 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The effects of medium components affecting lipase production by Aspergillus niger AS-02 using sheanut cake (SNC) were studied based on the one-factor-at-a-time (OFAT) method and the face-centred central composite design (FCCCD). Of the six medium components analysed by OFAT, three (Tween-80, (NH4)2SO4 and Na2HPO4) were subjected to FCCCD. Under optimized conditions, the obtained lipase production showed good agreement between the experimental (49.37 U/g) and predicted (48.81 U/g) yields, with a coefficient of determination (R2) of 0.96. The optimum medium components were 1.0% (v/w) Tween-80, 0.35% (w/w) (NH4)2SO4 and 0.40% (w/w) Na2HPO4. The findings in this study showed that, in addition to lipase production, the fermented SNC had reduced tannin and saponin contents, which are the major components that limit the use of SNC in feed formulations.
1 Introduction
Renewable agricultural residues have been used to synthesize different products, including biopolymers [Citation1], enzymes [Citation2] and biofuels [Citation3]. The valorization of these residues is based on their abundance, comparative low cost and environmental friendliness. This is justified by the number of reports on the utilization of agricultural residues for hydrolytic enzyme production (e.g., proteases, lipases and carbohydrases), which constitutes more than 95% of the total enzyme market [Citation4].
Lipases (triacylglycerol acyl hydrolases (EC 3.1.1.3), which account for up to 10% of the enzyme market, are widely used in different processes of industrial importance based on their ability to catalyse both synthetic and hydrolytic reactions [Citation5,Citation6]. Some of the industrial applications that employ the use of lipases include biofuel production, detergent formulation, fine chemical synthesis, perfumery and cosmetics, leather processing, pharmaceuticals and medical diagnostics, food and feed processing [Citation7,Citation8]. Thus, the uniqueness of lipases over other hydrolases could be related to their use as model for studying the regulation of interfacial enzyme-catalysed reactions; biocatalytic synthesis associated with transesterification, esterification, and aminolysis in organic solvents; chemo-, regio-, and enantiospecificities; as well as medical significance [Citation5,Citation9]. However, one of the major obstacles affecting the utilization of lipase technology is the production cost, where the carbon source was reported to account for more than half of the total production costs [Citation10].
Based on this, several agricultural residues, including brans (wheat, rice, soybean, barley), cakes (soy, olive, gingelly, babassu), and bagasse (sugarcane), have been reported to be used for lipase production [Citation11]. Thus, oil cakes (the by-products obtained after oil extraction) are among the most widely utilized substrates as they contain some residual oils that aid in inducing lipase production [Citation12]. The use of babassu cake and castor meal for lipase production by Penicillium brevicompactum led to maximum yields of 48.6 U/g and 87.7 U/g, respectively [Citation13]. However, lower extracellular lipase activities of 4.5, 2.35, 1.33, 1.68 and 1.24 U/g were reported by Chaturvedi et al. [Citation14] using groundnut oil cake, cocoanut oil cake, neem oil cake, mustard oil cake and linseed oil cake, respectively, in the presence of Bacillus subtilis. In the case of castor bean waste, statistical optimization of the medium and process parameters in the presence of Penicillium simplicissimum led to enhanced lipase production of 44.8 U/g [Citation15].
Sheanut cake (SNC) is a residue obtained after extraction of fat from sheanuts (Vitellaria paradoxa, Gaertn.), and it is highly abundant in most West African countries. The annual production of shea kernels exceeds 2.5 million tons and approximately 55% ended up as SNC [Citation16]. Shea butter has been utilized for cooking purposes, cosmetic formulations (moisturizer), cocoa butter substitutes and other edible fat spreads [Citation17]. However, SNC is basically used in feed formulation with a less than 10% inclusion rate in swine [Citation18] and poultry feed [Citation19]. Thus, wider utilization of SNC has been limited due to its anti-nutritional constituents such as tannin, saponin and theobromine [Citation20].
Using SNC as the main substrate for lipase production may boost its economic value, which can be better than its current potential as an animal feed ingredient. This study involves the use of the one-factor-at-a-time (OFAT) method and statistical experimental design (face-centred central composite design (FCCCD)) to optimize the lipase production by A. niger based on the identified medium components affecting the lipase production as previously reported by Salihu et al. [Citation21]. Thus, A. niger was selected as a potential strain because of its robustness, high yield of extracellular enzymes, non-pathogenicity, non-toxicity and GRAS (generally regarded as safe) nature as accorded by US Food and Drug Administration. Additionally, this strain showed the potential to reduce the levels of anti-nutrients and to produce lipase via solid state fermentation techniques.
2 Materials and methods
2.1 Sample collection
Sheanut cake (SNC), a by-product of shea butter fat extraction, was obtained from local producers in Zaria, Nigeria. The cakes were properly washed and dried at 60 °C in an oven. The dried samples were then milled and sieved through standard mesh sieves to obtain 1 mm particle sizes, which were stored in air tight plastic bags.
2.2 Microorganism and inoculum preparation
Aspergillus niger AS-02 obtained from the culture stock of Mycology Laboratory, Department of Crop Protection, Ahmadu Bello University, Zaria, Nigeria was used in this study. The inoculum preparation was based on the method described by Alam et al. [Citation22], where a culture plate containing A. niger was washed with 25 ml of sterile distilled water using a bent glass rod and the suspension was allowed to pass through Whatman No. 1 filter paper. The filtrate is made up of a spore suspension that was counted using a hemocytometer, and an inoculum concentration of 1 × 108 spores per ml was obtained.
2.3 Lipase production by solid state fermentation
Bioconversion experiments were carried out in 250-ml conical flasks containing 20 g of fermentation media (wet basis) using SNCs with particle size of 1 mm as the main substrate and the other medium constituents at varying concentrations, setting the initial moisture content to 60%. The flasks were autoclaved at 121 °C (15 psi) for 20 min. A. niger AS-02 inoculum (108 spores per ml; 5% (v/w)) was added into each flask, followed by incubation at 30 °C for 7 days. Following the fermentation, 0.05 M phosphate buffer (pH 7.0) was added to each flask and the contents were shaken on a rotary shaker (180 rpm) for 1 h at room temperature. The supernatant was used as a source of extracellular enzymes, which was obtained by centrifugation at 5000 × g for 10 min.
2.4 Determination of the optimum concentration of different medium components by the one-factor-at-a-time (OFAT) method
The Plackett–Burman design for screening different medium components that affect the lipase production by A. niger AS-02 using SNC showed that six medium components (sucrose, (NH4)2SO4, Na2HPO4, MgSO4, Tween-80, and olive oil) contributed positively to production [Citation21]. Thus, the OFAT approach was used to determine the optimum levels of these medium components; the concentration ranges selected were 0.2–1.2% for Tween-80, olive oil (v/w) and sucrose (w/w), 0.1–0.6% (w/w) for (NH4)2SO4 and Na2HPO4, and 0.05–0.2% (w/w) for MgSO4. The experiments were prepared accordingly in 250-ml conical flasks, setting the initial moisture content, temperature and inoculum concentration at 60%, 30 °C and 5%, respectively.
2.5 Optimization of media components affecting lipase production
Based on the OFAT results, Design Expert software (Stat-Ease Inc., Minneapolis, USA) was used to determine the optimum concentrations of three important parameters ((NH4)2SO4, Na2HPO4 and Tween-80) influencing the lipase production using face-centred central composite design (FCCCD) under the response surface methodology (RSM). The design consists of 20 experimental runs (eight star points, six axial points and six centre points) which were randomized to reduce all sets of variabilities that can affect the response. The selected parameters were studied at three levels, low, medium and high, as indicated in . The relationship between the dependent and independent variables is expressed in terms of second-order polynomial equation:(1)
(1) where Y represents the dependent variable (lipase production); X1, X2 and X3 are independent variables ((NH4)2SO4, Na2HPO4 and Tween-80); β0 is an intercept term; β1, β2 and β3 are linear coefficients; β12, β13 and β23 are the interaction coefficients; and β11, β22 and β33 are quadratic coefficients.
Table 1 Face-centred central composite design matrix with the experimental and predicted responses for lipase production.
The coefficient of determination (R2), lack of fit, and F-values obtained from the analysis of variance were used to determine the adequacy of the developed model. Thus, some sets of experiments were also performed to validate the model with respect to all three parameters in order to compare the experimental values with those predicted by the model.
2.6 Lipase activity assay
The titrimetric method described by Freire et al. [Citation23] was used to determine the lipase activity. The assay involves the incubation of an 18 ml emulsion of 10% olive oil and 5% acacia gum arabic in 0.05 M phosphate buffer (pH 7.0) with 2 ml of the supernatant at 37 °C for 15 min in a temperature-controlled orbital shaker. A mixture of acetone and ethanol (1:1) was used to stop the reaction and to extract the fatty acids. This was then titrated with 0.05 M NaOH. One unit of lipase activity was defined as the amount of enzyme that produced 1 μmol of fatty acids per minute under the assay conditions. The results were expressed in units per gram of SNC (U/g).
2.7 Determination of anti-nutritional contents
The utilization of SNC is strongly affected by the presence of tannin and saponin as major anti-nutritional compounds. Thus, the saponin content was determined based on AOAC [Citation24], which involved sequential extraction using acetone and methanol in a Soxhlet apparatus; while spectrophotometric method described by Burns [Citation25] was used to estimate the tannin contents at an absorbance of 605 nm using tannic acid as the standard.
3 Results and discussion
3.1 Selection of the nutritional components influencing lipase production
The effects of different nutritional components on lipase production using SNC were studied to develop a medium that requires minimal nutritional supplementation for enhanced enzyme production.
Different concentrations of sucrose (0.2–1.2%) were studied because of its positive contribution to enhancing lipase production using a Plackett–Burman experiment [Citation21]. Carbon sources, such as sucrose, are required during microbial fermentation because of their contribution in microbial cellular synthesis and metabolism [Citation26]. shows an initial increase in lipase production with an increase in sucrose concentration from 3.03 U/g at 0.2% to 4.46 U/g at 0.4%, and a further increase in sucrose concentration resulted in a decrease in enzyme activity. Most studies showed that glucose and other glucose-containing compounds (e.g., sucrose) have a negative influence on lipase production. However, de Azeredo et al. [Citation27] showed that these carbon sources could influence the lipase activity in Penicillium restrictum using solid state fermentation due to its minimum catabolite repression. In the case of Tween-80 (), the maximum lipase activity of 7.36 U/g coincided with a 1.0% concentration of Tween-80. This is in agreement with what has been reported by Maliszewska and Mastalerz [Citation28], where lipase production by Penicillium citrinum was enhanced by Tween-80. Similarly, the presence of Tween-80 resulted in 150.8% enhancement in lipase activity by Pseudozyma hubeiensis HB85A [Citation29]. While the presence of olive oil did not result in higher lipase production (), this could be related to its oleic acid constituent, which is also present in Tween-80.
Fig. 1 Effects of different concentrations of Tween-80, olive oil and sucrose (0.2–1.2%) on lipase activity by A. niger using SNC as the main substrate.
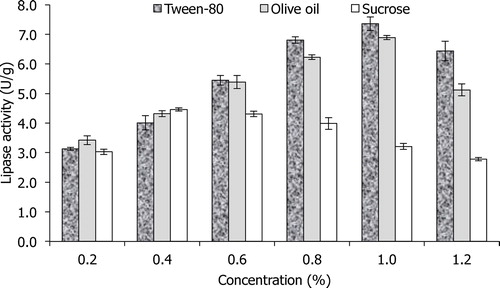
Among these three carbon sources, Tween-80, whose carbon skeleton contains oleic acid, was found to be the best carbon source and it had the potential to act as an inducer for lipase production by A. niger AS-02 in SNC medium. Based on these results, Tween-80 was selected for optimization using FCCCD, while the other components were fixed at their optimum concentrations.
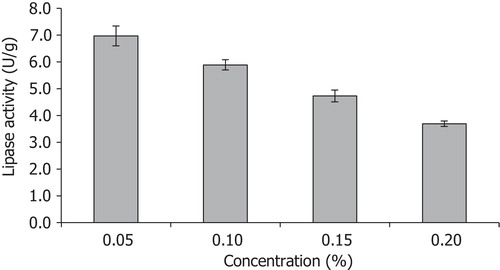
The trend of lipase activity in different mineral salts showed that both (NH4)2SO4 and Na2HPO4 contributed greatly to lipase production (), where maximum lipase activities of 7.41 U/g and 7.04 U/g were recorded at 0.4% (w/w) each for (NH4)2SO4 and Na2HPO4, respectively. The finding of this study agrees with what was reported by Iftikhar and Hussain [Citation30], where 0.4% ammonium sulphate resulted in higher lipase production by Rhizopus oligosporous Tuv-31 during solid state bioconversion of almond meal. Additionally, Alkan et al. [Citation31] reported that ammonium nitrate was the best nitrogen source for lipase production by Bacillus coagulans. In case of P. citrinum, supplementation of vegetable oil waste with ammonium chloride and ammonium sulphate influenced its lipase production [Citation32]. Similarly, He and Tan [Citation33] developed a soybean-based medium that contained some inorganic salts (K2HPO4, KH2PO4, (NH4)2SO4, MgSO4) for enhanced lipase production by Candida sp. 99–125. The results showed that absence of any of these salts affected both the microbial growth and lipase activity. Maximum lipase production by Hendersonula toruloidea was achieved in the presence of MgSO4, (NH4)2SO4, NaCl, K2HPO4 and BaCl2 [Citation34]. Thus, in this study lowest concentration of MgSO4 (0.05% w/w) resulted in maximum lipase production (), and a further increase in the concentration affected the overall yield. This showed that low exogenous magnesium salt addition coupled with its reported concentration of 1.43 ± 0.65 mg/kg in SNC [Citation16] was sufficient for lipase production.
Fig. 2 Effects of different concentrations of (NH4)2SO4 and Na2HPO4 (0.1–0.6%) on lipase activity by A. niger using SNC as the main substrate.
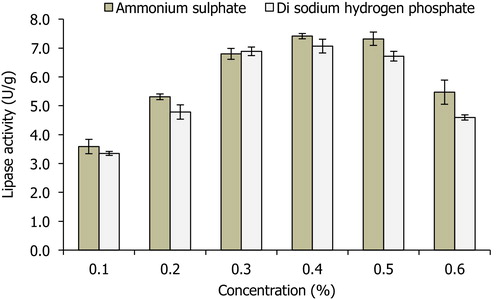
Fig. 3 Effects of different concentrations of MgSO4 (0.05–0.20%) on lipase activity by A. niger using SNC as the main substrate.
To develop an optimized medium with minimal supplementation, three factors (Tween-80, (NH4)2SO4 and Na2HPO4) were subjected to statistical optimization using FCCCD.
3.2 Optimization of the SNC-based medium by FCCCD
This design was selected based on its ability to determine the effects of the selected parameters as well as their feasible interactions within a limited sets of experiments. shows the design matrix for lipase production; both the experimental and predicted responses were indicated for each run. Based on the design, lipase production ranged from 41.53 to 49.44 U/g, and the runs representing the centre points showed the highest production (runs 15–20), while the lowest was found in run 4.
The relationship between each response and the selected parameters was established by the second-order polynomial model represented in the equation:(2)
(2) where A, B and C represent Tween-80, (NH4)2SO4 and Na2HPO4, respectively.
The analysis of variance (ANOVA) of the developed quadratic model was found to be significant at p < 0.05 as shown in . The model F value of 27.158 with a p-value of <0.0001 indicated an only 0.01% chance for the model F-value to occur due to noise.
Table 2 ANOVA of the quadratic model developed for the optimization of lipase production.
Thus, all of the linear terms (Tween-80 (A), (NH4)2SO4 (B) and Na2HPO4 (C)), two of the quadratic terms (A2 and C2) and one of the interaction terms were statistically significant. The non-significant lack of fit (p-value = 0.1649) from the developed quadratic model was desirable, as it is determined based on the comparison of residual error to the pure error. This indicates that the experimental responses obtained in this study adequately fit with the model. Additionally, the coefficient of determination (R2) is used to check the fitness of the model, a value closer to 1 shows better correlation between the experimental and predicted responses. In this study, R2 of 0.9607 and Adj R2 of 0.9253 were obtained, which indicate that more than 96% of the variations could be accounted for by the model equations, leaving less than 4% as unaccounted. Thus, Haaland [Citation35] indicated that a coefficient of determination (R2) greater than 0.75 was considered adequate and could explain most of the variability associated with any model. Adequate precision values of 17.802 showed the desirability of the model because values greater than 4 are required. In the case of the coefficient of variation, lower values are preferred because they indicate the precision and reliability of the experimental responses, and a value of 1.15 was obtained.
Two and three dimensional surface plots illustrated in – showed the interactions of the selected parameters for maximum lipase production. The plots were based on the function of concentrations of two parameters while fixing the third parameter at its optimum concentration. The elliptical or saddle nature of the plots indicates the level of significance of the interactions [Citation36]. In all of the figures (–), low and high levels of the selected parameters did not give higher enzyme yields. The elliptical contours indicated the synergic interaction between the selected parameters, which resulted in maximum lipase production by A. niger AS-02 using SNC. The most significant interaction based on the p-value (0.0044) was between Na2HPO4 and (NH4)2SO4 (). Thus, lipase production is affected up to a certain extent by increasing the concentrations of Tween-80, Na2HPO4 and (NH4)2SO4.
Fig. 4 Plots showing the effects of Tween-80 and (NH4)2SO4 at a fixed concentration of Na2HPO4 on lipase production by A. niger using SNC as the main substrate. (a) Response surface 3D and (b) contour plot (2D).
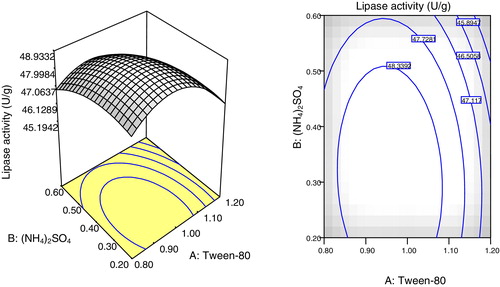
Fig. 5 Plots showing the effects of Tween-80 and Na2HPO4 at a fixed concentration of (NH4)2SO4 on lipase production by A. niger using SNC as the main substrate. (a) Response surface 3D and (b) contour plot (2D).
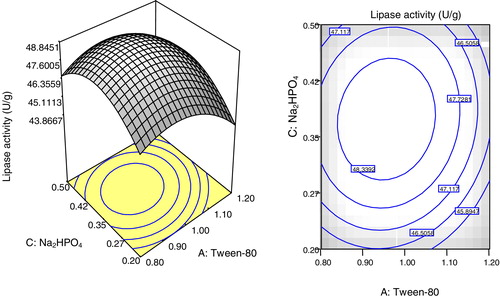
Fig. 6 Plots showing the effects of Na2HPO4 and (NH4)2SO4 at a fixed concentration of Tween-80 on lipase production by A. niger using SNC as the main substrate. (a) Response surface 3D and (b) contour plot (2D).
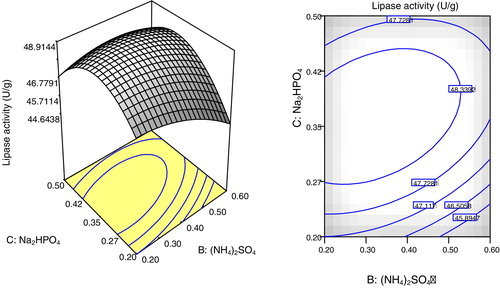
The applicability of the developed model was verified by the sets of experiments represented in . The results showed good agreement between the experimental and predicted responses, and the maximum lipase production was 49.37 U/g at 1.0% (v/w) of Tween-80, 0.35% (w/w) of (NH4)2SO4 and 0.40% (w/w) of Na2HPO4. Thus, statistical optimization using FCCCD resulted in higher enzyme production, which was several fold higher than the unoptimized medium. The lipase production obtained in this study could be considered promising, especially with regard to the substrate (SNC) used. The findings were in agreement with previous reports of lipase production from several oil cakes. Bioconversion of gingelly oil cake for lipase production by the A. niger strain MTCC 2594 resulted in a maximum lipase activity of 363.6 U/g after 72 h [Citation37]. Imandi et al. [Citation38] reported the application of Plackett–Burman design to screen different medium components for enhanced lipase production by Yarrowia lipolytica NCIM 3589 using Niger seed oil cake (Guizotia abyssinica). Following the screening and optimization procedures, a maximum lipase production of 26.42 U/g was recorded. Similarly, lipase production of 57.89 U/g by Y. lipolytica NCIM 3589 was realized using mustard oil cake [Citation39]. However, low lipase production of 4.5 U/g was observed when B. subtilis MTCC 6008 was cultured in groundnut oil cake-based medium [Citation14]. In the case of P. restrictum, supplementation of babassu oil waste with peptone and olive oil significantly improved the lipase production to 27.8 U/g [Citation40].
Table 3 Validation of the experimental model.
3.3 Effect of fermentation on tannin and saponin contents
The fermentation process tends to lower the concentration of anti-nutrients in different residues [Citation41]. Thus, A. niger was reported to improve the nutritive value of SNC by reducing its saponin and tannin contents [Citation42]. In this study, the trend of saponin and tannin reduction was monitored by analysing the samples every 24 h for a period of 7 days as shown in . Thus, an approximately 68% reduction in saponin and 43% reduction in tannin contents were obtained based on their initial concentrations of 4.99 ± 0.12 mg/g and 242 ± 3.41 mg/g, respectively. Additionally, the crude protein content of fermented SNC (18.51 ± 0.78%) was found to be higher than that of the unfermented SNC (13.68 ± 0.18%). The protein content of SNC increases with an increase in lipase production, and a more than 35% increase in protein content was observed after 7 days of fermentation ().
Fig. 7 Reductions in tannin and saponin concentrations (%) and an increase of protein concentration (%) with time during the bioconversion of SNC for lipase production by A. niger.
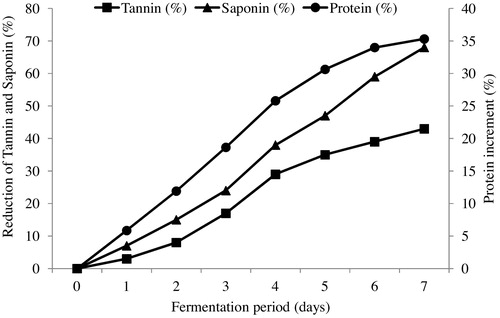
Based on this, the reduction in tannin content could be attributed to the potential of A. niger to produce several hydrolytic enzymes, including tannase, which hydrolyse tannin to glucose and gallic acid [Citation43]. Moreover, the organic acids produced by the organism during the fermentation process aid in reducing the saponin contents as described by Annongu et al. [Citation44]. The findings in this study showed that, in addition to the lipase production, the fermented SNC could be incorporated in feed formulation based on its crude protein concentration and its reduced tannin and saponin contents because high concentrations of these anti-nutrients in SNC greatly affect its incorporation in animal feed [Citation20].
4 Conclusion
This study involves the use of OFAT and FCCCD for determining the possible optimum levels of the medium components affecting lipase production by A. niger AS-02 using SNC as the main substrate. Following the validation of the statistical technique, the medium components that led to the maximal lipase production of 49.37 U/g were 1.0% (v/w) Tween-80, 0.35% (w/w) (NH4)2SO4 and 0.40% (w/w) Na2HPO4. The second-order model developed here showed a good correlation of the experimental and predicted responses with R2 and adjusted R2 values of 0.9607 and 0.9253, respectively. The findings in this study showed that the inexpensive raw material SNC could be used for lipase production, and the fermented residue contains residual nutrients with reduced anti-nutritional components that make it suitable for incorporation in feed formulation.
Acknowledgement
The authors are grateful to the Department of Biotechnology Engineering for providing the laboratory facilities.
Notes
Peer review under responsibility of Taibah University.
References
- A.BiswasS.KimG.W.SellingH.N.ChengConversion of agricultural residues to carboxymethylcellulose and carboxymethylcellulose acetateInd. Crops Prod.602014259265
- P.D.DelabonaR.D.P.Buzon-PirotaC.A.CodimaC.R.TremacoldiA.RodriguesC.S.FarinasUsing amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymesBiomass Bioenergy372012243250
- C.S.GohK.T.TanK.T.LeeS.BhatiaBioethanol from lignocellulose: status, perspective and challenges in MalaysiaBioresour. Technol.101201048344841
- J.VakhluA.KourYeast lipases: enzyme purification, biochemical properties and gene cloningElectron. J. Biotechnol.920066981
- N.N.GandhiApplications of lipaseJ. Am. Oil Chem. Soc.741997621634
- E.HabaO.BrescoC.FerrerA.MarquesM.BusquetsA.ManresaIsolation of lipase secreting bacteria by deploying used frying oil as selective substrateEnzym. Microb. Technol.2620004044
- M.MasomianR.N.Z.R.A.RahmanA.B.SallehM.A.BasriA new thermostable and organic solvent tolerant lipase from Aneurinibacillus thermoaerophilus strain HZProcess Biochem.482013169175
- J.F.M.BurkertF.MaugeriM.I.RodriguesOptimization of extracellular lipase production by Geotrichum sp. using factorial designBioresour. Technol.9120047784
- R.K.SaxenaW.S.DavidsonA.SheoranB.GiriPurification and charactrization of an alkaline thermostable lipase from Aspergillus carneusProcess Biochem.392003239247
- O.A.MirandaA.A.SalgueiroM.C.B.PimentelJ.L.Lima FilhoE.H.M.MeloN.DuranLipase production by a Brazilian strain of Penicillium citrinum using an industrial residueBioresour. Technol.691999145147
- A.SalihuM.Z.AlamM.I.AbdulKarimH.M.SallehLipase production: an insight in the utilization of renewable agricultural residuesResour. Conser. Recycl.5820123644
- R.R.SinghaniaC.R.SoccolA.PandeyApplication of tropical agro-industrial residues as substrate for solid-state fermentation processesA.PandeyR.R.SoccolC.LarrocheCurrent Development in Solid-State Fermentationvol. 42008412442
- M.F.SilvaD.M.G.FreireA.M.de CastroM.D.LuccioM.A.MazuttiJ.V.OliveiraH.TreichelD.OliveiraProduction of multifunctional lipases by Penicillium verrucosum and Penicillium brevicompactum under solid state fermentation of babassu cake and castor mealBioprocess Biosyst. Eng.342011145152
- M.ChaturvediM.SinghM.R.ChughS.PandeyLipase production from Bacillus subtilis MTCC 6808 by solid state fermentation using ground nut oil cake as substrateRes. J. Microbiol.52010725730
- M.G.GodoyM.L.E.GutarraF.M.MacielS.P.FelixJ.V.BevilaquaO.L.T.MachadoD.M.G.FreireUse of a low-cost methodology for biodetoxification of castor bean waste and lipase productionEnzyme Microb. Technol.442009317322
- I.Abdul-MumeenH.D.ZakpaaF.C.Mills-RobertsonBiochemical and microbiological analysis of shea nut cake: a waste product from shea butter processingJ. Agric. Biotechnol. Sus. Dev.520136168
- P.N.LovettN.HaqEvidence for anthropic selection of the Sheanut tree (Vitellaria paradoxa)Agroforest. Sys.482000273288
- S.W.A.RhulePerformance of pigs on diets containing detoxified sheanut cakeTrop. Anim. Health Product.3119994553
- H.K.DeiS.P.RoseA.M.MackenzieShea nut (Vitellaria paradoxa) meal as a feed ingredient for poultryWorld Poult. Sci. J.632007611624
- C.C.AtuaheneA.DonkohF.AsanteValue of sheanut cake as a dietary ingredient for broiler chickensAnim. Feed Sci. Technol.721998133142
- A.SalihuM.BalaS.M.BalaApplication of Plackett-Burman experimental design for lipase production by Aspergillus niger using shea butter cakeISRN Biotechnol.20131510.5402/2013/718352
- M.Z.AlamA.Fakhru’l-RaziA.H.MollaEvaluation of fungal potentiality for bioconversion of domestic wastewater sludgeJ. Environ. Sci.162004132137
- D.M.FreireE.M.TelesE.P.BonG.L.Sant’AnnaJr.Lipase production by Penicillium restrictum in a bench-scale fermenter: effect of carbon and nitrogen nutrition, agitation, and aerationBiochem. Biotechnol.63/651997409421
- AOACOfficial methods of analysisK.HelrichMethod 969.3315th ed.1990Association of Official Analytical ChemistsArlington
- R.B.BurnsMethods of estimation of tannin in the grain SorghumAgro. J.631971511512
- P.F.StanburyA.WhitakerS.J.HallPrinciples of Fermentation Technology2nd ed.1997AdityaNew Delhi, India93105
- L.A.I.de AzeredoP.M.GomesG.L.Sant’AnnaJr.L.R.CastilhoD.M.G.FreireProduction and regulation of lipase activity from Penicillium restrictum in submerged and solid-state fermentationsCurr. Microbiol.542007361365
- I.MaliszewskaP.MastalerzProduction and some properties of lipase from Penicillium citrinumEnzyme Microb. Technol.141992190193
- R.BussamaraA.M.FuentefriaE.S.de OliveiraL.BroettoM.SimcikovaP.ValenteA.SchrankM.H.VainsteinIsolation of a lipase-secreting yeast for enzyme production in a pilot plant scale batch fermentationBioresour. Technol.1012010268275
- T.IftikharA.HussainEffects of nutrients on the extracellular lipase production by Mutant strain of Rhizopus oligosporous Tuv-31J. Biotechnol.120021520
- H.AlkanZ.BaysalF.UyarM.DogruProduction of lipase by a newly isolated Bacillus coagulans under solid state fermentation using melon wastesAppl. Biochem. Biotech.1362007183192
- O.A.MirandaA.A.SalgueiroM.C.B.PimentelJ.L.LimaFilhoE.H.M.MeloN.Dur’anLipase production by Brazillian strain of Penicillium citrinum using an industrial residueBioresour. Technol691999145147
- Y.HeT.TanUse of response surface methodology to optimize culture medium for production of lipase with Candida sp. 99-125J. Mol. Catal. B: Enzym.432006914
- F.J.C.OdiboU.O.OkerekeC.A.OyekaInfluence of culture conditions on the production of lipase of Hendersonula toruloideaBioresour. Technol.5419958183
- P.D.HaalandExperimental Design in Biotechnology1989Marcel Dekker Inc.New York259 pp
- R.V.MuralidharR.R.ChirumamilaR.MarchantP.NigamA response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sourcesBiochem. Eng. J.920011723
- N.R.KaminiJ.G.S.MalaR.PuvanakrishnanLipase production from Aspergillus niger: by solid-state fermentation using gingelly oil cakeProcess Biochem.331998505511
- S.B.ImandiS.K.KaranamH.R.GarapatiOptimization of process parameters for the production of lipase in solid state fermentation by Yarrowia lipolytica from Niger seed oil cake (Guizotia Abyssinica)J. Microb. Biochem. Technol.220102833
- S.B.ImandiS.K.KaranamH.R.GarapatiUse of Plackett–Burman design for rapid screening of nitrogen and carbon sources for the production of lipase in solid state fermentation by Yarrowia lipolytica from mustard oil cake (Brassica napus)Braz. J. Microbiol.442013915921
- M.B.PalmaA.L.PintoA.K.GombertK.H.SeitzS.C.KivatinitzL.R.CastilhoD.M.G.FreireLipase production by Penicillium restrictum using solid waste of industrial babassu oil production as substrateAppl. Biochem. Biotechnol.84/86200011371146
- N.R.ReddyM.D.PiersonReduction in antinutritional and toxic components 5 in plant foods by fermentationFood Res. Inter.271994281290
- H.K.DeiS.P.RoseA.M.MackenzieEffects of fungal (Aspergillus niger or Ceriporiopsis subvermispora) fermentation on the nutritive value of shea nut (Vitellaria paradoxa) meal for broiler chicksBritish Poult. Sci.492008360367
- M.A.Ramirez-CoronelG.Vinigera-GonzalezI.A.DarvilleC.AugurA novel tannase from Aspergillus niger with beta-glucosidase activityMicrobiology149200329412946
- A.A.AnnonguU.TermeulenJ.O.AttehD.F.ApataToxicological assessment of native and industrial fermented shea butter cake in nutrition of broilersArchiv. fur Geflugelkunde601996221226
