?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this work, the kinetics of the photocatalytic decolourization of methylene blue (MB) is investigated using different surface morphologies of multilayer TiO2 coating onto a glass plate under irradiation from a 55-W household florescent lamp. A simple direct dip-coating technique was used, and the coating properties of TiO2 powder were improved by adding epoxidized natural rubber (ENR) as an organic binder in the coating formulation. The effects of the fundamental parameters that govern the kinetics of the photocatalytic decolourization of MB, such as the mass of TiO2 coated onto the glass plate, the pH and the TiO2 surface morphology, were also studied. The kinetics of the MB decolourization in all cases was found to be pseudo-first-order kinetics and was fitted to the Langmuir–Hinshelwood model. The degraded part of the ENR binder led to generating pores within the surface of the TiO2/ENR film and converting it into porous form, as confirmed by SEM analysis. Furthermore, TGA, FTIR and leachability analyses were conducted to further confirm the depletion of the ENR from the TiO2/ENR film. The kinetics of the MB decolourization and the efficiency of the MB colour removal indicated that the porous TiO2/ENR film becomes approximately twice as fast as the non-porous TiO2/ENR film.
1 Introduction
Among all of the semiconductor photocatalysts, titanium dioxide (Anatase) currently stands as an ideal benchmark photocatalyst in environmental photocatalysis applications because of its many desirable properties, such as being inexpensive and readily available, biologically and chemically inert, and having good photoactivity compared to other semiconductors [Citation1,Citation2]. However, there are two major technical challenges that have remarkably limited its potential field applications. First is the relatively wide band gap of TiO2, which absorbs only 3–4% of the energy of the solar spectrum and has restricted its applications to UV excitation sources [Citation3]. Second, the effective slurry or suspension mode application of TiO2 needs a post-treatment catalyst-recovering step, which is normally difficult, energy consumptive and not cost-effective [Citation4]. Moreover, TiO2 powder has a strong tendency to aggregate, especially at high concentrations, which provides a real limitation to applications to continuous flow systems and suffers from the scattering of incident UV light by the suspended particles [Citation5]. Another important factor that must be considered in photocatalysis applications is the pH of the solution; the pH of the aqueous solution significantly affects TiO2, including the charge on the particles, the size of the aggregates it forms, and the positions of the conductance and valence bands [Citation6,Citation7].
Immobilization of TiO2 powder on solid supports is an alternative convenient method for solving the problem of the post-treatment catalyst powder recovery and for facilitating the photocatalyst usage for long-term applications [Citation8]. However, the photocatalytic efficiency of the immobilized TiO2 system could be less than that of the slurry system due to having less surface area that is accessible for photocatalytic reactions as well as low porosity of the supported catalyst layer [Citation9,Citation10]. In addition, cracking and peeling off of the catalyst layer can also be expected because of the poor adherence of the photocatalyst/support. Thus, it was proven that adding an organic binder, such as epoxidized natural rubber (ENR) combined with phenol-formaldehyde resin (PF) in an immobilizing solution can produce a good quality coating formulation of TiO2 powder onto a solid support [Citation11]. However, mixing more than one organic binder for TiO2 powder immobilization is responsible for generating different types of transformation products and undesirable intermediates during the photocatalytic reaction [Citation12].
In recent years, a large amount of attention has been paid to using a compact household fluorescent lamp as an alternative light source to replace a conventional UV light source. It was found that a very low UV content in a compact fluorescent lamp is sufficient to induce TiO2 particles for a wide range of applications, such as photocatalytic oxidation of biopolymers [Citation13], crosslinked biopolymers [Citation14,Citation15], colourless organic water pollutants [Citation11,Citation12] and cationic dye [Citation16]. Therefore, the aim of this work is to study the decolourization kinetics of methylene blue on the surface of an immobilized TiO2 layer under most significant experimental parameters, such as the mass of TiO2 coated onto a glass plate, pH and surface morphology of the immobilized TiO2 film. Among various types of cationic dyes, MB was chosen as a probe in this study due to its complicated chemical structure and because it is not easy to be removed from wastewater by conventionally used techniques such as biological treatments and chemical precipitation.
2 Materials and methods
2.1 Materials
Titanium (IV) oxide (99% anatase) powder with a particle size of ≤150 μm was obtained from Sigma–Aldrich. Epoxidized natural rubber (ENR50) was obtained from Kumpulan Guthrie Sdn. Bhd., Malaysia. Toluene and acetone were purchased from BDH Analar. Hydrochloric acid (HCl) obtained from R & M Chemicals and sodium hydroxide (NaOH) pellets purchased from Mallinkoot were used to adjust the pH. Methylene Blue dye (c.a. 98%, Colour Index Number: 52015) was purchased from R & M, and summarizes the chemical structure, molecular weight and λmax for MB. All of the materials were used as received without further purification. Ultra-pure water (18.2 MΩ cm−1) was used to prepare all solutions in this study. The chemicals used in this experiment were all of the analytical grade and were used without further purification.
Table 1 Characteristics of Methylene blue dye.
2.2 Instruments and methods
A Daihan Ultrasonic cleaner model WUC-D06H (50–60 KHz) from Scientific Co., Inc. Company was used to homogenize TiO2 dip-coating solutions. A spectrophotometer, model DR 2800 from HACH, was used to determine the concentration of MB. The chemical oxygen demand (COD) was determined by using a high-range digestion solution according to an analytical procedure [Citation17]. In this analysis, the samples were placed in vials that contained a high range of COD digestion solution and a small amount of mercury sulfate. The vials were inverted many times to ensure complete mixing before being placed in a COD reactor (HACH 2000), which was preheated to 150 °C. The samples were kept in the COD reactor for 2 h. The samples were then withdrawn from the reactor and cooled down to room temperature, followed by COD measurement at 620 nm by a DR 2800 HACH spectrophotometer. Digested ultrapure water was used as the blank reference solution, and the difference between the absorbance of the digested blank sample and the digested sample was the COD measurement of the sample. The peel-off rate of the immobilized TiO2/ENR film was observed using sonication according to a published procedure [Citation12]. The leaching ratio (LR) of TiO2/ENR film was calculated according to a published procedure [Citation13]. A scanning electron microscope (SEM) analyser model LEO SUPRA 50 VP Field Emission SEM was used to investigate the surface morphology of the TiO2 layer. The Fourier transform infrared spectroscopy analyser model Nicolet, from Thermo Electron Corporation, was used to study the changes in the functional groups in the TiO2 formulation. A thermogravimetric analyser (TGA) from TA Instruments, model STDQ600, was used to determine the composition of the polymeric binders that were used in TiO2 dip-coating formulation at a heating rate of 10 °C min−1, and scanning was performed from 30 °C to 800 °C under an inert N2 gas flow.
2.3 Preparation of the TiO2 formulation
The TiO2 photocatalyst formulation was obtained by adding a fixed amount of 5 g ENR solution (11.32% solution of ENR in toluene) into an amber bottle that contained 12 g of TiO2 powder. Afterward, 60 mL of acetone was poured into the bottle before being homogenized by a sonication process for 5 h. A simple dip-coating method was used to immobilize the TiO2 formulation onto a glass plate. Glass plates with smooth surfaces on one side with dimensions of 45 mm (width) × 85 mm (height) were used as inert solid support materials for immobilizing the photocatalyst formulation. Varying amounts of TiO2 photocatalyst were produced by dipping and drying a glass plate for a varying number of times in the TiO2 formulation. In the dip-coating method, it was found that the catalyst loading was directly proportional to the number of dips into the TiO2 formulation.
2.4 Photo-reactor and light source
A custom-made glass photo-reactor cell with a length of 58 mm, width of 10 mm and height of 90 mm was used for all photocatalytic experiments. A compact 55-W fluorescent lamp (Qusun) with a UV content of 7.32 Wm−2 and visible 441 Wm−2 was used as the light source. The UV and visible content in a compact 55-W fluorescent lamp was measured using a radiometer (Solar light co. PMA 2100) connected to a UV-A and UV-B broadband detector (PMA 2130) and a visible light detector (PMA 2132). This lamp was placed vertically in contact with the outer surface of the photo reactor cell. Then, 20 mL of 20 mg L−1 MB solution was poured inside the photo-reactor cell. The initial pH of the MB solution was adjusted by adding either 0.1 M HCl or NaOH until the desired pH value was obtained by monitoring with a Metrohm, 827 pH lab. Afterward, an immobilized TiO2/ENR film was placed upright inside the photo-reactor cell and exposed directly to the light source. The aeration flow rate was maintained at 50 mL min−1 throughout the photocatalytic degradation process. The schematic diagram of the complete photo-reactor and light source setup was presented in our previous work [Citation14]. To generate open pores on the surface of an immobilized TiO2/ENR film, the TiO2/ENR film was irradiated in 20 mL ultra-pure water with a 55-W compact fluorescent lamp, and an aeration of 50 mL min−1 was performed for 5 h continuously. The degraded part of the ENR binder during the photocatalytic process acts as a pore-forming agent and converts the surface of the immobilized TiO2/ENR film to be more porous. To monitor the ENR by-product mineralization, the TiO2/ENR film was immersed in ultra-pure water under irradiation with a 55-W compact fluorescent lamp. After 1 h of irradiation, the water sample was collected and replaced with another fresh batch of ultra-pure water using the same TiO2/ENR film until 5 h of irradiation was reached. The collected water samples were subjected to COD testing.
2.5 Photocatalytic efficiency and kinetic study
The change in the concentration of MB during the photocatalytic decolourization process was measured for its respective absorbance using a DR 2800 HACH spectrophotometer, and the results were converted into the corresponding concentrations (C). The photocatalytic efficiency was calculated using the following (Eq. Equation(1)(1)
(1) ):
(1)
(1) where Co and Ct (both in mg L−1) are the initial and remaining concentration of MB in solution, respectively, and Ct is the absorbance at any irradiation time t (min).
The kinetics of the photocatalytic decolourization rate of MB was determined using the Langmuir–Hinshelwood kinetics model, as given in the following (Eq. Equation(2)(2)
(2) ):
(2)
(2)
The pseudo-first-order rate constant, kapp (min−1), was calculated from the slope of ln (Co/Ct) versus irradiation time t.
3 Results and discussion
3.1 Adhesion strength of the TiO2 layer
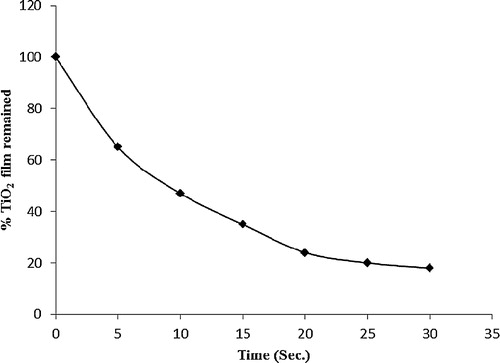
shows the percentage of the TiO2 film that remains attached on the glass plate at different sonication times. As observed, the gradual decreases in the catalyst amount occurred with increasing sonication time. At 30 s of sonication, the percentage of TiO2 that remains is approximately 20%. This relative strength can be attributed to the role of ENR organic binder, which offers good elasticity and adhesion. Thus, ENR acted as an adhesive to strengthen the coating conditions of the immobilized catalyst.
3.2 Effect of TiO2 loading on the MB decolourization kinetics
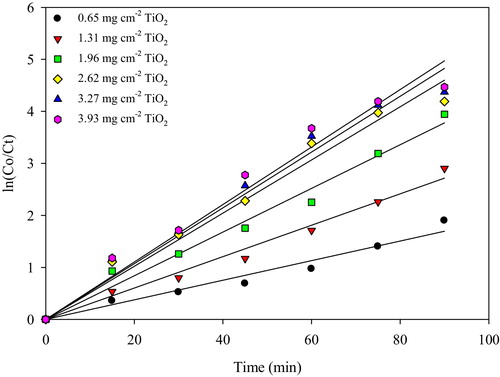
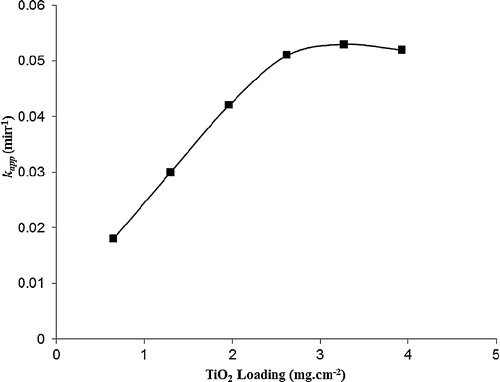
The optimization of TiO2 loading is an important issue that is significant for avoiding the use of excessive TiO2 and ensuring the maximum absorption of photon light. A series of TiO2 loadings that ranged from 0.65 to 3.93 mg cm−2 were obtained by varying the number of dip-coatings on the glass plate. In the dip-coating process, the TiO2 loading and thickness increase almost linearly with an increase in the number of dip coatings [Citation18]. shows that the photocatalytic decolourization of MB was fitted to the Langmuir–Hinshelwood model by plotting ln (Co/Ct) against the irradiation time (t) to obtain straight lines with linear regression coefficients (R2). The pseudo-first-order rate constants, kapp (min−1), were calculated from the slopes of the plots and are presented in . Furthermore, shows the pseudo-first-order rate constants for the photocatalytic decolourization of MB by TiO2/ENR film at different loadings onto the glass plate. From , it can be concluded that the pseudo-first-order rate constant becomes 2.8 times faster by increasing the TiO2 loading from 0.65 to the optimum loading of 2.62 mg cm−2. The SEM analysis shows that the thickness of the TiO2 film at the optimum loading of 2.62 mg cm−2 was 34.31 μm (image not shown). After this loading of 2.62 mg cm−2, no remarkable change in the pseudo-first-order rate constants can be observed. This observation can be attributed to the limited surface area of the solid support (glass plate); in addition, loading more TiO2 leads to increasing the thickness of the immobilized catalyst on the glass plate without significantly increasing the number of photo-induced catalyst particles. A similar observation was also reported by Jawad and Nawi [Citation12] of the photocatalytic performances of the glass/chitosan/TiO2 interface composite film for the degradation of phenol. Below the optimal TiO2 loading, there are insufficient TiO2 particles immobilized on the glass surface, and therefore, less light absorption will occur and there will be less production of oxidative radicals due to the thin TiO2 layer.
Table 2 Linearity of ln Co/Ct versus time for photocatalytic decolourization of 20 mg L−1 MB at different TiO2/ENR loading on glass plate.
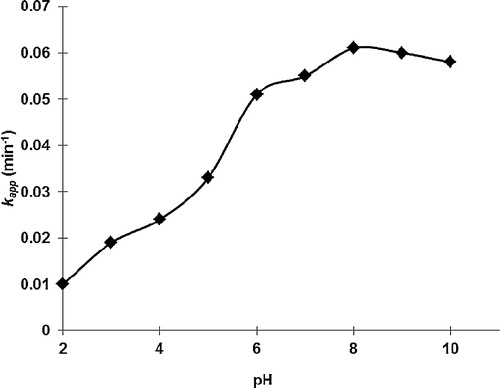
3.3 Effect of pH on the MB decolourization kinetics
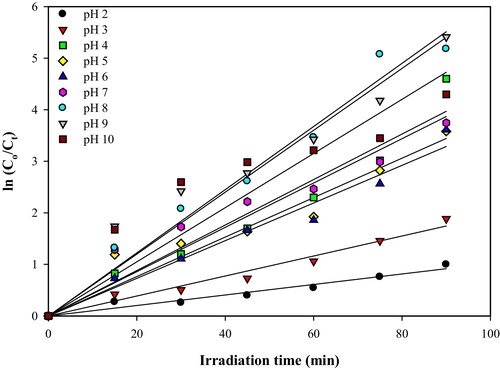
It is well known that pH will influence both the surface state of titanium and the ionization state of the ionizable dye molecules [Citation4]. Thus, shows that the photocatalytic decolourization of MB under a wide range of pH values was fitted to the Langmuir–Hinshelwood model by plotting ln (Co/Ct) versus irradiation time (t). The pseudo-first-order rate constants, kapp (min−1), were calculated from the slopes of the plots and are presented in . The influence of the initial pH of the solution on the calculated pseudo-first-order rate constant for the photocatalytic decolourization of MB is depicted in . As observed, the pseudo-first-order rate constants (kapp) remarkably increased from 0.0102 min−1 to 0.0485 min−1. In other words, the pseudo-first-order rate constant becomes almost five times faster by increasing the initial pH of the MB solution from 2.0 to 8.0, and no more noticeable changes can be observed with further increasing of the pH up to 10.0. Thus, the optimal pH that was obtained was found to be pH ∼8.0. In fact, the point of zero charge (pHpzc) of TiO2 is 6.8 [Citation19]. Therefore, the TiO2 surface becomes positively charged with the species TiOH2+ at pH < pHpzc, negatively charged with the species TiO− at pH > pHpzc, and neutral with the species TiOH at pH = pHpzc [Citation20]. Furthermore, the protonation and deprotonation condition of the photocatalyst surface by taking TiO2 as a model can be shown in Eqs. Equation(3)(3)
(3) and Equation(4)
(4)
(4) :
(3)
(3)
(4)
(4)
Table 3 Linearity of ln Co/Ct versus pH solution of 20 mg L−1 MB at fixed TiO2/ENR loading on glass Plate 2.62 mg cm−2.
In alkaline medium, the TiO2 surface is negatively charged (TiO−) and MB is basically a cationic dye; therefore, a strong electrostatic interaction can be achieved. Another reason is that increasing the pH of the solution increases the amount of hydroxyl radicals generated (because more hydroxyl ions are available on the surface of the TiO2). For both of the above-mentioned reasons, the rate constant of the photocatalytic activity for MB decolourization is expected to increase at high pH values.
3.4 Preparation of porous TiO2/ENR film
The main advantage of using an organic binder for immobilization of the photocatalyst is to generate pores and enhance the surface area of the immobilized photocatalyst by systematic degradation of the binder during the photocatalytic reaction. Moreover, the transformation by-products that are generated from the degraded binder in the treated water can be effectively and totally mineralized by the photocatalytic reaction [Citation21]. Thus, to investigate the effect of the porosity and change in the surface morphology of an immobilized TiO2/ENR film on the photocatalytic decolourization of MB, an experiment was conducted by irradiating TiO2/ENR film in 20 mL ultra-pure water for 5 h. In fact, during the 5-h irradiation process in the presence of ultra-pure water, a partial degradation of ENR catalyst binder will take place [Citation11]. The degraded or depleted part of the ENR will act as a pore-forming agent on the TiO2 surface. To confirm the formation of porous structure on the surface of the TiO2 film and the depletion of the ENR binder, analysis of the surface morphology of the TiO2 in addition to quantitative and qualitative analyses were conducted as described below.
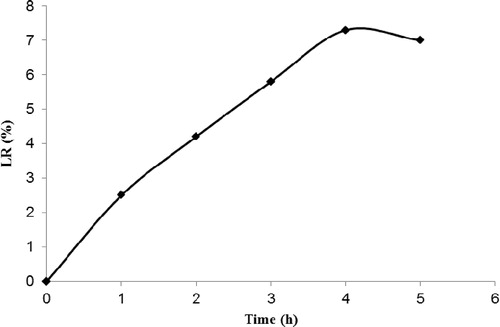
3.4.1 Leaching ratio (LR) measurements
shows the weight loss of the irradiated TiO2/ENR film in ultra-pure water in the form of the leaching ratio (LR) at different times of irradiation. As observed, the maximum LR of the irradiated TiO2/ENR film was 7.3% after 4 h of irradiation, and no remarkable changes can be observed by extending the irradiation time. The weight loss of the TiO2/ENR film was assumed to be due to the leaching or degradation of some of the ENR binder within the TiO2/ENR film. The transformation of the ENR by-products into minerals during the photocatalytic-oxidation process will be discussed separately in detail in the upcoming mineralization study section.
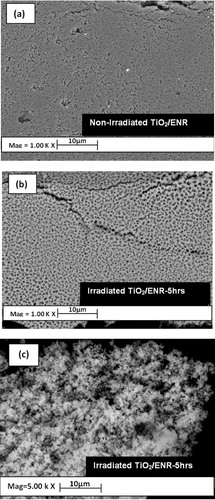
3.4.2 SEM analysis
shows representative SEM images of TiO2/ENR film before and after 5 h of the irradiation process in ultra-pure water. As observed, the surface morphology of the irradiated TiO2/ENR film has remarkably changed to be more porous after the irradiation process. The high proportion of pores generated on the immobilized TiO2 surface was attributed to the degraded part of the ENR binder.
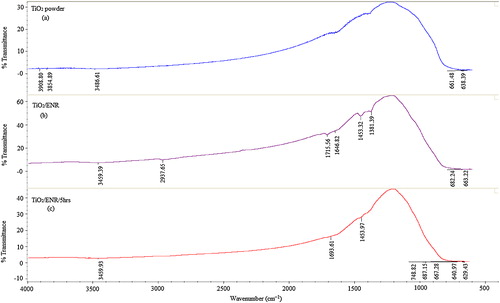
3.4.3 FTIR and TGA analysis
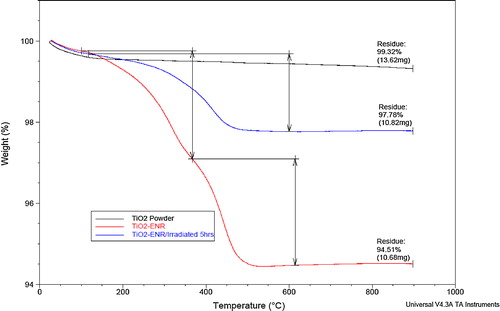
The FTIR spectra of the TiO2 powder, non-irradiated immobilized TiO2/ENR film (non-porous TiO2/ENR film) and irradiated immobilized TiO2/ENR (porous TiO2/ENR film) for 5 h in ultra-pure water are shown in (a)–(c), respectively. In the case of non-porous TiO2/ENR film (spectrum b), the broad band characteristic at 3459.39 cm−1 is attributed to the O–H stretch in the TiO2/ENR formulation. The peak at 2937.65 cm−1 is assigned to asymmetric and symmetric stretching vibration of the methyl (–CH3) group. The band at 1715.56 cm−1 is assigned to the C=O in ketones and saturated aliphatic. The band at 1646.82 cm−1 is attributed to C=C stretching. The bands at 1453.32 cm−1 and 1381.39 cm−1 are assigned to CH2 and CH deformations of the hydrocarbon backbone [Citation22,Citation23]. After 5 h of light irradiation, the intensity of the bands contributed by the ENR polymer was significantly reduced (see spectrum c). This obvious reduction in the band intensities of spectrum (b) indicated clearly the depletion of the ENR binder in the TiO2 formulation during the photocatalytic process. For further confirmation, TGA analysis was conducted for the same samples, and the TGA curves are given in . As observed, the stage degradation pattern of the ENR in the TiO2 formulation occurred at a peak temperature of approximately 380–600 °C. The weight loss was 5.49% and the residual was 94.51% for the non-irradiated TiO2 formulation. On the other hand, the weight loss was 2.22% and the residual 97.78% in the case of TiO2/ENR that was irradiated for 5 h with ultra-pure water. This difference in the weight loss of the TiO2/ENR formulation before and after irradiation indicates clearly that a considerable amount of ENR binder was degraded during the photocatalytic reaction. Thus, the degraded part of the ENR binder acted as a pore-forming agent with the immobilized TiO2 surface.
3.5 Photocatalytic efficiency and control testing
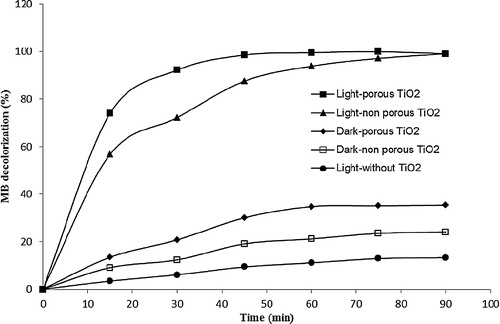
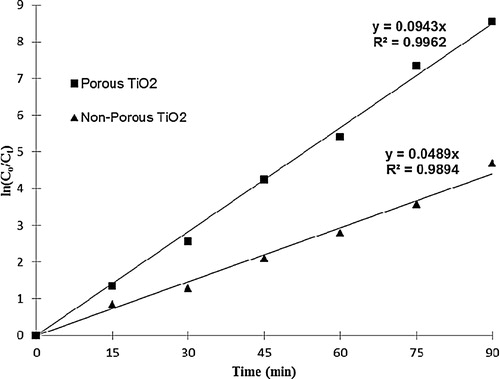
The effects of the key parameters on the decolourization of MB, such as photolysis (light-without TiO2), adsorption (dark-with TiO2), and photocatalysis (light-with TiO2) at different surface morphologies of TiO2/ENR film, were examined. The initial MB concentration was fixed at 20 mg L−1 at the optimal conditions with TiO2 loading at 2.62 mg cm−2 and pH 8.0, as shown in . As observed, the highest removal of MB colour can be achieved by porous TiO2/ENR film in both the processes of photocatalysis and adsorption compared to non-porous TiO2/ENR film. It was found that almost 99.07% of MB decolourization was achieved after 45 min of irradiation using porous TiO2/ENR film. Almost the same percentage (99.01%) of MB decolourization was also reached but after 90 min of irradiation using non-porous TiO2/ENR film. Regarding the adsorption process, the maximum MB colour removal was recorded as 35.42% and 24.08% after 45 min of contact time by using porous TiO2/ENR film and non-porous TiO2/ENR film, respectively. Moreover, the photolysis process was extremely poor, with only 13.25% removed by irradiation with light only and without using TiO2/ENR film. The kinetics study shows the straight lines with linear regression coefficients (R2) that are obtained by plotting ln (Co/Ct) versus irradiation time (t), which indicates that the kinetics of the photocatalytic decolourization of MB by both non-porous and porous TiO2/ENR films was fitted to the Langmuir–Hinshelwood model, as given in . The calculated value of the pseudo-first-order rate constant shows that the photocatalytic decolourization of MB by porous TiO2/ENR film becomes approximately twice as fast as the non-porous TiO2/ENR film, and the pseudo-first-order rate constant value was increased from 0.048 min−1 to 0.094 min−1. The kinetic result was in good agreement with the percentage of MB colour removal. This enhancement of the photocatalytic decolourization and the percentage of decolourization of MB by porous TiO2/ENR film is mainly attributed to the generation of the macro-pores on the surface of the TiO2/glass system. This generation of the macro-pores on the TiO2 surface offers better diffusion of the MB molecules and hydroxyl radicals across the inner layer of the TiO2 [Citation11].
3.6 Mineralization study
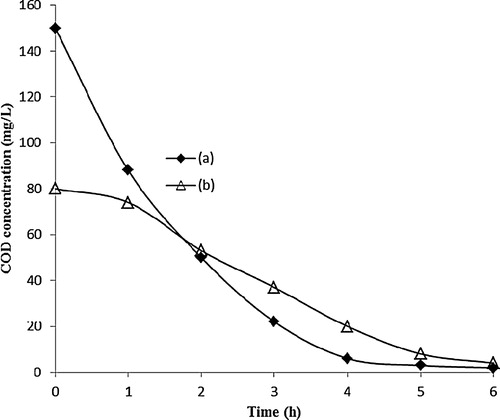
The heterogeneous photocatalytic process usually involves partial or complete mineralization of the organic substances by the oxidizing species that exist on the surface of titanium oxide. In this study, the main advantage of using ENR as an organic binder for immobilization of the TiO2 photocatalyst is that it can be regularly decomposed by the photocatalytic reaction to generate pores and enhance the surface area of the immobilized TiO2 film. Therefore, further verification of the photocatalytic degradation of this ENR binder is required. However, the photocatalytic decolourization of MB by the porous TiO2/ENR film does not necessarily indicate a full mineralization of the MB into CO2 and H2O and some types of mineral acids. In some circumstances, the intermediates produced during the photocatalytic reaction could be even more toxic than the starting materials. Therefore, the chemical oxygen demand (COD) test was conducted as a function of the presence of dissolved organic matter that is attributed to the degradation of ENR within the TiO2 film as well as MB and its by-products during the photocatalytic reaction. In the first step, the immobilized TiO2 film was immersed in ultra-pure water under the irradiation of a 55-W compact fluorescent lamp. After 1 h of irradiation, the water sample was withdrawn and replaced with fresh ultra-pure water using the same immobilized TiO2 film until 6 h of irradiation. The withdrawn water samples were subjected to the COD test. (a) presents the detected COD values of the treated water due to the degraded part of the ENR polymer inside the immobilized TiO2 film system. In the second step, the irradiated TiO2 film that was obtained from step 1 was taken to evaluate the ability of the porous TiO2 in the mineralization of 20 mg/L MB and its intermediates, as shown in (b). It can be observed that MB and its main by-products were mostly mineralized after 5 h of irradiation using an immobilized porous TiO2 film. This finding can be attributed to the porous structure of the immobilized TiO2 film, which led to enhanced MB and hydroxyl radical diffusion into the inner layers of the TiO2 film [Citation11]. In fact, once the porous TiO2 film is illuminated with a 55-W fluorescent lamp with a UV leakage of 7.32 Wm−2, the electrons are excited from the lower valence band (VB) towards the higher conduction band (CB), which leads to the formation of a paired positive hole and negative electron on the surface of the TiO2 film. The absorbed oxygen and molecular water on the surface of the TiO2 film can directly react with the photogenerated electron-hole pair to give O2•−, HO2• or •OH as products (Eqs. (5)–(8)) [Citation24,Citation25]:(5)
(5)
(6)
(6)
(7)
(7)
(8)
(8)
Fig. 12 COD values of (a) treated ultra-pure water sample of irradiated TiO2/ENR film, and (b) of the 20 mg/L irradiated in the presence of TiO2/ENR film.
The .OH radicals that are derived from an irradiated TiO2 film (Eq. (8)) would directly oxidize MB dye in the bulk solution, as in Eq. Equation(9)(9)
(9) :
(9)
(9)
4 Conclusions
The kinetic characteristics of the photocatalytic decolourization of MB using an immobilized TiO2/glass were experimentally determined with respect to the different TiO2 loading, pH conditions and surface morphology of the TiO2 film. A kinetic model was proposed according to the operational parameters. The apparent pseudo-first-order rate constant increased when there was an increase in the loaded amount of TiO2 formulation on the glass surface and remained at almost a plateau after reaching the optimal amount. The same observation was obtained by increasing the pH value towards basic media. It was also found that depletion of the ENR binder from the TiO2 formulation during the photocatalytic process plays a significant role in changing the surface morphology of TiO2 to be more porous and enhancing the diffusion of the MB molecules and increasing the hydroxyl radical penetration to reach the inner layer of the immobilized TiO2. The apparent pseudo-first-order rate constant and decolourization of MB using the porous structure of the TiO2 film became almost two times faster than for the non-porous TiO2.
Acknowledgments
The authors would like to thank the Faculty of Applied Sciences, Universiti Teknologi MARA, Arau Campus, for facilitating this work. Special thanks go to Miss Nur Lia Ali for facilitating the TGA analysis.
Notes
Peer review under responsibility of Taibah University.
References
- M.N.ChongB.JinC.W.K.ChowC.SaintRecent developments in photocatalyticwater treatment technology: a reviewWater Res.44201029973027
- S.Mohammadi-AghdamR.MarandiM.E.OlyaA.A.Mehrdad SharifKinetic modeling of BB41 photocatalytic treatment in a semibatch flow photoreactor using a nano composite filmJ. Saudi Chem. Soc.201310.1016/j.jscs.2013.09.012
- D.B.HamalK.J.KlabundeSynthesis, characterization, and visible light activity of new nanoparticles photocatalysis based on silver, carbon, and sulfur-doped TiO2J. Colloids Interface Sci.3112007514522
- Ch.SuwanchawalitCh.SriwongS.WongnawaRecyclable rubber sheets impregnated with potassium oxalate doped TiO2 and their uses in decolorization of dye-polluted watersInt. J. Environ. Res.42010615628
- L.AndronicA.DutaTiO2 thin films for dyes photodegradationThin Solid Films515200762946297
- S.AhmedM.G.RasulW.N.MartensR.BrownM.A.HashibAdvances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a reviewWater Air Soil Pollut.2152011329
- S.MalatoP.Fernández-IbáñezM.I.MaldonadoJ.BlancoW.GernjakDecontamination and disinfection of water by solar photocatalysis: recent overview and trendsCatal. Today1472009159
- V.VijayabalanK.SelvamR.VelmuruganM.SwaminathanPhotocatalytic activity of surface fluorinated TiO2-P25 in the degradation of reactive orange 4J. Hazard. Mater.1722009914921
- G.MascoloR.ComparelliM.L.CurriG.LovecchioA.LopezA.AgostianoPhotocatalytic degradation of methyl red by TiO2: comparison of the efficiency of immobilized nanoparticles versus conventional suspended catalystJ. Hazard. Mater.1422007130137
- V.K.GuptaR.JainA.NayakS.AgarwalM.ShrivastavaRemoval of the hazardous dye Tartrazine by photodegradation on titanium dioxide surfaceMater. Sci. Eng. C31201110621067
- M.A.NawiA.H.JawadS.SabarW.S.W.NgahImmobilized bilayer TiO2/chitosan system for the removal of phenol under irradiation by a 45 watt compact fluorescent lampDesalination2802011288296
- A.H.JawadM.A.NawiFabrication, optimization and application of an immobilized layer-by-layer TiO2/chitosan system for the removal of phenol and its intermediates under 45-W fluorescent lampReact. Kinet. Mech. Catal.106201220124965
- M.A.NawiA.H.JawadS.SabarS.NgahW.W.S.Photocatalytic-oxidation of solid state chitosan by immobilized bilayer assembly of TiO2–chitosan under a compact household fluorescent lamp irradiationCarbohydr. Polym.83201111461152
- A.H.JawadM.N.NawiCharacterizations of the photocatalytically-oxidized cross-linked chitosan-glutaraldehyde and its application as a sub-layer in the TiO2/CS-GLA bilayer photocatalyst systemJ. Polym. Environ.202012817829
- A.H.JawadM.A.NawiOxidation of crosslinked chitosan-epichlorohydrine film and its application with TiO2 for phenol removalCarbohydr. Polym.9020128794
- A.H.JawadA.F.M.AlkarkhiN.S.Abdul MubarakPhotocatalytic decolorization of methylene blue by an immobilized TiO2 film under visible light irradiation: optimization using response surface methodology (RSM)Desalin. Water Treat.52201411210.1080/19443994.2014.934736
- HACHWater Analysis Handbook2nd ed.1992HACH CompanyUSA
- Y.ChenD.D.DionysiouCorrelation of structural properties and film thickness to photocatalytic activity of thick TiO2 films coated on stainless steelAppl. Catal. B: Environ.6920062433
- N.KashifF.OuyangParameters effect on heterogeneous photocatalysed degradation of phenol in aqueous dispersion of TiO2J. Environ. Sci.212009527533
- C.H.ChiouC.Y.WuR.S.JuangPhotocatalytic degradation of phenol and m-nitrophenol using irradiated TiO2 in aqueous solutionsSep. Purif. Technol.622008559564
- M.A.NawiS.M.ZainEnhancing the surface properties of the immobilized Degussa P-25 TiO2 for the efficient photocatalytic removal of methylene blue from aqueous solutionAppl. Surf. Sci.258201261486157
- W.L.TanM.Abu BakarSynthesis, characterization and impedance spectroscopy study of magnetite/epoxidized natural rubber nanocompositesJ. Alloys Compd.56120134047
- W.A.K.MahmoodM.M.R.KhanM.H.AzarianlSol–gel synthesis and morphology, thermal and optical properties of epoxidized natural rubber/zirconia hybrid filmsJ. Non-Cryst. Solids3782013152157
- U.I.GayaA.H.AbdullahHeterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problemsJ. Photochem. Photobiol. C: Rev.92008112
- K.ElghnijiM.KsibiE.ElalouiSol–gel reverse micelle preparation and characterization of N-doped TiO2: efficient photocatalytic degradation of methylene blue in water under visible lightJ. Ind. Eng. Chem.182012178182