Abstract
The aim was to study the influence of albumin supplementation on the changes of the kidney function and structure in cirrhotic rats induced by common bile duct ligation (BDL). Twenty-four male albino rats weighing 200–250 g were divided into Group I: 6 rats underwent laparotomy alone, and the bile duct was only dissected from the surrounding tissue; Group II: 6 rats underwent a sham operation and received 2% albumin in their drinking water; Group III: 6 rats were subjected to bile duct ligation only; and Group IV: 6 rats were subjected to bile duct ligation and received a daily albumin 2% in drinking water. All rats were sacrificed after 4 weeks. We measured the liver and kidney functions and oxidative stress markers in the renal tissue and conducted a histological evaluation of the liver and kidney. The liver enzymes were decreased, but there was no significant difference in the bilirubin levels in group IV compared to group III. There was a significant elevation of serum creatinine in group III compared to group II, and serum creatinine was attenuated in group IV. The renal tissue catalase activity and reduced glutathione, as well as the nitric oxide levels, were significantly increased in group IV and were elevated in group III. Histologically, the livers of group IV showed degeneration and inflammatory cell infiltration with regeneration areas in which normal hepatocytes appeared. The kidneys of group IV showed recovery as well as areas of inflammatory cell infiltration. Some tubules appeared with normal epithelial lining. In conclusion, the results suggest that albumin partially improves the renal functions and structures after their disturbances as a result of BDL.
1 Introduction
Cholestatic (Obstructive) jaundice occurred within 2 weeks of common bile duct ligation (BDL) and progressed to cirrhosis within 4 to 6 weeks [Citation1]. The patients with cholestatic jaundice may have a higher incidence of renal dysfunction, and approximately 6–8% of the patients suffer from acute renal injury, with mortality over 68% [Citation2]. Renal dysfunction secondary to liver cirrhosis, which was experimentally induced by BDL, is known as hepatorenal syndrome (HRS) [Citation3].
The oxidative stress and generation of reactive oxygen species (ROS) secondary to cholestasis could give rise to the involvement of remote organs other than the liver, such as the kidney with alterations in its function [Citation4]; antioxidant administration improves these alterations [Citation5]. Experimentally, the in vitro addition of excess bile acids or bilirubin to a mixture of reactive enzymes inhibited mitochondrial oxidative enzymes. Thus, high concentrations of these substances in the blood may explain the development of renal failure during cholestatic liver disorders [Citation6]. The blockage of the biliary pathway in cholestasis may cause an overload on the kidney with a consequent disturbance of the kidney function, which may progress to renal failure [Citation7].
Albumin is a liver protein; its production depends on food intake and the integrity of liver cells. The main function of albumin is the maintenance of the colloidal osmotic pressure of the plasma and interstitial fluid [Citation8]. Moreover, albumin plays a significant antioxidant role because of its ability to reduce the production of free radicals by leukocytes [Citation9]. Patients with a reduced serum albumin level are more liable to the dangers of oxidative stress and excess free radicals [Citation10]. Recently, albumin infusion was shown to improve renal function in decompensated cirrhotic patients with acute kidney injury by impacting on the renal blood flow autoregulation [Citation11].
This study was designed with the aim of evaluating the renal function parameters and renal histological changes in rats subjected to a ligation of the extrahepatic bile duct and the influence of albumin supplementation in these animals.
2 Materials and methods
2.1 Animals
The present study was performed on adult male albino rats weighing 200–250 g. The rats were purchased from Vacsera Animal House (Helwan) and were maintained in the Physiology Department Animal House under standard conditions of boarding and feeding. The given diet consisted of bread, milk and green vegetables, and the diet and water were provided ad libitum. All surgical procedures were performed with diethyl ether inhalation.
2.2 Experimental protocol
2.2.1 Animal groups
Twenty-four male albino rats were divided into four groups:
| • | Group I: sham/untreated (n = 6) rats underwent laparotomy with handling of the bile ducts, but without ligation of the bile duct and were sacrificed 4 weeks after the manipulation. | ||||
| • | Group II: sham/treated (n = 6) rats underwent a sham operation and received 2% albumin (aqueous solution) ad libitum and were sacrificed 4 weeks after manipulation. | ||||
| • | Group III: BDL/untreated (n = 6) rats underwent laparotomy with ligation of the common bile duct and were sacrificed 4 weeks after the common bile duct ligation. | ||||
| • | Group IV: BDL/treated (n = 6) rats underwent laparotomy with ligation of the common bile duct, were treated with 2% (albumin aqueous solution) ad libitum [Citation12], and were sacrificed 4 weeks after the common bile duct ligation. | ||||
Rats in the sham-operated group were subjected to a 1.5-cm upper midline abdominal incision, and the common bile duct was isolated from the surrounding tissues. The rats in the BDL group underwent the same procedure and the common bile duct was doubly ligated with a 4–0 silk suture and transected between the two ligatures. The rats were subjected to either bile duct ligation (BDL) or the sham operation using aseptic techniques, as previously described by [Citation13].
During the period of the study, the animals were kept at room temperature and were subjected to a light/dark cycle of 12 h each. All animals received rat chow and water ad libitum, whereas in the case of the animals in the treated groups (groups II and IV), the water contained 2% albumin.
After the end of the experimental period, animals were anaesthetized by an overdose of diethyl ether and were sacrificed, blood was collected, and the liver and kidney were excised and weighed immediately and preserved in parafilm and 10% formalin for biochemical and histological analyses, respectively.
2.2.2 Blood samples collection
Initially, a few drops of blood were collected in a heparinized capillary tube that was centrifuged by microcentrifuge to determine the haematocrit value. Most of the blood collected was then centrifuged to separate the serum, which was stored at −20 °C for later measurements of bilirubin, aminotransferases and alkaline phosphatase, as well as measurements of blood urea nitrogen (BUN) and serum creatinine and albumin.
2.2.3 Tissue samples
Tissue samples were obtained from the liver and the kidney. The renal tissue level of malondialdehyde (MDA) and glutathione (GSH), as well as catalase activity, were determined.
Hepatic and renal tissue samples were examined under a light microscope for histopathological changes.
2.2.4 Liver function parameters
Measurement of the serum levels of albumin [Citation14], total bilirubin (TB) and direct bilirubin (DB) [Citation15]; alkaline phosphatase enzyme (ALP) [Citation16]; alanine aminotransferase (ALT), and aspartate aminotransferase (AST) [Citation17].
2.2.5 Renal function parameters
BUN (mg/dl) [Citation18] and serum creatinine (mg/dl) [Citation19] were measured by the colorimetric method using kits supplied by Biodiagnostic (Egypt).
2.2.6 Measurement of the oxidative stress parameters and nitric oxide in renal tissue
The level of lipid peroxides, measured as malondialdehyde (MDA) [Citation20], in addition to the level of reduced glutathione (GSH) [Citation21], was determined in the kidney homogenates. The units used to express these concentrations were μmol/g of renal tissue.
2.2.6.1 Catalase activity (CAT)
The catalase activity was determined by the method of Aebi [Citation22]. The enzyme activity is expressed as units per milligram of protein (U/mg protein). The kits used in the tissue analysis were supplied by Biodiagnostic (Egypt).
2.2.6.2 NO determination
As a NO measurement is very difficult to perform in biological specimens, the tissue nitrite (NO2) plus nitrate (NO3) concentrations were estimated as an index of the NO production. A method for determining the tissue NO2 plus NO3 levels based on the Griess reaction was used [Citation23]. The total nitrite (NOX) (NO2 + NO3) was measured by a spectrophotometer at 545 nm. The results are expressed as nanomole per gram of wet hepatic tissue.
2.2.7 Histological analysis
Liver and kidney tissue were fixed in 10% buffered formaldehyde and were then processed and embedded in paraffin and sectioned (5 μm). These sections were stained and viewed by light microscopy.
2.3 Statistical analysis
Data were entered and analyzed using SPSS version 9.0. All of the values were expressed as the mean ± SEM. The significance of the data obtained was evaluated by using analysis of variance (ANOVA). P values of less than 0.05 were considered significant.
3 Results
Four weeks after the BDL, the animals had typical signs of cirrhosis, such as decreased growth and jaundice. After death, an abdominal inspection revealed diffuse yellow-green pigmentation in the abdominal organs and a small amount of ascites in many rats in the BDL group. Furthermore, all animals presented with mesenteric oedema and an enlarged liver and spleen (indirect evidence of portal hypertension).
3.1 General parameters
As shown in , there was no significant difference in the haematocrit values between the different groups. All of the BDL rats (group III and IV) had higher liver and kidney-to-body weight ratios than the sham control groups (groups I and II). No significant change was observed between the sham control groups.
Table 1 Haematocrit value and ratios of the liver and kidney-to-body weight in the different experimental groups.
3.2 Liver and kidney functions parameters
As shown in , the levels of total and direct bilirubin is commonly used in patients to evaluate cholestasis. Total and direct bilirubin were increased in the BDL group compared to the sham group. The administration of albumin did not influence significantly the level of bilirubin in the serum.
Table 2 Kidney and liver function parameters in the different experimental groups.
The AST, ALT and ALP serum levels are considered to be markers of hepatocyte destruction. The AST, ALT, and ALP levels were significantly increased in group III in comparison with the sham groups. However, the AST, ALT, and ALP levels were decreased in group IV compared to group III, but were still higher than for the sham control group. Serum albumin decreased significantly in the BDL group and was nearly corrected after albumin supplementation in group IV.
There was a tendency to an increased level of creatinine in group III compared to the sham control group. The albumin supplementation significantly decreased the creatinine level towards normal. There was no significant change in the BUN between the different experimental groups.
3.3 Renal tissues levels of ROS and NO
The MDA levels were significantly greater in the kidney homogenates prepared from the BDL rats than those measured in the kidney homogenates from the sham control groups. These levels were reduced in the BDL rats with the albumin treatment. However, there were no significant differences between the two groups of sham control rats ().
Fig. 1 Levels of renal tissue MDA in different experimental groups. The results are the means and standard errors. *Significance compared to the sham control groups.
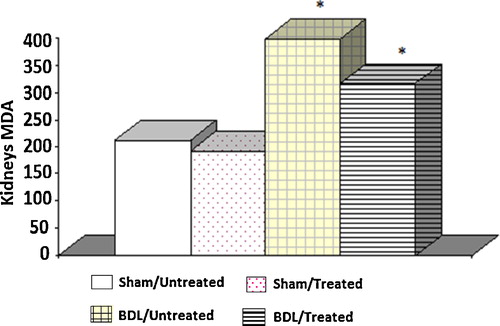
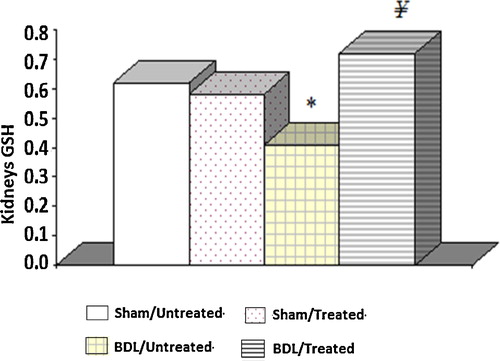
The mean renal level of GSH in the BDL group was significantly lower than in the sham control groups. These levels were increased in the BDL rats with the albumin treatment. Furthermore, there were no significant differences between the two groups of sham control rats ().
Fig. 2 Tissue glutathione levels in different experimental groups. The results are the means and standard errors. *Significance compared to the sham control groups. ¥Significance between groups III and IV.
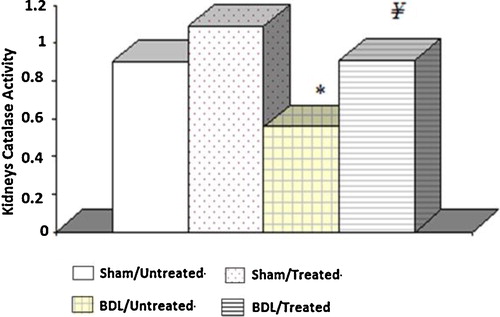
Catalase activity in the BDL group was significantly lower than in the sham control groups. However, this activity was increased in the BDL rats receiving the albumin treatment. Furthermore, there were no significant differences between the two groups of sham control rats ().
Fig. 3 Tissue catalase activity in the different experimental groups. The results are the means and standard errors. *Significance compared to the sham control groups. ¥Significance between groups III and IV.
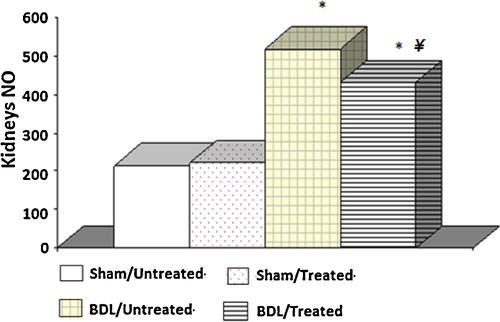
NO levels were significantly greater in the kidney homogenates prepared from the BDL rats than those measured from the sham control groups. These levels were reduced in the BDL rats with the albumin treatment. Furthermore, there were no significant differences between the two groups of sham control rats ().
3.4 Histopathological examination
3.4.1 Liver
The liver of the sham control rats presented with a normal histological structure; the hepatic lobules were intact with a normal arrangement of the hepatic plates that radiated from the central vein (a). The hepatocytes appeared regular, with normal cytoplasmic density and normal nuclear morphology. The bile ducts were intact (b).
Fig. 5 (a) Photomicrograph of a liver of a sham untreated albino rat showing normal hepatic lobulation and normal hepatic plates (H&E 400×). (b) Photomicrograph of a liver of a sham treated albino rat showing normal hepatic plates; note hepatocytes with normal cytoplasmic density and normal nuclei (H&E 1000×). (c) Photomicrograph of a liver of an albino rat 4 weeks after BDL showing severe hepatic fibrosis (H&E 400×). (d) Photomicrograph of a liver of an albino rats 4 weeks after BDL showing the hepatic lobules replaced with collagenic fibrosis (c) and the newly formed bile ducts (D). (Crossmon's trichrome 400×). (e) Photomicrograph of a liver from a BDL/Albumin treated albino rat showing degenerated areas with inflammatory cells infiltration (D) and some normal areas (n) (H&E 200×). (f) Photomicrograph of a liver from a BDL/Albumin treated albino rat showing some normal hepatocytes (arrow) (H&E 400×).
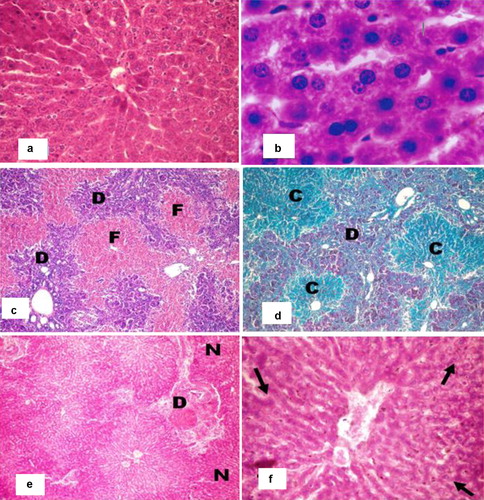
Four weeks after bile duct ligation, the livers of the rats showed severe centrilobular fibrosis and prominent bile duct proliferation in the portal tracts (c). The hepatocytes in the areas of the proliferating bile ducts were atrophied with an increase of collagen in the hepatic parenchyma. Generally, this followed the proliferation process of the bile ducts 30 days after the BDL (d).
The liver of the rats in the albumin treated group showed some degree of degeneration and inflammatory cell infiltration (e) with some areas of regeneration in which normal hepatocytes appeared (f).
3.4.2 Kidney
The kidney of the rat in the control group appeared with normal renal parenchyma, spherical shaped renal corpuscles with normal glomerulus and intact Bowman's capsules. Additionally, the proximal and distal convoluted tubules appeared with intact healthy lining epithelium and the loop of Henle (a) and the collecting tubules appeared with normal epithelium (b).
Fig. 6 (a) Photomicrograph of a renal medulla of a sham untreated albino rat showing normal thin segments of the loop of Henle (arrow) and collecting tubules (c) (H&E 320×). (b) Photomicrograph of a kidney of a sham treated albino rat showing normal thick segments of the loop of Henle (arrow) (H&E 200×). (c) Photomicrograph of a kidney from a BDL/Albumin treated albino rat showing some recovery in some renal medulla with decrease the interlobular fluid and some focal area of lymphocytic infiltration (arrow). (H&E 400×). (d) Photomicrograph of a kidney from an albino rat 4 weeks after BDL showing severe renal damage. Note the swelling of renal corpuscles (R), haemorrhage (arrow) and eosinophilic fluid (arrow head) of granulose cells (arrow head) (H&E 100×). (e) Photomicrograph of a kidney from an albino rat 4 weeks after BDL showing swelling in the renal corpuscles (R) with vacuolated and damaged epithelium of the tubules (arrow), some of which appeared to be ruptured (arrow head) (H&E 400×). (f) Photomicrograph of a kidney from a BDL/Albumin treated albino rat showing recovery in some renal corpuscles (R) and renal tubules (arrow head). Note the infiltration of the inflammatory cell (arrow) and wide tubules (w) (H&E 400×).
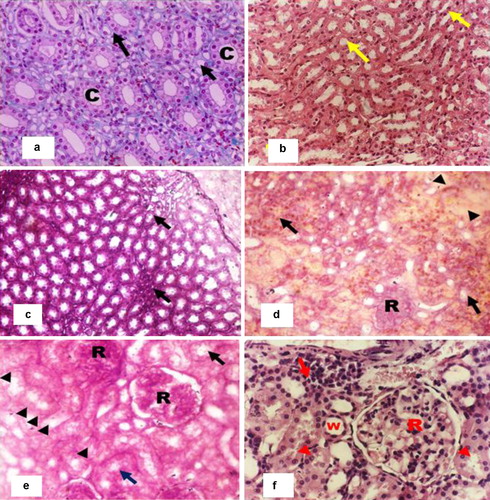
Four weeks after bile duct ligation, the kidneys of the rats showed severe damage. The renal medulla presented some regenerated tubules with inflammatory cell infiltration in some parts (c). The renal corpuscles showed thickening in some parts with swelling in the glomerulus, and the cortical capillaries appeared engorged with blood; some areas of haemorrhage were also observed. Eosinophilic homogenous fluid was observed in the spaces between the renal tubules (d). Also swelling in the tubules and hypertrophy in their epithelium was observed. Some of the tubules were completely ruptured (e).
The kidneys of the rats in the albumin-treated group showed some recovery as the homogenous fluid between the tubules decreased. There were areas of inflammatory cell infiltration, and some tubules showed a wide lumen. Some tubules appeared with a somewhat normal epithelial lining (f).
4 Discussion
The present study confirms the association between liver injury and oxidative stress. In this study, albumin administration caused a significant reduction in the destructive parameters of cholestasis and in the activities of hepatic injury enzymes. Furthermore, albumin significantly improved the parameters of oxidative stress and caused a significant increase in the antioxidant enzyme activities in the renal tissue.
The BDL rats showed marked disturbances in liver function that was manifested in this study by elevated serum levels of AST; ALT; bilirubin, mainly the direct portion; and ALP. The elevated liver function parameters can be considered to be an indicator of the success of the surgery. This finding is in agreement with many other studies [Citation24]. The serum liver enzymes decreased significantly in the albumin-treated rats, but the serum bilirubin remained elevated despite the albumin administration. However, the serum albumin level decreased significantly in the BDL rats, most likely due to the increased leakage outside the vascular system and a lack of compensation due to the hepatic damage [Citation25]. Such a decline was corrected by albumin administration. Previous studies also found a decline in the levels of albumin in rat plasma starting two weeks after common bile duct ligation [Citation26].
It was demonstrated that HRS is characterized by functional rather than structural disarrangement of the kidneys in the presence of progressive liver disease [Citation27].
In the current work, the BDL rats showed a disturbance in renal function, as revealed by elevated serum creatinine levels. This is in agreement with previous studies [Citation28,Citation29]. The BDL/Albumin treated rats showed mild improvement in renal function, as evidenced by a significant decrease in serum creatinine, which, was still more than the control value. It has been reported that at early stages of hepatic injury, renal compensatory mechanisms still remain operating with no observed disturbance of renal function [Citation30]. It was also demonstrated that the administration of albumin prevents the deterioration of renal function and reduces mortality in these patients [Citation31].
In the present study, we found reduced levels of GSH and catalase activity, but the MDA content in the kidney homogenate of the BDL rats was elevated compared with the controls. These results suggest enhanced lipid peroxidation, which causes tissue damage, in addition to the failure of the antioxidant defence mechanisms that prevent the formation of excessive free radicals. The results suggest the development of liver necrosis and cholestasis, which is evident after 72 h; however, lipid peroxidation started after 1–2 weeks of BDL [Citation32]. Our results are in agreement with Ercin et al. [Citation33], who reported that in BDL rats, the activity of the antioxidant systems diminished, which is responsible for the inhibition of the free oxygen radicals. The administration of albumin significantly increased the catalase activity and GSH level as well as reduced the MDA level and thus reduced reactive free radical induced oxidative damage, protected the tissues from highly reactive hydroxyl radicals and attenuated lipid peroxidation in the kidney of the BDL rats.
Regarding the NO content in the kidney tissue, there was a significant decrease in the NO in the renal tissue of the BDL rats with albumin treatment after its elevation in the BDL rats. The excessive generation of NO has been observed both in experimental cholestasis [Citation34,Citation35] and in primary biliary cirrhosis patients [Citation36,Citation37]. Renal impairment occurs in cirrhotic patients with a high concentration of NO in the plasma and ascitic fluid. Such impairment was improved by the inhibition of NO synthase, thus decreasing NO [Citation38]. The excess NO in the BDL rats may be one of the factors responsible for the observed renal disturbances. The peripheral arterial vasodilation produced by the NO with relative arterial underfilling activates the sympathetic nervous system with severe renal vasoconstriction and consequent renal ischaemia that may end in renal failure in the advanced stage. Thus, in the present work, the albumin decreased nitric oxide in the BDL rats, which would be expected to attenuate the development of the renal disturbances resulting from excess nitric oxide. It was also demonstrated that the superoxide anion can rapidly react with NO, resulting in peroxynitrite formation, which can subsequently initiate lipid peroxidation [Citation39].
In the BDL rats, the liver showed severe centrilobular fibrosis and prominent bile duct proliferation. The hepatocytes in the areas of the proliferating bile ducts were atrophied with an increase of collagen in the hepatic parenchyma. It was reported that BDL in rats stimulates the proliferation of biliary epithelial cells and hepatocyte progenitors, resulting in proliferating bile ductules with accompanying portal inflammation and fibrosis. Cholangiocyte proliferation is initiated after the BDL at the edge of the portal tract [Citation1]. However, the liver of rat in the BDL/albumin treated group showed some degree of degeneration and inflammatory cell infiltration with some areas of regeneration in which normal hepatocytes appeared.
In the BDL rats, the renal corpuscles showed thickening in some parts with swelling in the glomerulus, and the cortical capillaries appeared to be engorged with blood with haemorrhagic areas. Additionally, swelling in the tubules and hypertrophy in their epithelium were observed. Some of the tubules were completely ruptured. However, some researchers demonstrated that no alterations in the renal histology were observed in any of the bile duct-ligated rats compared to the sham-operated animals [Citation3,Citation27]. The kidneys of the BDL/albumin-treated rats showed some recovery with inflammatory cell infiltration. Some regenerated tubules appeared in the renal medulla.
5 Conclusion
Albumin decreased tubulointerstitial lesions in the kidney induced by bile duct ligation in rats. These effects of albumin may be related to improved liver function or to their impact on oxidative stress, which is the most common cause of remote organ damage in liver cirrhosis.
Conflict of interest
There is no conflict of interest.
Support Information
Download PDF (209.8 KB)Acknowledgements
This study was supported by the Department of Physiology, Faculty of Medicine, Ain Shams University, Cairo.
Notes
Peer review under responsibility of Taibah University
References
- T.G.MarquesE.ChaibJ.H.da FonsecaA.C.LourençoF.D.SilvaM.A.RibeiroF.H.GalvãoL.A.D’AlbuquerqueReview of experimental models for inducing hepatic cirrhosis by bile duct ligation and carbon tetrachloride injectionActa Cir. Bras.272012589594
- S.Raicević SibinovićA.NagorniV.BrzackiM.RadisavljevićPrediction of renal dysfunction in patients with obstructive icterusMed. Pregl.649–102011503506
- R.M.PereiraR.A.dos SantosE.A.OliveiraV.H.LeiteF.L.DiasA.S.RezendeL.P.CostaL.S.BarcelosM.M.TeixeiraA.C.Simoes e SilvaDevelopment of hepatorenal syndrome in bile duct ligated ratsWorld J. Gastroenterol.14200845054511
- M.MiyazonoD.ZhuR.NemenoffE.R.JacobsE.P.CarterIncreased epoxyeicosatrienoic acid formation in the rat kidney during liver cirrhosisJ. Am. Soc. Nephrol.14200317661775
- M.C.OrtizM.C.ManriquezK.A.NathD.J.LagerJ.C.RomeroL.A.JuncosVitamin E prevents renal dysfunction induced by experimental chronic bile duct ligationKidney Int.642003950961
- B.KalerT.KarramW.A.MorganP.H.BachI.M.YousefA.BomzonAre bile acids involved in the renal dysfunction of obstructive jaundice? An experimental study in bile duct ligated ratsRenal Fail.2652004507516
- P.GentiliniG.La VillaLiver-kidney pathophysiological interrelationships in liver diseasesDig. Liver Dis.40122008909919
- J.P.NicholsonM.WolmaransG.R.ParkThe role of albumin in critical illness review articleBr. J. Anaest.852000599610
- B.RuotF.BéchereauG.BayleD.BreuilléC.ObledThe response of liver albumin synthesis to infection in rats varies with the phase of the inflammatory processClin. Sci.1022002107114
- G.Pereira-FilhoC.FerreiraA.SchwengberC.MarroniC.ZettlerN.MarroniRole of N-acetylcysteine on fibrosis and oxidative stress in cirrhotic ratsArq. Gastroenterol.452008156162
- R.Garcia-MartinezL.NoiretS.SenR.MookerjeeR.JalanAlbumin infusion improves renal blood flow autoregulation in patients with acute decompensation of cirrhosis and acute kidney injuryLiver Int.10201412528
- J.F.LessaL.S.RangelN.J.CostaJr.O.Castro e SilvaC.A.CruzJ.B.SousaEffects of albumin administration in serum liver enzymes of rats in the presence of extrahepatic biliary obstructionActa Cir. Bras.26Suppl. 220117073
- Z.AckermanF.KarmeliG.PizovI.Ben-DovO.PappoRenal effects of gentamicin in chronic bile duct ligated ratsDig. Dis. Sci.5122006406415
- M.WalterH.GeradeBilirubin direct/totalMicrochem. J.151970231233
- B.T.DoumasW.A.WatsonH.G.BiggsAlbumin standards and the measurement of serum albumin with bromcresol greenClin. Chim. Acta31119718796
- A.BelfieldD.M.GoldbergRevised assay for serum phenyl phosphatase activity using 4-amino-antipyrineEnzyme121971561573
- A.ReitmanS.FrankelA colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminasesAm. J. Clin. Path.28119575663
- J.K.FawcettJ.E.SocttA rapid and precise method for the determination of ureaJ. Clin. Path.131960156159
- J.SchirmeisterH.WillmanH.KieferFor and against the usefulness of endogenous creatinine clearance in functional kidney diagnosisDtsch. Med. Wochenschr.89196416401647
- H.OhkawaW.OhishiK.YagiAssay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnal. Biochem.9551979351358
- E.BeutlerO.DuronM.B.KellyImproved method for the determination of blood glutathioneJ. Lab. Clin. Med.611963882888
- H.AebiCatalase in vitroMethods Enzymol.1051984121126
- N.K.CortasN.W.WakidDetermination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction methodClin. Chem.36199014401443
- G.TahanF.ErenO.TarçinH.AkinV.TahanH.ŞahınO.ÖzdoğanN.İmeryüzÇ.ÇelıkelE.AvşarN.TözünEffects of a long-acting somatostatin analogue lanreotide on bile duct ligation-induced liver fibrosis in ratsTurk. J. Gastroenterol.212010287292
- S.KrähenbühlU.MartiI.GrantP.J.GarlickP.E.BallmerCharacterization of mechanisms causing hypoalbuminemia in rats with long-term bile duct ligationJ. Hepatol.2319957986
- M.A.RothschildM.OratzD.ZimmonS.S.SchreiberA.V.CaneghemAlbumin synthesis in cirrhotic subjects with ascites studied with carbonate-*4CJ. Clin. Invest.481996344350
- A.CardenasP.GinesHepatorenal syndromeClin. Liver Dis.1022006371385 ix–x
- P.GentiliniF.VizzuttiA.GentiliniM.ZipoliM.FoschiR.G.RomanelliUpdate on ascites and hepatorenal syndromeDig. Liver Dis.342002592605
- A.CárdenasHepatorenal syndrome: a dreaded complication of end-stage liver diseaseAm. J. Gastroenterol.10022005460467
- V.ArroyoW.JimenezComplications of cirrhosis II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problemJ. Hepatol.322000157170
- J.D.GrangéX.AmiotNitric oxide and renal function in cirrhotic patients with ascites: from physiopathology to practiceEur. J. Gastroenterol. Hepatol.1662004567570
- M.ParolaG.LeonarduzziG.RobinoE.AlbanoG.PoliM.U.DianzaniOn the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasisFree Radic. Biol. Med.201996351359
- C.N.ErcinZ.YesilovaA.KorkmazA.OzcanC.OktenliA.UygunThe effect of iNOS inhibitors and hyperbaric oxygen treatment in a rat model of experimental colitisDig. Dis. Sci.54120097579
- M.GraebeL.BrondS.ChristensenS.NielsenN.V.OlsenT.E.JonassenChronic nitric oxide synthase inhibition exacerbates renal dysfunction in cirrhotic ratAm. J. Physiol. Renal Physiol.28622004F288F297
- M.M.ArrieroA.Lopez-FarreO.FryeiroJ.A.Rodríguez-FeoS.VelascoM.García-DuránJ.FortesJ.C.De La PintaL.E.MuñozA.CeldránExpression of inducible nitric oxide synthase in the liver of bile duct-ligated Wistar rats with modulation by lymphomononuclear cellsSurgery1292001255266
- I.GrattaglianoP.PortincasaV.O.PalmieriG.PalascianoMutual changes of thioredoxin and nitrosothiols during biliary cirrhosis: results from humans and cholestatic ratsHepatology452007331339
- L.M.López-SánchezF.J.CorralesM.BarcosI.EspejoJ.R.Muñoz-CastañedaA.Rodríguez-ArizaInhibition of nitric oxide synthesis during induced cholestasis ameliorates hepatocellular injury by facilitating S-nitrosothiol homeostasisLab. Invest.9012010116127
- J.BeckmanW.KoppenolNitric oxide, superoxide, and peroxynitrite; the good the bad and the uglyAm. J. Physiol. Cell Physiol.2711996C1424C1437
- G.La VillaG.BarlettaP.PantaleoR.Del BeneF.VizzuttiS.VecchiarinoE.MasiniF.PerfettoR.TarquiniP.GentiliniG.LaffiHemodynamic, renal, and endocrine effects of acute inhibition of nitric oxide synthase in compensated cirrhosisHepatology34120011927
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jtusci.2015.01.001.