TianY.-F.YaoJ.-H.ShaoB.LiuYtterbium triflate as an efficient catalyst for one-pot synthesis of substituted imidazoles through three-component condensation of benzil, aldehydes and ammonium acetateJ. Fluor. Chem.127200615701573
- M.M.HeraviKh.BakhtiariH.A.OskooieS.TaheriSynthesis of 2,4,5-triaryl-imidazoles catalysed by NiCl2·6H2O under heterogeneous systemJ. Mol. Catal. A: Chem.2632007279281
- A.ShaabaniR.RahmatiE.FarhangiZ.BadriSilica sulfuric acid promoted the one-pot synthesis of trisubstituted imidazoles under conventional heating conditions or using microwave irradiationCatal. Commun.8200711491152
- K.F.ShelkeS.B.SapkalM.S.ShingareUltrasound-assisted one-pot synthesis of 2,4,5-triarylimidazole derivatives catalyzed by ceric (IV) ammonium nitrate in aqueous mediaChin. Chem. Lett.202009283287
- H.N.RoyM.M.RahmanP.K.PramanickRapid access of some trisubstituted imidazoles form benzils condensed with aldehydes and ammonium acetate catalyzed by l-sisteineIndian J. Chem.52B2013153159
- B.SadeghiB.B.F.MirjaliliM.M.HashemiBF3·SiO2: an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazolesTetrahedron Lett.49200825752577
- S.SamaiG.Ch.NandiP.SinghM.S.Singhl-Proline: an efficient catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazolesTetrahedron6520091015510161
- A.BamoniriB.F.MirjaliliS.NazemianN.Y.MahabadiNano silica phosphoric acid as an efficient catalyst for one-pot synthesis of 2,4,5-tri-substituted imidazoles under solvent free conditionBulg. Chem. Commun.4620147984
- B.MalekiS.Sedigh AshrafiN-Bromosuccinimide catalyzed three component one-pot efficient synthesis of 2,4,5-triaryl-1H-imidazoles from aldehyde, ammonium acetate, and 1,2-diketone or α-hydroxyketoneJ. Mex. Chem. Soc.5820147681
- J.SafariN.NasehZ.ZarnegarZ.AkbariApplications of microwave technology to rapid synthesis of substituted imidazoles on silica-supported SbCl3 as an efficient heterogeneous catalystJ. Taibah Univ. Sci.201410.1016/j.jtusci.2014.01.007
- J.SafariS.Gandomi RavandiZ.AkbariSonochemical synthesis of 1,2,4,5-tetrasubstituted imidazoles using nanocrystalline MgAl2O4 as an effective catalystJ. Adv. Res.42013509514
- J.ZhuH.BienaymeMulticomponent Reactions2005Wiley-VCHWeinheim, Germany
- W.G.DongG.H.KimJ.Y.ChoiJ.R.YoonY.H.KimA study of the curing behavior of polyamic acid coated on a Cu or Zn layerColloids Surf. A3242008122125
- Z.A.LewickaW.W.YuB.L.OlivaE.Q.ContrerasV.L.ColvinPhotochemical behavior of nanoscale TiO2 and ZnO sunscreen ingredientsJ. Photochem. Photobiol. A: Chem.26320132433
- A.K.GuptaM.GuptaSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials26200539954021
- J.Y.LaoJ.Y.HuangD.Z.WangZ.F.RenZnO nanobridges and nanonailsNano Lett.32003235238
- S.ÖztürkN.KılınçN.TaşaltınZ.Z.ÖztürkFabrication of ZnO nanowires and nanorodsPhysica E44201210621065
- P.X.GaoY.DingZ.L.WangElectronic transport in superlattice-structured ZnO nanohelixNano Lett.92009137143
- D.ChandraS.MridhaD.BasakA.BhaumikTemplate directed synthesis of mesoporous ZnO having high porosity and enhanced optoelectronic propertiesChem. Commun.20092384
- A.B.DjurišićA.M.C.NgX.Y.ChenZnO nanostructures for optoelectronics: material properties and device applicationsProg. Quantum Electron.342010191259
- W.WanJ.HuangL.ZhuL.HuZh.WenL.SunZh.YeDefects induced ferromagnetism in ZnO nanowire arrays doped with copperCrystEngComm15201378877894
- T.ZhaiX.FangM.LiaoX.XuH.ZengB.YoshioD.GolbergA comprehensive review of one-dimensional metal-oxide nanostructure photodetectorsSensors9200965046529
- M.K.HossainS.C.GhoshY.BoontongkongC.ThanachayanontJ.DuttaGrowth of zinc oxide nanowires and nanobelts for gas sensing applicationsJ. Metastable Nanocryst. Mater.2320052730
- S.K.GuptaA.JoshiM.KaurDevelopment of gas sensors using ZnO nanostructuresJ. Chem. Sci.12220105762
- G.SinghE.M.JoyceJ.BeddowT.J.MasonEvaluation of antibacterial activity of ZnO nanoparticles coated sonochemically on to textile fabricsJ. Microbiol. Biotechnol. Food Sci.22012106120
- D.DasB.C.NathP.PhukonA.KalitaS.K.DoluiSynthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activityColloids Surf. B1112013556560
- A.GhoshR.SharmaA.GhuleV.S.TaurR.A.JoshiD.J.DesaleY.G.GudageK.M.JadhavS.H.HanLow temperature LPG sensing properties of wet chemically grown zinc oxide nanoparticle thin filmSens. Actuators B14620106974
- J.QiuW.YuX.GaoX.LiSol–gel assisted ZnO nanorod array template to synthesize TiO2 nanotube arraysNanotechnology17200646954698
- Ch.FauteuxR.SmiraniJ.PegnaM.A.El KhakaniD.TherriaultFast synthesis of ZnO nanostructures by laser-induced chemical liquid depositionAppl. Surf. Sci.255200953595362
- L.SchlurA.CartonP.LévêqueD.GuillonG.PourroyOptimization of a new ZnO nanorods hydrothermal synthesis method for solid state dye sensitized solar cells applicationsJ. Phys. Chem. C117201329933001
- S.CimitanS.AlbonettiL.ForniF.PeriD.LazzariSolvothermal synthesis and properties control of doped ZnO nanoparticlesJ. Colloid Interface Sci.32920097380
- A.K.SinghV.ViswanathV.C.JanuSynthesis, effect of capping agents, structural, optical and photoluminescence properties of ZnO nanoparticlesJ. Lumin.1292009874878
- L.S.PanchakarlaM.A.ShahA.GovindarajC.N.R.RaoA simple method to prepare ZnO and Al(OH)3 nanorods by the reaction of the metals with liquid waterJ. Solid State Chem.180200731063110
- M.Hosseini-SarvariS.EtemadNanosized zinc oxide as a catalyst for the rapid and green synthesis of β-phosphono malonatesTetrahedron64200355195523
- M.Hosseini-SarvariH.SharghiS.EtemadNanocrystalline ZnO for Knoevenagel condensation and reduction of the carbon, carbon double bond in conjugated alkenesHelv. Chim. Acta912008715724
- M.L.KantamK.B.S.KumarCh.SridharNanocrystalline ZnO as an efficient heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazolesAdv. Synth. Catal.347200512121214
- P.GoswamiDually activated organo- and nano-cocatalyzed synthesis of coumarin derivativesSynth. Commun.39200922712278
- M.Hosseini-SarvariAn efficient and eco-friendly nanocrystalline zinc oxide catalyst for one-pot, three component synthesis of new ferrocenyl aminophosphonic esters under solvent-free conditionCatal. Lett.1412011347355
- M.Hosseini-SarvariM.TavakolianPreparation, characterization, and catalysis application of nano-rods zinc oxide in the synthesis of 3-indolyl-3-hydroxy oxindoles in waterAppl. Catal. A: Gen.441–44220126571
- S.PaulP.BhattacharyyaA.R.DasOne-pot synthesis of dihydropyrano[2,3-c]chromenes via a three component coupling of aromatic aldehydes, malononitrile, and 3-hydroxycoumarin catalyzed by nano-structured ZnO in water: a green protocolTetrahedron Lett.52201146364641
- A.AzarifarR.Nejat-YamiD.AzarifarNano-ZnO: an efficient and reusable catalyst for one-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones and pyrazolo[1,2-a][1,2,4]triazole-1,3-dionesJ. Iran. Chem. Soc.102013297306
- M.AbaszadehM.SeifiA.AsadipourUltrasound promotes one-pot synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives, catalyzed by ZnO nanoparticlesRes. Chem. Intermed.201410.1007/s11164-014-1711-9
- S.SadjadiM.EskandariUltrasonic assisted synthesis of imidazo[1,2-a]azine catalyzed by ZnO nanorodsUltrason. Sonochem.202013640643
- H.SachdevaR.SarojZnO nanoparticles as an efficient, heterogeneous, reusable, and ecofriendly catalyst for four-component one-pot green synthesis of pyranopyrazole derivatives in waterSci. World J.20132013680671
- B.H.SoniM.P.DeshpandeS.V.BhattN.GargS.H.ChakiStudies on ZnO nanorods synthesized by hydrothermal method and their characterizationJ. Nano Electron. Phys.5201340774082
- R.N.GayenR.BharA.K.PalSynthesis and characterization of vertically alined zno nanorodes wit controlled aspect ratioIndian J. Pure Appl. Phys482010385393