Abstract
A highly selective and sensitive spectrophotometric method has been developed for the determination of trace amounts of bismuth in various samples. The method is based on the adsorption of Bi(III) after complexation with 5-(2′-bromophenylazo)-6-hydroxy pyrimidine-2,4-dione (BPAHPD) in a pH range of 2.0–2.5 on amberlite XAD-2 resin in the presence of emulsifier-OP. The retained analyte on the resin recovered with 5.0 mL of 2.0 mol L−1 hydrochloric acid and bismuth was analyzed spectrophotometrically at λmax 579 nm. Beer’s law is obeyed in the concentration range of 0.1–7.5 μg mL−1 Bi(III) in the measured solution. For more accurate results, the Ringbom optimum concentration range was determined to be 0.25–7.25 μg mL−1. The linear regression equation was A = 0.123C (μg mL−1) + 0.002 (r = 0.9996). The molar absorptivity was calculated to be 2.56 × 105 L mol−1 cm−1 at 579 nm, and the Sandell sensitivity was determined to be 8.15 ng cm−2. Various parameters, such as the effect of pH, reagent concentration, surfactant, and flow rate, were studied. The interference of various metal ions on the determination of bismuth has been studied in detail to optimize the conditions for the determination of bismuth in biological samples (i.e., National Institute for Environmental Studies (NIES, No. 5 human hair, NIES, No. 7 tea leaves)) and water samples (i.e., spring water, river water, underground water, and sea and tap water).
1 Introduction
Bismuth has been used in medicines for the treatment of helicobacter pylori-induced gastritis [Citation1,Citation2]. Bismuth and its compounds are also used in semiconductors, cosmetic preparations, alloys and metallurgical additives and in the preparation and recycling of uranium nuclear fuels [Citation3]. As the uses of bismuth in medicine increase, bismuth has spread in the environment, and the chance of exposure of organisms to bismuth has increased [Citation1]. Several methods have been developed for the determination of bismuth. These methods include hydride generation inductively coupled plasma atomic emission spectrometry (HG-ICPAES) [Citation4], electrothermal vaporization ICP mass spectrometry (ETV-ICP-MS) [Citation5], atomic absorption spectrometry (AAS) [Citation6,Citation7], electrothermal atomic absorption spectrometry (ETAAS) [Citation8] potentiometric stripping analysis (PSA) [Citation9], anodic stripping voltammetry [Citation10,Citation11], cathodic stripping voltammetry (CSV) [Citation12] and amperometry [Citation13]. However, due to the presence of low levels of bismuth in environmental samples, the separation of bismuth from other elements and the use of preconcentration are typically necessary. Conventional solvent extraction and separation of Bi in the presence of co-extracting ligands, such as bis(2,4,4,-trimethylpentyl) monothiophosphinic acid [Citation14] and pyrrolidine dithiocarbamate [Citation15], has attracted considerable attention. The disadvantages of liquid/liquid extraction include the use of large volumes of organic solvents, cumbersome glassware and cost. However, several other techniques for preconcentration of Bi have been proposed including preconcentration using Mg–W cell electrodeposition [Citation16], preconcentration with sodium di-n-propyl dithiophosphinate and activated carbon in a batch method, a flow injection on-line two stage solvent extraction [Citation17], a flow injection on-line sorption preconcentration [Citation18–Citation20], a fluorescence quenching method [Citation21] and solid phase extraction [Citation22–Citation28].
The spectrophotometric method is a sensitive technique for the determination of trace amounts of bismuth(III) in aqueous media [Citation29–Citation36]. Preconcentration methods of bismuth in environmental samples, such as preconcentration using Mg–W cell-electrodeposition [Citation16], flow injection on-line two stage solvent extractions [Citation17], solid-phase extraction by silica gel [Citation37], microcrystalline benzophenone [Citation38], and carbon nanotubes [Citation39–Citation42], have been reported. Typically, solid phase extraction replaces liquid/liquid extraction as a sample preconcentration tool and provides a method that is simple and safe to use. Amberlite XAD adsorption resins exhibit good physical properties, such as porosity, uniform pore size distribution, and high surface area as chemically homogeneous nonionic structures for large amounts of uncharged compounds, and these resins have been used as solid sorbents for the enrichment/separation of metals [Citation43–Citation46]. In this study, solid-phase extraction followed by the spectrophotometric determination of bismuth is reported. The method is simple, sensitive, and selective and allows for the determination of Bi(III) at concentrations as low as 0.1 μg mL−1 in water samples.
2 Experimental
2.1 Apparatus
A Perkin-Elmer Lambda 12 UV–Vis spectrophotometer (Waltham, MA, USA) with a 10 mm quartz cell was used for all of the spectral measurements. A funnel tipped glass tube (60 mm × 6 mm) was used as a column for preconcentration. The laboratory glassware (Superior, Germany) and column were maintained overnight in a 5.0% nitric acid solution. A Perkin Elmer atomic absorption spectrometry model AAnalyst 300 (Waltham, MA, USA) was used for all of the GFAAS measurements. An Orion research model 601 A/digital ionalyzer pH meter (Tokyo, Japan) was used for confirming the pH of the solutions.
2.2 Reagents
All of the chemicals were of analytical reagent grade. Bismuth(III) nitrate (Loba Chemie Pvt Ltd, Mumbai, 132, India) was dissolved in a minimum volume of concentrated nitric acid followed by dilution to 1000 mL with bidistilled water in a standard flask and standardized by known methods [Citation47]. BPAHPD was synthesized according to a previously reported method [Citation48]. A 2 × 10−3 mol L−1 stock solution was prepared by dissolving an appropriate weight of the pure reagent in the least amount of ethanol (15 mL) followed by dilution to the mark in a 100-mL measuring flask with ethanol (Merck, Darmstadt, 140 Germany). A buffer solution at a pH of ∼2.5 was prepared by mixing of 0.1 mol L−1 potassium hydrogen phthalate and 0.1 mol L−1 hydrochloric acid (Sigma, St 143 Louis, MO, USA) solutions in an appropriate ratio. The solutions of the alkali metal salts (1.0%) and various metal salts (0.1%) (Sigma, St 143 Louis, MO, USA) were used to study the interference of anions and cations, respectively.
2.3 Preparation of the amberlite XAD-2 column loaded with BPAHPD
Amberlite XAD-2 (Merck, Darmstadt, 140 Germany) was treated with an ethanol–hydrochloric acid–water (2:1:1) solution overnight. Then, the resin was rinsed with deionized water until the pH of the supernatant water (5.0 mL of phosphate buffer solution) became neutral. The packing of the column must be performed using ethanol as an eluent because water makes the resin beads float. The resin was saturated with the reagent using 1.2 mL of a 2 × 10−3 mol L−1 BPAHPD solution in ethanol and 2.5 mL of a 5.0% emulsifier-OP solution (Sigma, St 143 Louis, MO, USA) at a flow rate of 0.5 mL min−1. Then, the resin was washed with water until the reagent excess was eliminated from the resin. All of the experiments were performed in a funnel-tipped glass tube (60 mm × 6 mm), which was used as a column for preconcentration. This tube was plugged with glass wool and filled with XAD-2 to a height of 1.0–1.2 cm. Prior to sample loading, the column must be preconditioned by using a buffer solution.
2.4 Procedure for the sorption of bismuth on the column
An aliquot of the bismuth solution (containing 0.1–37.5 μg) was placed in a 50-mL beaker contained 3.0 mL of the buffer solution (pH ∼ 2.5) followed by dilution to 25 mL with bidistilled water. This solution was passed through the column at a flow rate of 2.0 mL min−1. Next, the column was eluted with 5.0 mL of deionized water. The adsorbed bismuth(III) complex on the column was eluted with 5.0 mL of a 2.0 mol L−1 hydrochloric acid solution at a flow rate of 1.0 mL min−1. The eluent was collected in a 5.0-mL measuring flask, and bismuth was determined spectrophotometrically at a λmax of 579 nm against a reagent blank that was similarly treated.
3 Results and discussion
3.1 Absorption spectra
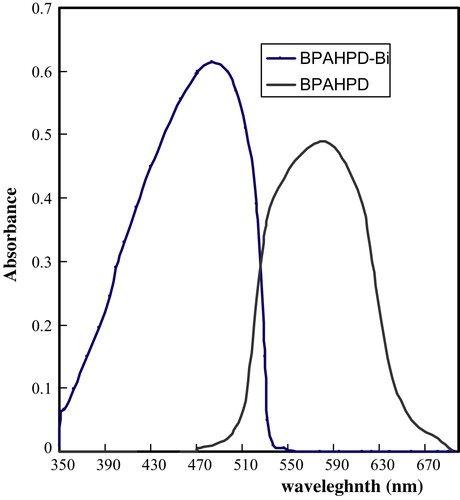
The absorption spectra of BPAHPD and its Bi(III) complex are shown in . The absorption bands of BPAHPD and its complex in acidic media are located at 484 nm and 579 nm, respectively.
3.2 Reaction conditions
The reaction conditions were investigated with 4.0 μg mL−1 bismuth. The adsorption was carried out at different pH values, and the other variables were held constant. The bismuth complex was quantitatively adsorbed on the amberlite XAD-2 resin in a pH range of 2.0–2.5. In subsequent studies, the pH was maintained at approximately 2.5. The addition of 1.5–5.0 mL of the buffer did not affect the retention of bismuth, and the use of 3.0 mL of buffer is recommended.
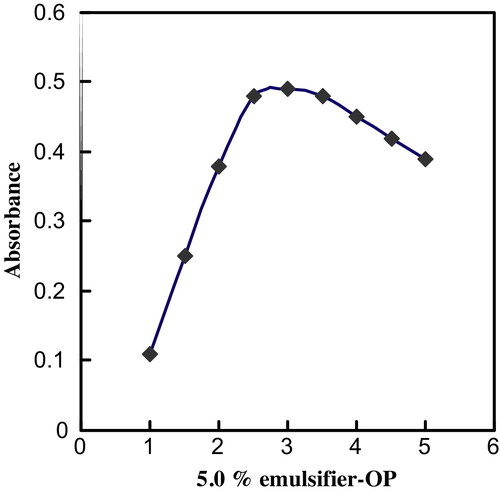
The effects of surfactants on the Bi(III)–BPAHPD system were investigated. The results indicated that in the absence of surfactants (i.e., anionic or cationic surfactants) the Bi(III)–BPAHPD chromogenic system exhibits a low absorption. However, in the presence of nonionic surfactants, the absorption of the chromogenic system increases substantially. Various nonionic surfactants enhance the absorbance in the following order: emulsifier-OP > Triton X-100 > Tween-80 > Tween-60 > Tween-20. Emulsifier-OP was determined to be the best additive, and the use of 2.0–3.0 mL of a 5.0% emulsifier-OP solution yielded a constant and maximum absorbance (). Therefore, the use of 2.5 mL of this additive is recommended.
Fig. 2 Effect of emulsifier-OP on the complexation of 4.0 μg mL−1 Bi(III) with 2.4 × 10−4 mol L−1 BPAHPD and at the optimum experimental conditions.
For up to 4.0 μg of Bi(III), the use of 1.2 mL of a 2 × 10−3 mol L−1 BPAHPD solution was sufficient for to obtain a complete reaction. Therefore, 1.2 mL of a 2 × 10−3 mol L−1 BPAHPD solution was added in all additional measurements.
The flow rate was varied from 0.1 to 5 mL min−1. A flow rate of 0.1–3.0 mL min−1 did not affect adsorption. A flow rate of 2.0 mL min−1 is recommended for use in all of the experiments.
The volume of the aqueous phase was varied from 2.0 to 100 mL under the optimum conditions, and the other variables were held constant. The highest absorbance value was nearly constant up to 25 mL. However, for convenience, all of the experiments were carried out with 25 mL of the aqueous phase.
Preliminary observations indicated that bismuth was completely desorbed with 5.0 mL of a 2.0 mol L−1 hydrochloric acid. Therefore, 5.0 mL of a 2.0 mol L−1 hydrochloric acid was used in the current study.
3.3 Sorption capacity of the resin for the ligand and bismuth
The sorption capacity of the amberlite XAD-2 resin for the ligand and bismuth was also evaluated using the Langmuir model [Citation49]. The XAD-2 resin has a sorption capacity of 1.0 and 0.4 mg g−1 for the ligand and bismuth, respectively.
3.4 Stoichiometric ratio
The nature of the complex was established at the optimum conditions described above using the molar ratio and continuous variation methods. The plot of the absorbance as a function of the BPAHPD to Bi(III) molar ratio, which was obtained by varying the BPAHPD concentration, exhibited an inflection at a molar ratio of 3.0, indicating the presence of three BPAHPD molecules in the formed complex. In addition, the Job method indicated a BPAHPD to Bi(III) ratio of 3.0. Therefore, the results indicated that the stoichiometric ratio was (3:1) [BPAHPD:Bi(III)]. The conditional formation constant (log K), which was calculated using the Harvey and Manning equation applying the data obtained from the two methods, was determined to be 6.22 but the true constant was 6.10.
3.5 Effect of foreign ions
Various salts and metal ions were individually added to a solution containing 10 μg of bismuth, and the general procedure was applied. The tolerance limit was set to the concentration of the diverse ion required to cause ±5.0% error in the determination of bismuth. The tolerance limit (error < 5.0%) is given in . Most of the studied salts did not interfere at the gram or milligram level except for EDTA, which may be due to the high formation constant of the Bi–EDTA complex compared to the bismuth–BPAHPD complex. Therefore, the method is highly selective and has been successfully applied to the trace determination of bismuth in various biological and water samples without any previous separation.
Table 1 Effect of various salts and metal ions at the optimum experimental conditions.
3.6 Calibration curve and sensitivity
The calibration curve indicated that the system obeys Beer’s law in a concentration range of 0.1–7.5 μg Bi(III) per mL of the measured solution. For more accurate results, the Ringbom optimum concentration range was determined to be 0.25–7.25 μg Bi(III) per mL in the measured solution. The linear regression equation was A = 0.123C (μg mL−1) + 0.002 (r = 0.9996). The molar absorptivity was calculated to be 2.56 × 105 L mol−1 cm−1 at 579 nm, and the Sandell sensitivity was determined to be 8.15 ng cm−2. The standard deviations of the absorbance measurements were calculated from a series of 13 blank solutions. The limits of detection (K = 3) and quantification (K = 10) of the method were established [Citation50] and are listed in according to the IUPAC definitions (i.e., C1 = KSo/s where C1 is the limit of detection, So is the standard error of blank, s is the slope of the standard curve and K is the constant related to the confidence interval). The relative standard deviation was 1.11%, which was obtained from a series of 10 standards that each contained 4.0 μg mL−1 of Bi(III).
Table 2 Analytical parameters at the optimum experimental conditions.
The sensitivity expressed as the molar absorptivity of the proposed method was compared to that of published spectrophotometric methods (). The higher sensitivity of the proposed method is notable and even greater than that of other methods [Citation29–Citation36] based on spectrophotometry. Although the measurable concentration range is from approximately 5.0–150 ng L−1 using graphite furnace atomic absorption spectrometry, the proposed method was very simple and accurate.
Table 3 Comparison of reagents for spectrophotometric determination of bismuth.
3.7 Analysis of bismuth in biological materials and water samples
The accuracy and applicability of the proposed method have been investigated for the determination of bismuth in National Institute for Environmental Studies (NIES) No. 5 human hair and NIES No. 7 tea leaves. A 0.1 g sample was placed in a beaker and dissolved in concentrated nitric acid (∼5 mL) with heating. The solution was cooled, diluted and filtered. Because no standard biological sample containing bismuth was available, the experiment was conducted by adding a known amount of bismuth to the standard biological sample. The filtrate was diluted to 50 mL with water in a calibrated flask. An aliquot (5.0–25 mL) of the sample solution was individually taken, and bismuth was determined using the general procedure. The results are listed in .
Table 4 Determination of Bi(III) in biological samples.
To confirm the applicability of the proposed method, the determination of microgram amounts of Bi(III) in real water samples has been performed. For the water samples, the samples were acidified with hydrochloric acid and filtered through a 0.45-μm filter. The bismuth content was analyzed according to the general procedure. An AAS method was used as a reference method, and the results are shown in .
Table 5 Determination of Bi(III) in water samples.
The performance of the proposed method was assessed by calculation of the t-value (for accuracy) and F-test (for precision) compared to the results from the AAS method [Citation7]. The mean values were obtained in Student’s t- and F-tests at 95% confidence limits for five degrees of freedom [Citation51]. The results indicated that the calculated values () did not exceed the theoretical values. A wider range of determination, higher accuracy, more stability and less time consuming are the advantages of the proposed method over the other method.
4 Conclusions
The proposed method has several advantages. First, BPAHPD is one of the most easily prepared highly pure, sensitive, and selective spectrophotometric reagents for Bi(III) determination. The molar absorptivity of the chelate was determined to be as high as 2.56 × 105 L mol−1 cm−1 at 579 nm in the measured solution. The higher sensitivity of the proposed method is notable and even greater than other methods based on spectrophotometry. Second, the detection and quantification limits are 31 and 99.5 ng mL−1, respectively, in the original sample. Third, most common ions do not interfere with the determination, demonstrating the high selectivity of the proposed method. Fourth, the successful application of the proposed method to the determination of low levels of bismuth in biological and water samples was carried out with good results. Finally, in addition to lower tolerance limits, the proposed method is simple and more sensitive at the nanogram level than other commonly used methods.
Notes
Peer review under responsibility of Taibah University.
References
- A.SlikkerveerF.A.de-WolfPharmacokinetics and toxicity of bismuth compoundsMed. Toxicol. Adverse Drug Exp.41989303307
- P.J.SadlerMetal complexes in medicine: design and mechanism of actionJ. Inorg. Biochem.671997411
- D.W.ThomasE.MerianMetals and Their Compounds in the Environment1991VCHWeinheim789/801
- A.MorrowG.WitshireA.HuvsthousAn improved method for the simultaneous determination of Sb, As, Bi, Ge, Se, and Te by hydride generation ICP-AES: application to environmental samplesAt. Spectrosc.1819972328
- M.V.HindsD.C.GregoieE.A.OzakiDirect determination of volatile elements in nickel alloys by electrothermal vaporization inductively coupled plasma mass spectrometryJ. Anal. At. Spectrom.121997131135
- S.TokaliogluS.KartalL.ElciDetermination of some trace elements in high-purity aluminium, zinc and commercial steel by AAS after preconcentration on amberlite XAD-1180 resinMicrochim. Acta1271997281286
- G.E.M.HallA.T.MaclaurinJ.C.PelchatG.GauthierComparison of the techniques of atomic absorption spectrometry and inductively coupled plasma mass spectrometry in the determination of Bi, Se and Te by hydride generationChem. Geol.13719977989
- H.AshkenaniM.A.TaherApplication of a new ion-imprinted polymer for solid-phase extraction of bismuth from various samples and its determination by ETAASInt. J. Environ. Anal. Chem.93201311321145
- P.OstapczukPresent potentials and limitations in the determination of trace elements by potentiometric stripping analysisAnal. Chim. Acta27319933540
- R.D.YeS.B.KhooContinuous flow and flow injection stripping voltammetric determination of silver(I), mercury(II), and bismuth(III) at a bulk modified graphite tube electrodeElectroanalysis91997481489
- C.M.WangQ.Y.SunH.L.LiVoltammetric behavior and determination of bismuth on sodium humate modified carbon paste electrodeElectroanalysis91997645649
- T.FerriS.PaciR.MorabitoUse of a modified chelating resin to study trace-metals in environmental aqueous matricesAnn. Chim.861996595603
- D.V.ReddyA.V.ReddyAmperometric determination of bismuth using gallacetophenone phenylhydrazone with the structural elucidation of complexE-J. Chem.7201012901295
- S.G.SarkarP.M.DhadkeSolvent extraction separation of antimony(III) and bismuth(III) with bis-(2,4,4-trimethylpentyl) monothiophosphinic acid (Cyanex 302)Sep. Purif. Technol.151999131138
- J.M.LoY.P.LinK.S.LinPreconcentration of trace metals in seawater matrix for inductively coupled plasma atomic emission spectrometryAnal. Sci.71991455459
- S.I.IthoS.KanecoK.OhtaT.MizunoDetermination of bismuth in environmental samples with Mg–W cell-electrothermal atomic absorption spectrometryAnal. Chim. Acta3791999169173
- J.WangE.H.HansenFlow injection on-line two-stage solvent extraction preconcentration coupled with electrothermal atomic absorption spectrometry for determination of bismuth in biological and environmental samplesAnal. Lett.33200027472766
- S.A.BarakatFlow injection extraction–spectrophotometric determination of bismuth with di-(hydrogenated tallow alkyl) dimethyl-ammonium chlorideTurk. J. Chem.262002345350
- E.IvanovaX.P.YanF.AdamsDetermination of bismuth in cod muscle, lake and river sediment by flow injection on-line sorption preconcentration in a knotted reactor coupled with electrothermal atomic absorption spectrometryAnal. Chim. Acta3541997713
- M.B.O.GiacomelliE.M.GanzarolliA.J.CurtiusAutomated flow injection system for the preconcentration of bismuth and lead from acid solutions of alloys and determination by electrothermal atomic absorption spectrometrySpectrochim. Acta (B)552000525533
- M.A.TaheraM.RahimiH.FazeliradA sensitive fluorescence quenching method for determination of bismuth with tironJ. Lumin.1452014976980
- S.MoyanoJ.A.GasquezR.OlsinaE.MarchevskyL.D.MartinezPre-concentration system for bismuth determination in urine using FI-ICP-AES with ultrasonic nebulizationJ. Anal. At. Spectrom.141999259264
- E.VassilevaL.ProinovaK.HadjiivanovSolid-phase extraction of heavy metal ions on a high surface area titanium dioxide (anatase)Analyst1211996607612
- R.KocjanM.GarbackaSilica-gel modified with methyl thymol blue for separation and preconcentration of trace amounts of heavy-metals from some saltsSep. Sci. Technol.291994799807
- J.B.B.SilvaM.B.O.GiacomelliA.J.CurtiusDetermination of bismuth in aluminium and in steels by electrothermal atomic absorption spectrometry after on-line separation using a minicolumn of activated carbonAnalyst124199912491253
- S.H.GaikwadS.V.MahamuniM.A.AnuseExtractive spectrophotometric determination of bismuth (III) in alloy sample using 1-amino-4,4,6-trimethyl (1H, 4H) pyrimidine-2-thiolIndian J. Chem. Technol.122005365368
- A.S.AminCloud-point extraction and spectrophotometric determination of trace quantities of bismuth in environmental water and biological samplesSpectrosc. Lett.442011424431
- N.AliH.SimaR.MajidSpectrophotometric determination of bismuth in water samples by dispersive liquid–liquid microextraction after multivariate optimization based on Box–BehnkenJ. Chil. Chem. Soc.58201318991901
- T.MadrakianA.AfkhamiA.EsmaeiliSpectrophotometric determination of bismuth in water samples after preconcentration of its thiourea–bromide ternary complex on activated carbonTalanta602003831838
- M.A.RaufM.IkramM.AhmadSpectrophotometric studies of ternary complexes of lead and bismuth with o-phenanthroline and eosinDyes Pigments522002183189
- H.-S.ZhangJ.-F.ZhangH.WangX.-Y.LiSynthesis and analytical application of 2,6-dichloroarsenazo as a new chromogenic reagent for bismuthAnal. Chim. Acta3801999101104
- C.TongyueG.JialongW.XiaomingOn the colour reaction between bismuth(III) and a thioazolylazo-derivativeMikrochim. Acta (Wien)I1985375381
- P.D.TzanavarasD.G.ThemelisA.EconomouSequential injection method for the direct spectrophotometric determination of bismuth in pharmaceutical productsAnal. Chim. Acta5052004167171
- M.S.El-ShahawiS.M.AldhaheriSpectrophotometric determination of bismuth(III and V) in water after ion-pair liquid–liquid extraction using tetramethylammonium cation as counter ionFresenius J. Anal. Chem.3541996200203
- G.B.KolekarT.N.LokhandeP.N.BhosaleM.A.AnuseExtraction, separation and spectrophotometric determination of bismuth(III) using 1(40-bromophenyl) 4,4,6-trimethyl (1H,4H)-pyrimidine-2-thiolAnal. Lett.31199822412254
- Z.NanDirect microspectrophotometric determination of bismuth in silver with semi-xylenol orangeMicrochim. Acta14620044347
- N.TokmanS.AkmanM.OzcanFlow injection spectrophotometric determination of fosfestrol, following on-line thermal induced digestion and using an orthophosphate calibration graphTalanta592003201205
- D.BurnsN.TungkananurukS.ThuwasinSpectrophotometric determination of bismuth after extraction of tetrabutylammonium tetraiodo bismuthate(III) with microcrystalline benzophenoneAnal. Chim. Acta41920004144
- A.DuranM.TuzenM.SoylakPreconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbentJ. Hazard. Mater.1692009466471
- M.TuzenK.O.SaygiC.UstaM.SoylakPseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ionsBiores. Technol.99200815631570
- M.TuzenK.O.SaygiM.SoylakSolid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubesJ. Hazard. Mater.1522008632639
- M.TuzenM.SoylakMultiwalled carbon nanotubes for speciation of chromium in environmental samplesJ. Hazard. Mater.1472007219225
- M.SoylakA.U.KaratepeL.ElçiM.DoganColumn preconcentration/separation and atomic absorption spectrometric determinations of some heavy metals in table salt samples using Amberlite XAD-1180Turk. J. Chem.272003235242
- L.ElciM.SoylakM.DoganPreconcentration of trace metals in river waters by the application of chelate adsorption on Amberlite XAD-4Fresenius J. Anal. Chem.3421992175178
- V.N.BulutA.GundogduC.DuranH.B.SenturkM.SoylakL.ElciM.TufekciA multi-element solid-phase extraction method for trace metals determination in environmental samples on Amberlite XAD-2000J. Hazard. Mater.1462007155163
- M.SoylakL.EiciM.DoganSolid phase extraction of trace metal ions with amberlite XAD resins prior to atomic absorption spectrometric analysisJ. Trace Microprobe Tech.192001329344
- A.I.VogelText Book of Quantitative Chemical Analysis6th ed.2000LongmanLondon381
- A.S.AminT.Y.MohammedA.A.MousaSpectrophotometric studies and applications for the determination of yttrium in pure and in nickel base alloysSpectrochim. Acta (A)59200325772584
- D.L.SparksEnvironmental Soil Chemistry1995Academic PressNew York
- IUPACNomenclature, symbols, units and their usage in spectrochemical analysis—II. Data interpretation Analytical chemistry divisionSpectrochim. Acta (B)331978241245
- J.N.MillerJ.C.MillerStatistics and Chemometrics for Analytical Chemistry5th ed.2005Prentice-HallLondon