Abstract
A new sensitive spectrophotometric method was developed to determine three carbapenem antibiotics: imipenem, meropenem and ertapenem. The proposed method was based on the formation of the coloured tris(o-phenanthroline)-iron(II) complex (ferroin) [Phen] or Fe (II)-2,2″-bipyridyl complex [Bip] in the reaction of the tested drugs with the corresponding iron (III)-complexes in an acetate pH 4 buffer. The formed coloured complexes showed maximum absorbance at 510 and 520 nm for [Phen] and [Bip], respectively. The reaction conditions, including the pH, reagent concentration, reaction time, temperature and stability of the formed coloured species, were optimized to achieve the highest sensitivity. Linear calibration curves were obtained in the concentration ranges from 0.2 to 10, 0.5 to 10 and 0.5 to 10 μg mL−1 for the aforementioned drugs in the same order. The developed method was successfully applied to determine the investigated carbapenems in their pharmaceutical formulations with average recoveries of 100.8, 99.8 and 99.4% for the Phen method and 98.9, 101.7 and 100.6% for the Bip method for imipenem, meropenem and ertapenem, respectively. A statistical comparison of the results with the reference method showed good concurrence and indicated no significant difference in accuracy or precision.
1 Introduction
Carbapenems are β-lactam antibiotics, such as penicillins and cephalosporins, that inhibit bacterial cell wall synthesis by binding to penicillin-binding proteins (PBP) [Citation1,Citation2]. The analogues of carbapenem (imipenem, {(5R,6S)-6-[(1R)-1-hydroxyethyl]-3-({2-[(iminomethyl)amino]ethyl}thio)-7-oxo-1-azabicyclo[3.2.0] hept-2-ene-2-carboxylic acid}), meropenem, ({3-[5-(dimethylcarbamoyl) pyrrolidin-2-yl] sulfanyl-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0] hept-2-ene-2-carboxylic acid), and ertapenem ({(4R,5S,6S)-3-[(3S,5S)-5-[(3-carboxyphenyl)carbamoyl]pyrrolidin-3-yl]sulfanyl-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid}), which are used in treatment, have an exceptionally broad spectrum of antibacterial activity. Since the late 1970s, when thienamycin was discovered, the next analogues of carbapenem have been introduced into therapeutic use, which include imipenem, panipenem, meropenem, ertapenem, biapenem and doripenem. The three carbapenems currently under investigation using the developed method are imipenem, meropenem and ertapenem ().
Several analytical techniques were published for carbapenem analysis [Citation3]. Currently, the most common techniques are chromatographic methods [Citation4–Citation8]. In addition to the chromatographic methods, capillary zone electrophoresis [Citation9,Citation10] and a microbiological assay [Citation11] were also reported. The determination of imipenem and its metabolites in human urine and pharmaceutical formulations using electrochemical methods were reported [Citation12,Citation13]. The electrochemical protocol was performed in phosphate buffer solutions over a pH range of 2.0 to 8.0 using differential pulse polarography, cathodic adsorptive stripping voltammetry, cyclic voltammetry, linear sweep voltammetry and adsorptive stripping voltammetry. The proposed methods have been used for the direct determination of imipenem in spiked human urine and real human-derived urine with good results and should be appropriate for monitoring purposes with a detection limit of 0.28 μg L−1 imipenem.
Spectrophotometric methods were also reported for the determination of carbapenems. Classic UV spectrophotometry has been used to estimate meropenem in powder for injection (wavelength is 298 nm) [Citation14], and first-derivative bivariate procedures have been applied to determine meropenem in the presence of its metabolite (open-ring degradation product) [Citation15]. The UV methods are notably simple, rapid and economical, and they enable drug determination with sufficient reliability.
Drug quality control is a branch of analytical chemistry with a wide impact on public health; thus, the development of reliable, quick and accurate methods for active-ingredient determination is notably important. Spectrophotometric methods are the most commonly used techniques and continue to enjoy wide popularity [Citation16–Citation18]. The common availability of the instrumentation, simplicity of the procedures, and speed, precision and accuracy of the technique make spectrophotometric methods attractive. Redox reactions have been used as the basis to develop simple and sensitive spectrophotometric methods to determine many pharmaceutical compounds [Citation19–Citation21]. In oxidimetric reactions, the most commonly used oxidizing agents is Fe (III), which reduces to Fe (II), followed by complexation with either 1,10-phenanthroline [Phen] or 2,2″-bipyridyl [Bip] with an absorbance at 510 or 520 nm, respectively. The present study was dedicated to investigate the application of these reagents in a new spectrophotometric determination of three carbapenem derivatives (imipenem, meropenem and ertapenem) in their pharmaceutical dosage forms. The proposed methods can be used for quality control analysis, where modern and expensive apparatuses, such as GLC, HPLC and HPTLC, are not available.
2 Experimental
2.1 Materials and reagents
All chemicals were of analytical reagent grade, and double-distilled water was used throughout the experiments. The carbapenem pharmaceutical preparation vials were purchased from the Kingdom Saudi Arabia local drug stores. The pharmaceutical preparations were as follows: Tienam (Tienam*-500 imipenem/cilastain sodium, Merck Sharp & Dohme B.V. Harlem, Netherlands), Meronem (Meronem™, meropenem trihydrte 500 mg, Astra Zeneca UK limited, Macclesfield, Cheshire, Sk 10 2NA, United Kingdom) and Ertapenem (Invanz®, 1-g vials, BN: NE20790, labelled to contain 1 g of ertapenem (equivalent to 1.046 g of ertapenem sodium, 175 mg of sodium bicarbonate). The concentrations of the corresponding active ingredients were estimated according to the official methods [Citation22,Citation24]. Drug stock solutions (100 μg m L−1) were always freshly prepared on the day of analysis and stored in a refrigerator to be used within 24 h.
Fe (III)-o-phenanthroline [Phen] reagent [Citation25] was prepared by mixing 0.198 g of 1,10-phenanthroline monohydrate (Fluka, Swiss) with 2.0 mL of 1.0 M HCl and 0.16 g of ferric ammonium sulphate dodecahydrate (Fluka, Swiss) and diluting with bidistilled water to 100 mL. The Fe(III)-bipyridyl [Bip] reagent was prepared by dissolving 0.16 g of 2,2″-bipyridyl in 2.0 mL of 1.0 M HCl and 0.16 g of ferric ammonium sulphate dodecahydrate and diluting with bidistilled water to 100 mL.
2.2 Apparatus
The preliminary spectrophotometric measurements were recorded on a Shimadzu UV-240 spectrophotometer (Kyoto, Japan) with 10-mm light-path cells. A JASCO V-570 (Jasco, Japan) double-beam spectrophotometer (Jasco) was used with 10-mm light-path cells for the absorbance measurements. The software package that accompanied this instrument (Spectra Manager for Windows version 1.21.00) was used for instrument control and data handle, whereas the software Spectra Analysis version 1.24.00 was used to convert to and save the spectra as ASCII-typed files. The pH measurements were performed with a Metrohm 692 pH-metre using a combined electrode. The measurements were performed in a double-jacket thermostated glass cell using a Haake thermosetting circulating water bath with a temperature stability of 70.0 ± 1.0 °C.
2.3 General procedure
Aliquots of different carbapenem standard solutions were transferred into a series of 10-mL calibrated flasks followed by adding 2 mL of [Phen] or [Bip] reagent solution and 2.0 mL of a pH 4.00 acetate buffer solution. The reaction mixture was heated on a water bath at 70 °C for 15 min. After cooling to room temperature, the volume was diluted to the mark with double-distilled water, and the formed coloured complexes were measured at 510 and 520 nm for the [Phen] and [Bip] methods, respectively, against a reagent blank, which was similarly treated. The absorbance values were recorded and plotted against the drug concentration in μg mL−1.
3 Results and discussion
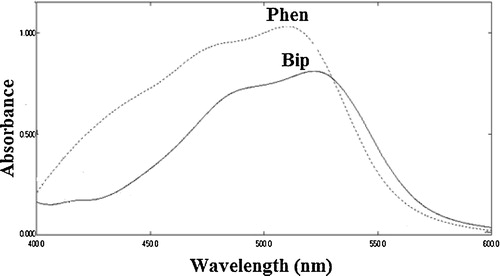
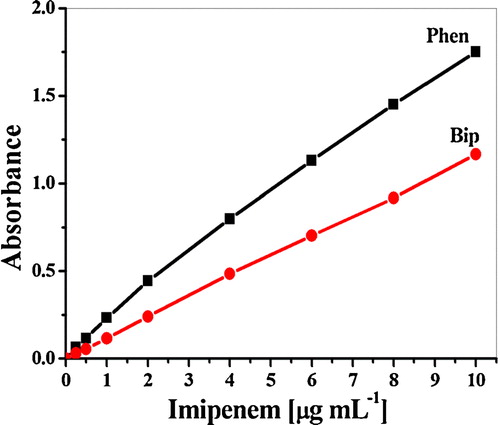
Ferric ions play a prominent role in the spectrophotometric determination of many pharmaceutical drugs by acting as oxidants, whereas a ferric ion is reduced to a ferrous ion in extent equivalent to the drug concentration. The liberated Fe (II) can be determined using selective chelating agents, such as 1,10-phenanthroline and 2,2-bipyridyl [Citation26]. The proposed methods are based on the formation of tris(o-phenanthroline)-iron(II) [Phen] or tris (bipyridyl)-iron(II) [Bip] chelates upon the reduction of the corresponding Fe (III) complexes with different carbapenem derivatives [Citation21,Citation25,Citation27–Citation29] with maximum absorptions at 510 and 520 nm, respectively (). Because of the critical role of the reaction conditions on the analysis performance, comprehensive studies were performed, for example, the effect of the measured wavelength, buffer, concentration and sequence of reagent addition were tested in detail to select the optimal measuring conditions.
3.1 Effect of pH
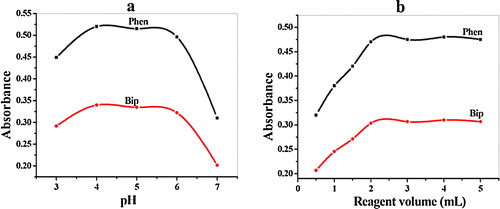
Different buffer media, such as universal, phosphate, borate, and acetate buffer solutions, were examined to achieve the maximum colour intensity at a fixed carbapenem concentration. The acetate buffer proved to be the most favourable buffer with the highest absorbance of the formed coloured complexes. A pH adjustment was necessary, particularly in an acidic medium, because of the precipitation of ferric ions. The studied pH values were from 3.0 to 8.0, and the optimum pH value was 4.00 ± 0.20 (a). Moreover, a 2.0 ± 0.1 mL buffer solution in the reaction medium was sufficient for complete colour development.
3.2 Effect of the reagent concentration
Different amounts of Phen or Bip reagents were added to the reaction mixture, which contained 4 μg mL−1 of imipenium, and the absorbance of the formed coloured complexes was subsequently measured at 510 and 520 nm, respectively. An addition of 2.0 ± 0.2 mL of each reagent was sufficient to obtain the maximum and reproducible absorbance (b). Smaller volumes of the reagents led to incomplete complex formation, whereas higher volumes did not improve the method sensitivity.
3.3 Effect of the reaction temperature and heating time
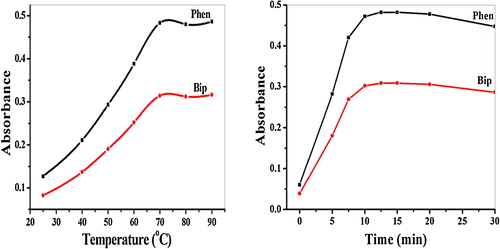
The effects of the temperature and heating time on the development of the coloured complexes were studied under the optimal pH and reagent concentration conditions. The reaction of imipenem with both reagents slowly proceeded at room temperature and was accelerated by heating (). The maximum absorbance was obtained after heating at 70.0 ± 1.0 °C for approximately 15.0 ± 2.0 min. Further heating caused no appreciable change in colour. Moreover, the formed coloured complexes were stable for more than 24 h.
3.4 Method validation
The consistency with Beer’s law was studied by measuring the absorbance values of solutions with various drug concentrations. The analytical parameters, such as the molar absorptivity, Sandell’s sensitivity, detection limit, quantification limit, slope, intercept, and correlation coefficients, were described (). The calibrations graphs are described by the regression equation: Y = a + bX (where Y = absorbance, a = intercept, b = slope and X = concentration in μg mL−1), which was obtained using the method of least squares.
Table 1 Characteristics of different calibration graph for carbapenem derivatives by developed Phen and Bip methods.
The calibration graphs () were rectilinear in the concentration range of 0.2 to 10 μg mL−1, and the molar absorptivities were 3.50 × 104 to 8.70 × 104. In addition, a detection limit of 0.042 μg mL−1 was obtained for meropenem using the Phen method.
3.5 Accuracy and precision
The accuracy and precision of the methods (within-assay and between assays) were determined at different imipenem concentrations (). The within-assay precision was assessed by analysing five replicates of each sample as a batch in a single assay run, and the between-assays precision was assessed by analysing the same sample in triplicate in two separate assay runs. The average recoveries were 95.0–104%, and the relative standard deviations (RSD) were less than 1.3% (). The obtained level of precision was suitable for the quality control analysis of imipenem.
Table 2 Accuracy and precision of the proposed methods for analysis of imipenem (n = 5).
3.6 Interference studies
In pharmaceutical analysis, it is important to test the selectivity of the method for the excipients that are commonly added to pharmaceutical preparations, such as glucose, starch, talc, lactose, and sucrose. The proposed spectrophotometric methods have the advantages that the measurements are performed in the visible region, away from the UV-absorbing interfering substances that may be present in the dosage forms. Regarding the interference of the common excipients and additives in pharmaceutical formulations (sodium lauryl sulphate, magnesium stearate, starch sodium glycolate, lactose spray dried, carboxymethylcellulose PA 102, talc, titanium dioxide, microcrystalline cellulose, hydroxypropylcellulose and pre-gelatinized starch), no change in the absorbance of the formed complex was observed in the presence of such interferents, which indicates the high selectivity of the proposed methods.
3.7 Analytical applications
The obtained satisfactory validation results made the proposed methods suitable for the routine quality control analysis of carbapenem derivatives (imipenem, ertapenem and meropenem) in their pharmaceutical preparations. The results of the proposed methods were statistically compared with those of the official pharmacopoeia method. The obtained mean values of the labelled amounts were 98.90 and 101.7% using the Phen and Bip methods, respectively, as recorded in . In the t- and F-tests, no significant difference was found between the calculated and theoretical values of both the proposed and reported methods at the 95% confidence level [Citation22–Citation24]. These results evidently show that all of the proposed methods can be applied to analyze different carbapenems in their formulations with a comparable analytical performance.
Table 3 Determination of carbapenem in pharmaceutical preparations by the proposed and official method.
4 Conclusion
The presently proposed method represents simple and sensitive new spectrophotometric measurements of different carbapenem derivatives in their pharmaceutical formulations. The redox reaction of carbapenem using Fe3+ and the formation of Fe2+-Phen and Fe2+-Bip complexes were used to develop a simple, accurate, highly sensitive, and selective spectrophotometric method. The proposed methods can be used in quality control laboratories as alternative methods to the official ones for the routine determination of the aforementioned formulations.
Acknowledgments
The authors acknowledge the support from the project 9030104 NRC.
Notes
Peer review under responsibility of Taibah University
References
- J.KohlerK.L.DorsoK.YoungG.G.HammondJ.RosenH.KroppL.SilverIn vitro activities of the potent, broad-spectrum carbapenem MK-0826 (L-749,345) against broad-spectrum β-lactamase-and extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli clinical isolatesAntimicrob. Agents Chemother.431999117011760
- R.MendezT.AlemanyJ.Martin-VillacortaStability of the Penems SCH 29482 and FCE 22101 in aqueous solutionChem. Pharm. Bull.401992204420488
- J.Cielecka-PiontekK.MichalskaP.ZalewskiS.ZasadaComparative review of analytical techniques for determination of carbapenemsCurr. Anal. Chem.8201291115
- T.LegrandS.ChhunE.ReyB.BlanchetJ.ZaharF.LanternierdG.PonsaV.JullienSimultaneous determination of three carbapenem antibiotics in plasma by HPLC with ultraviolet detectionJ. Chromatogr. B8752008551556
- A.K.MajumdarD.G.MussonK.L.BirkC.J.KitchenS.HollandJ.McCreaG.MistryM.HesneyL.XiS.X.LiR.HaesenR.A.BlumR.L.LinsH.GreenbergS.WaldmanP.DeutschJ.D.RogersPharmacokinetics of ertapenem in healthy young volunteersAntimicrob. Agents Chemother.462002350635011
- Y.OzkanI.KucukguzelS.A.OzkanH.Y.Aboul-EneinA rapid, sensitive high performance liquid chromatographic method for the determination of meropenem in pharmaceutical dosage form, human serum and urineBiomed. Chromatogr.152001263266
- D.G.MussonK.L.BirkC.J.KitchenJ.ZhangJ.Y.HsiehW.FangA.K.MajumdarJ.D.RogersAssay methodology for the quantitation of unbound ertapenem, a new carbapenem antibiotic, in human plasmaJ. Chromatogr. Anal. Technol. Biomed. Life Sci.783200319
- J.B.GordienE.BoselliC.FleureauB.AllaouchicheG.JanvierO.LalaudeM.C.SauxD.BreilhDetermination of free ertapenem in plasma and bronchoalveolar lavage by high-performance liquid chromatography with ultraviolet detectionJ. Chromatogr. Anal. Technol. Biomed. Life Sci.8302006218223
- Y.MrestaniR.NeubertF.NagelCapillary zone electrophoresis determination of meropenem in biological media using a high sensitivity cellJ. Pharm. Biomed. Anal.201999899903
- S.TaniguchiK.HamaseA.KinoshitaK.ZaitsuSimple and rapid analytical method for carbapenems using capillary zone electrophoresisJ. Chromatogr. B7271999219225
- M.A.Al-MeshalM.A.RamadanK.M.LotfiA.M.ShiblDetermination of meropenem in plasma by high-performance liquid chromatography and a microbiological methodJ. Clin. Pharm. Ther.201995159163
- R.Fernandez-TorresM.NavarroB.LopezM.MochonJ.SanchezUrea as new stabilizing agent for imipenem determination: electrochemical study and determination of imipenem and its primary metabolite in human urineTalanta772008241248
- A.HilaliJ.C.JiménezM.CallejónM.A.BelloA.GuiraúmElectrochemical study of imipenem’s primary metabolite at the mercury electrode: voltammetric determination in urineJ. Pharm. Biomed. Anal.382005768775
- N.HassanE.Abdel-MoetyN.ElragehyM.RezkSelective determination of ertapenem in the presence of its degradation productSpectrochim. Acta A722009915921
- N.A.ElragehyE.M.Abdel-MoetyN.Y.HassanM.R.RezkStability-indicating determination of meropenem in presence of its degradation productTalanta7720082836
- J.M.CalatayudL.L.ZamoraReference Module in Chemistry. Molecular Sciences and Chemical Engineering2013Elsevier
- D.P.TzanavarasD.G.ThemelisReview of recent applications of flow injection spectrophotometry to pharmaceutical analysisAnal. Chim. Acta588200719
- A.A.GoudaM.I.El-SayedA.S.AminSpectrophotometric and spectrofluorometric methods for the determination of non-steroidal anti-inflammatory drugs: a reviewArab. J. Chem.62013145163
- A.A.GoudaA.S.AminCopper(II)–neocuproine reagent for spectrophotometric determination of captopril in pure form and pharmaceutical formulationsArab. J Chem.32010159165
- A.S.AminI.S.AhmedH.A.DessoukiE.A.GoudaUtility of oxidation–reduction reaction for the determination of ranitidine hydrochloride in pure form, in dosage forms and in the presence of its oxidative degradatesSpectrochim. Acta Part A592003695703
- M.S.KamelE.KhaledA.M.HomodaB.N.BarsoumSpectrophotometric microdetermination of anti-diabetic drug metformin HCl in pharmaceutical formulation and biological fluidsAnal. Chem. Indian J.102011825830
- Monograph of Imipenem7th ed.2010European Pharmacopoeia22302231
- Monograph of Imipenem32nd ed.2008U.S. PharmacopeiaUSA26162617
- Monograph of Meropenem trihydrate7th ed.2010European Pharmacopoeia24502451
- A.S.AminM.ZakyH.M.KhaterA.M.El-BeshbeshyNew colorimetric methods for microdetermination of melatonin in pure and in dosage formsAnal. Lett.32199914211434
- Z.MarczenkoSpectrophotometric Determination of element.1986Ellis Horwood LimitedChichester, Englandp327
- H.A.OmaraA.S.AminSpectrophotometric microdetermination of anti-Parkinsonian and antiviral drug amantadine HCl in pure and in dosage formsArab. J. Chem.42011287292
- L.M.AbdellazizM.M.HosnyDevelopment and validation of spectrophotometric, atomic absorption and kinetic methods for determination of moxifloxacin hydrochlorideAnal. Chem. Insights620116778
- A.A.GoudaW.S.HassanSpectrophotometric determination of etodolac in pure form and pharmaceutical formulationsChem. Central J.220087