?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A simple, precise and accurate reverse phase high performance liquid chromatographic method was developed for the simultaneous estimation of bromhexine hydrochloride, chlorpheniramine maleate, dextromethorphan hydrobromide and guaiphenesin in their tablet dosage form. The chromatographic conditions were standardised using a Chromatopak C18 (25 cm × 4.6 mm i.d. × 5 μm) with UV detection at 265 nm, and the mobile phase consisted of methanol:acetonitrile:0.025 M phosphate buffer (50:25:25, v/v/v). The retention times of bromhexine hydrochloride, chlorpheniramine maleate, dextromethorphan hydrobromide and guaiphenesin were 16.254 min, 12.219 min, 6.156 min and 9.432 min, respectively. The calibration curves were linear with correlation coefficients of 0.9987, 0.9988, 0.9981 and 0.9981 over a concentration range of 4.0–24.0 μg/ml for bromhexine hydrochloride, 5.0–30.0 μg/ml for chlorpheniramine maleate, and 10.0–60.0 μg/ml for both dextromethorphan hydrobromide and guaiphenesin, respectively. The proposed method has been validated according to the ICH guidelines and was successfully applied to estimate the levels of four drugs in a combined formulation with good accuracy and precision.
1 Introduction
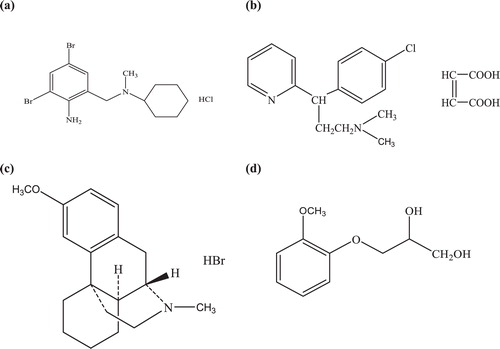
Combinations of decongestant and antihistamine preparations are widely used for cough and cold treatments. Bromhexine HCl (BROM), chemically named 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl benzenemethanamine hydrochloride (), is a mucolytic agent used in the treatment of respiratory disorders associated with viscid or excessive mucus [Citation1,Citation2]. This agent’s mechanism is to increase the production of serous mucus in the respiratory tract and it makes the phlegm thinner and less viscous. BROM is a mucous modifying drug that helps to improve the flow properties of bronchial mucous and eases expectoration. A literature survey reveals several HPLC methods that were reported for their simultaneous determination along with several other active ingredients, which exist as various combinations in cough–cold mixtures [Citation3]. These methods include liquid chromatography [Citation4], liquid gas chromatography [Citation5], gas chromatography with mass detection [Citation6], combined formulations using HPLC [Citation7–Citation9] and UV spectrophotometry [Citation10–Citation13]. Chlorpheniramine maleate (CHL), 3-(p-chlorophenyl)-3-(2-pyridyl)-N,N-dimethyl propylamine () is a powerful first-generation alkyl amine antihistamine that antagonises the H1-receptor and is widely used for symptomatic relief of the common cold and allergic rhinitis with weak sedative properties [Citation14]. The symptoms of allergic rhinitis include rash, watery eyes, itchy eyes and throat, cough, and sneezing. CHL is also effective against nausea and motion sickness, and its primary mechanism of action being is to reduce acetylcholine levels in the brain. A literature survey shows that several HPLC methods have been reported for chlorpheniramine maleate alone and in combination in pharmaceuticals, such as liquid chromatographic [Citation15–Citation17], HPTLC [Citation18], spectrophotometry [Citation19] and micellar electrokinetic chromatography [Citation20]. Dextromethorphan hydrobromide (DEX), [(+)-3-Methoxy-17-methyl-9α, 13α, 14α morphinan hydrobromide monohydrate] is a cough suppressant that is used for the relief of non-productive cough; it has a central action on the cough centre in the medulla [Citation21]. Dextromethorphan hydrobromide (DEX) is an antitussive drug that is used for pain relief and psychological applications [Citation22]. The chemical structures of DEX are shown in . The combination of these drugs is used as an antitussive and mucolytic in bronchitis and chronic pulmonary conditions. Several analytical techniques have been reported in the literature, most commonly liquid chromatography [Citation23,Citation24], first and second-derivative UV spectrophotometric techniques [Citation25–Citation27], capillary electrophoresis [Citation28], and gas chromatography [Citation29]. Guaiphenesin (GUA; ), (2RS)-3-(2-methoxyphenoxy) propane-1, 2-diol [Citation30], is reported to increase the volume and reduce the viscosity of tenacious sputum and is used as an expectorant for productive cough. GUA is the glyceryl ether of guaiacol (a constituent of guaiac resin from the wood of Guajacum officinale Linne) and acts as an expectorant by increasing the volume and reducing the viscosity of secretions in the trachea and bronchi. GUA is the component of numerous cough and cold preparations available worldwide. Additionally, GUA has been given to patients with altered nasal mucociliary clearance associated with HIV infection [Citation31]. A literature survey revealed that some techniques have been published for the determination of guaiphenesin either alone or in combinations with other drugs by capillary gas chromatography [Citation32], HPLC [Citation33], LC-MS [Citation34], and LC-MS/MS [Citation35,Citation36].
Fig. 1 Chemical structure of interacting compounds of (a) bromhexine hydrochloride (b) chlorpheniramine maleate (c) dextromethorphan hydrobromide (d) guaiphenesin.
There is no method reported for the simultaneous estimation of bromhexine hydrochloride (BROM), chlorpheniramine maleate (CHL), dextromethorphan hydrobromide (DEX) and guaiphenesin (GUA) in a combined dosage form. Therefore, we communicate here a rapid and cost-effective quality-control tool and a reliable method for the simultaneous assay of mixtures of these four drugs. The method should have sufficient accuracy and precision and permit a simple and time-saving assay for mixtures of BROM, CHL, DEX and GUA.
2 Materials and methods
2.1 Apparatus
To develop a suitable LC method for the analysis of BROM, CHL, DEX and GUA in their combined dosage form, different mobile phases were tried. The chromatographic system consists of a pump (Shimadzu LC 10AT VP) with a universal loop injector (Rheodyne 7725i) with an injection capacity of 20 μL. The detector consists of a photodiode array detector (PDA), a SPD-10 AVP UV-Visible detector and a Chromatopak C18 (25 cm × 4.6 mm i.d. × 5 μm) column. The equipment was controlled by a PC work station equipped with CLASS M 10-VP software (Shimadzu, Kyoto, Japan). A UV/Visible double beam spectrophotometer (Shimadzu Model 1700) was employed with a spectral bandwidth of 1 nm and a wavelength accuracy of 0.3 nm (with automatic wavelength correction using a pair of 1 cm matched quartz cells).
2.2 Reagents and materials
Pure drug samples of BROM, CHL, DEX and GUA were generously obtained as a gift from TABLIKE and SCHON Pharmaceutical (Indore, India). The tablet dose form, MARICOF (Label claim: 8.0 mg BROM, 2.0 mg CHL, 10.0 mg DEX and 100.0 mg GUA), was procured from the local market (manufactured by G.S. Pharmaceuticals Pvt. Ltd., Roorkee, India). HPLC grade methanol and acetonitrile were obtained from Merck (Mumbai, India).
2.3 Chromatography conditions
The solubility of the four drugs indicated that the reverse phase chromatographic method would be best option for the simultaneous estimation of BROM, CHL, DEX, and GUA. The mobile phase consists of an organic phase of methanol, acetonitrile and 0.025 M phosphate buffer at a ratio of 50:25:25 (v/v/v adjusted to pH 5.5 using orthophosphoric acid). The mobile phase and working solutions were filtered through a 0.2 μm nylon filter and degassed using a sonicator before use. To determine the appropriate wavelength for the simultaneous determination of BROM, CHL, DEX, and GUA, solutions of these compounds were scanned on a UV–vis spectrophotometer in the range 200–400 nm. The suitable wavelength to monitor these drugs was chosen from the overlaid UV spectra (265 nm).
2.4 Preparation of standard stock solutions
Standard stock solutions of BROM, CHL, DEX and GUA were prepared separately by accurately weighing 10.0 mg of each of BROM, CHL, DEX and GUA (reference standard), transferring it to a 100 ml volumetric flask and dissolving it in 20.0 ml of HPLC grade methanol. The solutions were sonicated in bath sonicator for 10 min to ensure complete solubilisation. After sonication, the volume was brought to 100 ml with same HPLC grade methanol at a final concentration of 0.1 mg/ml (100 μg/ml) of each reference standard.
A combined standard solution containing BROM, CHL, DEX and GUA was prepared by adding 160 mg, 40 mg, 200 mg and 2000 mg of each reference standard, respectively, transferring it to a 1000 ml volumetric flask, and adding 200 ml of HPLC grade methanol. The solution was sonicated in bath sonicator for 10 min to ensure complete solubilisation. After sonication, the volume was brought to 1000 ml with same diluent, to result in final concentrations of 160 μg/ml of BROM, 40 μg/ml of CHL, 2 μg/ml of DEX and 2000 μg/ml of GUA, respectively.
2.5 Estimation from pharmaceutical dosage form
Twenty tablets of MERICOF were weighed to calculate the average weight of one tablet. They were homogenised to a fine powder, transferred to a 1000.0 ml volumetric flask, dissolved in 200.0 ml of diluent (HPLC grade methanol) and sonicated in a bath sonicator for 20.0 min to ensure complete solubilisation. After sonication, the supernatant was transferred to a 1000.0 ml volumetric flask by filtering through Whatman #41 filter paper. The residue was washed three times with 10.0 ml of methanol and the combined filtrate was brought to 1000.0 ml with the same diluent to achieve final concentrations of 160.0 μg/ml of BROM, 40.0 μg/ml of CHL, 2.0 μg/ml of DEX and 2000.0 μg/ml of GUA, respectively.
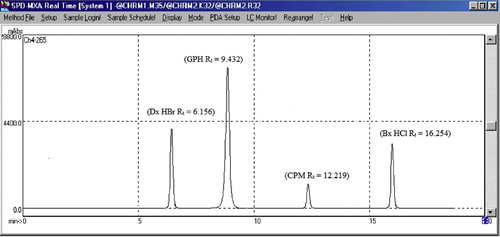
A constant volume of the sample solution was injected six times under the conditions described above. The chromatogram showed that the retention times of BROM, CHL, DEX and GUA were 16.254, 12.219, 6.156 and 9.432, respectively, with a resolution of 3.15 between DEX and GUA, 2.72 between GUA and CHL, and 3.98 between CHL and BROM. The capacity factor, tailing factor, theoretical plate number results are reported in . The total run time was 20 min. The peak areas were measured at 265 nm for BROM, CHL, DEX and GUA, respectively, and their concentrations in the samples were determined using a multi-level calibration curve and linear regression equation using the same conditions on the same HPLC system.
Table 1 Data for the evaluation of the system suitability.
2.6 Preparation of solutions to determine linearity
From the standard stock solution 1, 100.0 μg/ml of each (BROM, CHL, DEX and GUA), of the different working standards were prepared at the following concentrations to determine linearity: 4.0, 8.0, 12.0, 16.0, 20.0 and 24.0 μg/ml for BROM; 5.0, 10.0, 15.0, 20.0, 25.0 and 30.0 μg/ml for CHL; and 10.0, 20.0, 30.0, 40.0, 50.0 and 60.0 μg/ml for DEX and GUA, respectively. Six replicates of each different working standard were prepared for each drug. The peak areas were plotted against the corresponding concentrations to obtain the calibration graphs.
2.7 Analytical method validation
The method was validated for analytical procedures according to ICH guidelines to determine the linearity, sensitivity, precision and accuracy for the analyte. A system suitability test of the chromatography system was performed before each validation run. Five replicate injections of a system suitability standard and one injection of a test standard were made. Regression characteristics, validation and system suitability parameters for BROM, CHL, DEX and GUA in their pharmaceutical dosage form are shown in .
Table 3 Summary of validation parameters.
2.7.1 Linearity
The method was linear from 4.0 μg/ml to 24.0 μg/ml for BROM, 5.0 to 30.0 μg/ml for CHL, and 10.0 to 60.0 μg/ml for both DEX and GUA, respectively. The calibration curve was plotted using area vs. the concentration each compound, and had an R2 value of 0.9980 or greater.
2.7.2 Accuracy
A recovery study for MERICOF was carried out per ICH guidelines [Citation37], where a known concentration of all four standards solutions (equivalent to 80, 100, and 120% of total drug content) was added to a pre-analysed solution of the tablet formulation and the percentage of recovery was calculated.
2.7.3 Precision
Intra- and inter-day precision studies for MERICOF were calculated by assaying the sample solution (marketed formulation) on the same day and different days at different time intervals, respectively. The assay was performed with at least six replicates of the sample solution. An amount of the sample powder equivalent to 100% of the label claim of BROM, CHL, DEX and GUA was accurately weighed and assayed. Method repeatability was achieved by repeating the same procedure six times on the same day for intra-day precision. The intermediate (inter-day) precision of the method was checked by performing the same procedure on different days under the same experimental conditions.
2.7.4 Limit of detection and limit of quantitation (LOD and LOQ)
For LOD and LOQ, 10.0 μg/ml of all four standard solutions were prepared from each of the 100.0 μg/ml standard stock solutions (BROM, CHL, DEX and GUA): 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 μg/ml working dilutions for BROM and CHL; 0.3, 0.5, 0.7, 0.9, 1.1 and 1.3 μg/ml working dilutions for DEX; and 0.3, 0.4, 0.5, 0.6, 0.7, 0.8 μg/ml working dilutions for GUA. LOD and LOQ values were calculated to assess the detection limit of the method using the following equation, per ICH guidelines:where σ is the standard deviation of y-intercepts of regression lines and S is the slope of the calibration curve.
2.7.5 Selectivity and specificity
A combination of methanol:acetonitrile:0.025 M phosphate buffer, pH 5.5 (50:25:25, v/v) was used as a specific mobile phase, and 265.0 nm was selected as a specific analytical wavelength to simultaneously determine the levels of BROM, CHL, DEX and GUA in a marketed formulation (MERICOF) using an HPLC method. Specificity was assessed by a qualitative comparison between chromatograms obtained from sample, standard, blank and placebo solutions. The diluent was injected as a blank. A placebo () interference study was conducted by injecting a placebo solution prepared from the excipients most commonly used in pharmaceutical formulations, including starch, lactose monohydrate, and magnesium stearate.
3 Results and discussion
A new, rapid, sensitive and accurate RP-HPLC method was developed for the simultaneous estimation of bromhexine hydrochloride, chlorpheniramine maleate, dextromethorphan hydrobromide and guaiphenesin in pharmaceutical formulations. After trying different columns, the final choice for the stationary phase that gave satisfactory resolution and run time was the reverse-phase C18 Chromatopack Peerless (25 cm×4.6 mm i.d.×5 μm) column. There were many mobile phases that were tested to resolve all four chromatographic peaks, including methanol:water (80:20, v/v) and methanol:water (50:50, v/v), but the broadness of the peaks did not produce satisfactory results in these chromatograms. To improve the sharpness of the chromatographic peaks, we worked with slightly acidic acetonitrile and phosphate buffer. Finally, the mobile phase methanol:acetonitrile:0.025 M phosphate buffer (50:25:25, v/v/v) (pH: 5.5) adjusted with O-phosphoric acid was found to be satisfactory as it gave four symmetric peaks for BROM, CHL, DEX and GUA. The total run time was 20 min at a 1.0 ml min−1 rate and ambient temperature. The retention times of bromhexine hydrochloride, chlorpheniramine maleate, dextromethorphan hydrobromide and guaiphenesin were 16.254 min, 12.219 min, 6.156 min and 9.432 min (), respectively. The best fit for the calibration curve (peak area vs. respective concentrations) could be achieved by separate linear regression equations, which were y = 9040x + 9993 (BROM), y = 9579x − 308 (CHL), y = 25,935x + 25,500 (DEX) and y = 34,255x + 14,194 (GUA). The proposed method was evaluated for formulations containing BROM, CHL, DEX and GUA. Four replicate determinations were performed using capsules, and found 100.45% for BROM, 100.25% for CHL, 99.88% for DEX and 100.56% for GUA. Specificity was assessed by comparing the chromatogram of the tablet solution with the placebo solution and also with the chromatograms obtained from the standard drugs. The retention time of all four drugs was the same in the quaternary mixed standard solutions as well as the marketed formulation (MERICOF) solution, and there was also no interference from the excipients. This indicates the specificity of the method for quantitative estimation of BROM, CHL, DEX and GUA in a marketed formulation (). The recoveries of all of the components were between 99.0 and 102%. The LOD and LOQ values were 21.49 and 65.13 ng ml−1, 29.17 and 88.39 ng ml−1, 11.65 and 35.51 ng ml−1, and 8.1 and 24.57 ng ml−1 for BROM, CHL, DEX and GUA, respectively (). The excipients did not interfere with the peaks of interest. Hence, the proposed method is applicable for the routine simultaneous estimation of BROM, CHL, DEX and GUA in pharmaceutical dosage forms.
Table 2 Results from assay of the marketed formulation.
4 Conclusions
In the present work, we successfully and simultaneously analysed BROM, CHL, DEX and GUA in a marketed formulation (MERICOF) using an RP-HPLC method based on a literature survey. All of the critical steps for developing the method have been summarised and prioritised. To develop an effective RP-HPLC method, most of the effort should be spent in method development and optimisation, as this emphasis will improve the final method performance. The method validation, however, should be treated as an exercise to summarise or document the overall method performance for its intended purpose. Thus, the present method is rapid, easy and accurate for the simultaneous estimation of BROM, CHL, DEX and GUA in a commercially available pharmaceutical formulation.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors are thankful to TABLIKE and SCHON Pharmaceutical, Jumbu Di Hapsi Hatod Road, Indore, M.P, for generously providing drug samples and Head, School of Pharmacy, DAVV Indore for providing necessary facilities and chemicals. VJ thanks the All India Council for Technical Education (AICTE), New Delhi, India for financial support for this research. We are grateful to the referees for their valuable suggestions.
Notes
Peer review under responsibility of Taibah University.
References
- L.ParvezM.VaidyaA.SakhardandeS.SubburajT.G.RajagopalanEvaluation of antitussive agents in manPulm. Pharm.91996299308
- D.M.CobbinF.M.ElliottA.S.RebuckThe mucolytic agent bromhexine (bisolvon) in chronic lung disease. A double-blind crossover trialAust. N. Z. J. Med.11971137140
- J.P.RauhaH.SalomiesM.AaltoSimultaneous determination of bromhexine hydrochloride and methyl and propyl p-hydroxybenzoate and determination of dextromethorphan hydrochloride in cough-cold syrup by high-performance liquid chromatographyJ. Pharm. Biomed. Anal.151996287293
- E.V.RaoG.R.RaoS.RaghuveerP.KhadgapathiGas–liquid chromatographic and ion-pair high-performance liquid chromatographic determination of pseudoephedrine hydrochloride and bromhexine hydrochloride in pharmaceuticalsAnalyst1121987871874
- O.W.LauY.M.CheungSimultaneous determination of some active ingredients in cough–cold syrups by gas–liquid chromatographyAnalyst115199013491353
- C.E.BohJ.A.RudyL.R.SomaM.FennellL.MayR.SamsF.A.RailingJ.ShellenbergerM.KahlerCharacterization of bromhexine and ambroxol in equine urine: effect of furosemide on identification and confirmationJ. Pharm. Biomed. Anal.1919913339
- J.P.RauhaH.SalomiesM.AaltoSimultaneous determination of bromhexine hydrochloride and methyl and propyl p-hydroxybenzoate and determination of dextromethorphan hydrobromide in cough-cold syrup by high-performance liquid chromatographyJ. Pharm. Biomed. Anal.151996287293
- M.VasudevanS.RavisankarM.GeorgeJ.RaviSimultaneous estimation of terbutaline, bromhexine and guaiphenesin in soft gelatin capsules by HPLC methodIndian Drugs372000489492
- H.N.DaveR.C.MashruA.K.PatelThin layer chromatodraphy method for the determination of ternary mixture containing salbutamol sulphate, bromhexine hydrochloride and etofyllineJ. Pharm. Sci. Res.22010143148
- V.GuptaM.VermaU.MisraR.K.NemaSimultaneous spectrophotoetric estimation of bromhexine hydrochloride and pseudoephedrine hydrochloride in tablet dosagesAsian J. Chem.21200916331635
- A.K.GuptaS.G.KaskhedikarDerivative spectrophotometric estimation of amoxycillin and bromhexine hydrochloride in tabletsAsian J. Chem.152003977980
- S.K.PandaA.K.SharmaL.K.SahuSimultaneous analysis of phenylpropanolamine, chlorpheniramine and bromhexine in syrups by derivative spectrophotometryIndian J. Pharm. Sci.642002540544
- S.GangwalP.TrivediSimultaneous determination of terbutaline sulphate, bromhexine hydrochloride and guaiphenesin in three-component tablet formulation by UV spectrophotometryIndian J. Pharm. Sci.611999128130
- D.M.PatonD.R.WebsterClinical pharmacokinetics of H1-receptor antagonists (the antihistamines)Clin. Pharmacokinet.101985477497
- M.MaithaniR.RaturiG.VertikaD.KumarDevelopment and validation of RP-HPLC method for the determination of chlorpheniramine maleate and phenylephrine HCl in pharmaceutical dosage formInt. Res. J. Pharm.5201014
- D.B.WanjariV.V.ParasharS.N.LulayM.R.TajneN.J.GaikwadSimultaneous HPLC estimation of acetaminophen, chlopheniramine maleate, dextromethorphan hydrobromide and pseudoephedrine hydrochloride in tabletsIndian J. Pharm. Sci.662004345434
- H.SenyuvaT.ÖzdenSimultaneous high-performance liquid chromatographic determination of paracetamol, phenylephrine HCl, and chlorpheniramine maleate in pharmaceutical dosage formsJ. Chromatogr. Sci.40200297100
- N.HunanS.MultalDensitometric analysis of chlorpheniramine maleate, phenylephrine and acetaminophen by HPTLC methodAnal. Lett.19198678
- J.MurthaT.JulianG.RadebaughSimultaneous determination of pseudoephedrine hydrochloride, chlorpheniramine maleate and dextromethorphan hydrobromide by second-derivative photodiode array spectroscopyJ. Pharm. Sci.771988715717
- L.SuntornsukO.PipitharomeP.WilairatSimultaneous determination of paracetamol and chlorpheniramine maleate by micellar electrokinetic chromatographyJ. Pharm. Biomed.332003441449
- United States Pharmacopoeia, 25th Review, The National Formulary, 19th Review2002US Pharmacopoeia ConventionRockville, MD
- B.KuKanichM.G.PapichPlasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogsJ. Vet. Pharmacol. Ther.272004337341
- L.A.ShervingtonA quantitative simultaneous HPLC determination of pseudoephedrine HCl, guaifenesin and dextromethorphan hydrobromideAnal. Lett.301990927
- B.MistryJ.LeslieN.E.EddingtonA sensitive assay of metoprolol and its major metabolite alpha-hydroxy metoprolol in human plasma and determination of dextromethorphan and its metabolite dextrorphan in urine with high performance liquid chromatography and fluorometric detectionJ. Pharm. Biomed. Anal.16199810411049
- E.K.BendrissN.MarkoglouI.W.WainerHigh-performance liquid chromatography assay for simultaneous determination of dextromethorphan and its main metabolites in urine and in microsomal preparationsJ. Chromatogr. B. Biomed. Sci. Appl.7542001209215
- V.TantishaiyakulC.PoeaknapoP.SribunK.SirisupDerivative spectrophotometric determination of dextromethorphan HBr and bromohexine HCl in tabletJ. Pharm. Biomed. Anal.171998237243
- G.DavidsonL.M.M.MkojiSimultaneous assay of triprolidine, pseudoephedrine and dextromethorphan in combined preparation by derivative difference spectrophotometryJ. Pharm. Biomed. Anal.61988449460
- L.SuntornsukSeparation of cold medicine ingredients by capillary electrophoresisElectrophoresis222001139143
- M.StatheropoulosN.TzamtzisK.MikediShort column gas chromatography-mass spectrometry and principal component analysis for the identification of co eluted substances in doping control analysisJ. Chromatogr. B. Biomed. Sci. Appl.7061998245251
- The British PharmacopoeiaHer Majesty’s2007The Stationary OfficeLondon
- Martindale-Extra PharmacopoeiaThe Complete Drug References34th ed.2005The Pharmaceutical PressLondon, UK
- H.M.Sharaf MagedD.Stiff DwightDetermination of guiafenesin in human serum by capillary gas chromatography and electron capture detectionJ. Pharm. Biomed. Anal.352004801806
- A.El-GindyS.EmaraH.ShaabanDevelopment and validation of chemometrics assisted spectrophotometric and liquid chromatographic methods for the simultaneous determination of two multi-component mixtures containing bronchodilator drugsJ. Pharm. Biomed. Anal.432007973982
- J.WenH.ZhangC.XiaX.HuW.XuX.ChengJ.GaoY.XiongA sensitive liquid chromatography-electrospray ionization-mass spectrometry method for the simultaneous determination of pentoxyverine citrate and guifenesin in human plasma – application to pharmacokinetic and bioequivalence studiesBiomed. Chromatogr.242010351357
- J.FosterDetermination of guifenesin in human plasma using automated liquid–liquid extraction and tandem mass spectrometric detectionAAPS2004 Abstracts AM-2004; 002586
- T.H.EichholdD.L.McCauley-MyersD.A.KhambeG.A.ThompsonS.H.HokeSimultaneous determination of dextromethorphan, dextrorphan and guaifenesin in human plasma using semi-automated liquid/liquid extraction and gradient liquid chromatography tandem mass spectrometryJ. Pharm. Biomed. Anal.432007586600
- International Conference on Harmonization (ICH)Q2B: Text on Validation of Analytical Procedures: Methodologyvol. 621997US FDA Federal register